Surgical Science
Vol. 3 No. 6 (2012) , Article ID: 19982 , 10 pages DOI:10.4236/ss.2012.36064
Expression of Cyclooxygenase-2 and Transforming Growth Factor-Beta 1 in Patients with the Early Recurrence of Hepatocellular Carcinoma Following Hepatectomy
1Second Department of Surgery, School of Medicine, Wakayama Medical University, Wakayama, Japan
2Department of Functional Diagnostic Science, Osaka University Graduate School of Medicine, Osaka, Japan
Email: *ma@wakayama-med.ac.jp
Received March 19, 2012; revised April 24, 2012; accepted May 16, 2012
Keywords: Cyclooxygenase-2; Transforming Growth Factor-Beta1; Hepatocellular Carcinoma; Early Recurrence; Hepatectomy
ABSTRACT
Background: Cyclooxygenase-2 (COX-2) and transforming growth factor-beta1 (TGF-β1) are modulated in variety cancers including Hepatocellular carcinoma (HCC). However, there is a paucity of data concerning their role in the pathologic process of recurrence of HCC following hepatectomy. We herein assessed the role of the hepatic expression of COX-2 and TGF-β as predictors for patients with early recurrence within 2 years of HCC diagnosis. Methods: Sixty patients with HCC who underwent curative hepatectomy between 2000 and 2003 were entered in the present study. The immunoreactivity and distribution patterns of COX-2 and TGF-β1 were examined in both the HCC and the adjacent nonHCC tissues of the liver. Risk factors of tumor recurrence within 2 years, including COX-2 and TGF-β1 expression, were investigated by univariate and multivariate analyses. Results: Among 60 patients, 31 patients had early recurrences within 2 years and 14 patients recurred after 2 years following surgery. Patients with low COX-2 expression in the HCC tissues and adjacent nonHCC tissues had favorable disease-free survival (p = 0.002 and p < 0.001, respectively) and patients with positive TGF-β1 expression in the nonHCC tissues had also longer disease-free survival (p = 0.045). Based on the expression patterns of COX-2 and TGF-β1, patients with low COX-2 and positive TGF-β1 expression in the nonHCC tissues had favorable overall and disease-free survival (p < 0.001, respectively). Conclusions: Increased COX-2 expression and decreased TGF-β1 signaling in nontumor tissues suggested high risk of recurrence and poor survival to the HCC patients following hepatectomy.
1. Introduction
Surgical resection for hepatocellular carcinoma (HCC) is a widely accepted and safe treatment with a low operative mortality as a result of advances in surgical techniques and peri-operative management [1,2]. However, the long-term survival remains unsatisfactory, mainly because of the high incidence of recurrence: a 2-year recurrence rate of up to 60% - 70% [3-5]. In HCC, there are two types at recurrence; intrahepatic metastasis and multicentric carcinogenesis derived from background liver disease. Intrahepatic metastasis occurs mainly within 2 years after hepatectomy [4].
In general, vascular invasion, number of tumors and large tumor size were thought to be conventional prognostic factors. However, some other biological molecules are also related to early intrahepatic recurrence in HCCs [5].
Cyclooxygenase-2 (COX-2) is induced by a variety of factors such as cytokines, growth factors, and carcinoma promoters [6] and has been connected to inflammation and carcinogenesis [7]. As key enzymes in the conversion of arachidonate to prostaglandin, COX-1 and COX-2 are involved in various biological processes from the production of cell specific prostaglandin, including the regulation of immune function, kidney development, reproductive biology, and gastrointestinal capability. The expression of COX-2 and the production of prostaglandins appear to provide a survival advantage to transformed cells through the inhibition of apoptosis [8], increased attachment to the extracellular matrix, increased invasiveness, and the stimulation of angiogenesis [9]. An increased COX-2 expression has been observed in several human tumor types and in selected animal and cell culture models of carcinogenesis, including hepatocellular carcinoma [6-9].
Transforming growth factor-beta (TGF-β) is a member of the multifunctional cytokine family and has been implicated in diverse cellular phenomena, including cell growth control, cell adhesion and motility, alteration of the cellular phenotype, production and degradation of the extracellular matrix protein, and apoptosis of hepatic cell lines [10]. TGF-β signaling plays an important role in the pathogenesis of fibrosis in liver cirrhosis, and hepatic stellate cells are the mediators of this protein. A large number of experimental and clinical studies have established that the TGF-β system can be activated as a tumor suppressor pathway, and that this pathway inhibits cellcycle progression during the G1 phase through the enhanced expression of cyclin-dependent kinase inhibitors such as p21.This signaling activates a tumor suppressor pathway by reversible arrest of cell proliferation [11]. However, these neoplastic epithelial cells often become resistant to TGF-β-mediated mitoinhibition, and the mechanisms for this alteration during carcinogenesis appear to be influenced by COX-2 expression [12]. COX-2 has been implicated in a number of carcinogenetic functions, but its relationship to TGF-β1-mediated HCCs is unknown. In past studies, TGF-β1 and epidermal growth factor (EGF) synergistically induced the expression of COX-2 production in mink lung epithelial cells [13]. Therefore, a major purpose of the present study was to assess the relation between TGF-β1 and COX-2 expression in the HCC and adjacent nonHCC tissues, and to assess the role of hepatic expression of COX-2 and TGF-β1 as predictors for patients with early recurrence of HCC.
2. Materials and Methods
2.1. Patients
This study was designed as a retrospective cohort study and was conducted in accordance with the Declaration of Helsinki and “ethical guidelines for clinical studies” from Ministry of Health, Labor and Welfare in Japan.
Sixty patients underwent curative liver resection and pathologically proved to be HCC between 2000 and 2003 in Wakayama Medical University Hospital were overviewed in this study. All the patients were routinely diagnosed by ultrasonography (US), contrast enhanced dynamic computed tomography (CE-CT) and tumor markers such as alpha fetoprotein (AFP). Fifty one patients underwent anatomical resection and 9 patients underwent non-anatomical resection. None of the patients received neoadjuvant and adjuvant chemotherapy or radiotherapy. Postoperative surveillance was performed every 2 to 3 months using US, CE-CT and blood examinations including tumor markers.
The presence of an intrahepatic recurrence was determined by the existence of a hypervascular nodule in early phase with a perfusion defect in the portal phase under CE-CT. If an extra-hepatic recurrence was suspected, lung CT or bone scintigraphy was performed. An extrahepatic recurrence was determined by the existence of a tumor. After detecting any recurrence, appropriate therapeutic modalities were administered, and the same surveillance was performed.
2.2. Data Collection
Clinical risk factors that may associate with early recurrence; the tumor size, preoperative blood chemical data, pathologic grading, vascular invasion, and high AFP values were reviewed.
2.3. Immunohistochemical Staining of COX-2
Tissue Samples were routinely fixed in 10% neutralbuffered formalin and were subsequently embedded in paraffin. Serial sections 4 μm thick were prepared from paraffin blocks and mounted on silanized slides (DAKO Japan, Kyoto, Japan). Sections were deparaffinized and hydrated by sequential immersion in xylenes and a graded alcohol series. Tissue sections were heated with a water bath in 0.01 M/L citric buffer (pH 6.0) at 95˚C for 40 minutes, and were washed in Tris-buffered saline containing 0.05% Tween 20 (TBS-T). Slides were incubated in 10% (vol/vol) H2O2 in methanol for 20 minutes to block the endogenous peroxidase activity and were treated with distilled water obtained from the same species in which the secondary antibody was developed for 25 minutes to block nonspecific staining. Subsequently, the slides were incubated with primary antibody [anti COX-2 (IBL, Gunma, Japan) at 1:50 for overnight at 4˚C]. After washing in TBS-T, immunostaining was performed using the avidin-biotin-peroxidase complex method. The slides were treated with a biotin-conjugated secondary antibody (Life Science, Tokyo, Japan) at 1:100 for 30 minutes followed by incubation with peroxidase-conjugated streptavidin (Life Science, Tokyo, Japan) for 30 minutes at room temperature.
The reaction products were visualized using 0.1% 3-3’-diamino-benzidine-tetrahydrochloride and 0.0005% H2O2 in 0.05 mol/L Tris-buffer (pH 7.6). Finally, the sections were counterstained with hematoxylin. All samples were stained twice to confirm the replication.
2.4. Immunohistochemical Staining of TGF-β
Samples sections were routinely treated as mentioned in the procedures for COX-2. Tissue sections were heated in a water bath in 0.05 M/L Trypsin buffered (pH 7.6) at 37˚C for 45 minutes, and were washed in TBS-T. The slides were incubated in 10% (vol/vol) H2O2 in methanol for 20 minutes to block the endogenous peroxidase activity. The slides were treated with distilled water obtained from the same species in which the secondary antibody was developed for 25 minutes to block nonspecific staining. Subsequently, slides were incubated with the primary antibody [anti TGF-β1 (Abcam, UK) at 1:100 for 18 h at room temperature].
After washing in TBS-T, all slides were treated as the same avidin-biotin-peroxidase complex method for COX- 2 staining and stained twice to confirm the replication.
2.5. Evaluation of COX-2 Expression
The immunoreactivity and distribution pattern of COX-2 were examined in both the HCC and adjacent nonHCC tissues of the liver. All immunostained sections were evaluated by two of us (NM, MU) who were blinded to clinical and pathologic information. Cases with discrepant results were re-evaluated jointly until agreement was reached. For each section, the intensity of staining was scored on a scale from 0 to 2, where 0 represented negative staining; 1, moderate; and 2, strong staining (Figures 1-2). Tissue samples with a score of 2 were defined as the high COX-2 group, and scores of 1 and 0 were defined as the low COX-2 group. COX-2 expression was very faint or undetectable in the vascular epithelium, whereas epithelial cells of the bile ducts generally expressed strong levels of COX-2. Accordingly, the latter level of staining was used as an internal control, which was designated arbitrarily as an intensity level of 2. COX-2 expression was generally homogeneous in each sample.
2.6. Evaluation of TGF-β Expression
The immunoreactivity and distribution pattern of TGF-β1 were also examined in both the HCC and adjacent nonHCC tissues of the liver by the same way as evaluation of COX-2 expression. For each section, the intensity of staining was scored on a scale from 0 to 2, where 0 represented negative staining; 1, moderate; and 2, strong staining (Figures 3-4). Tissue samples with scores of 2 and 1 were defined as the positive TGF-β1 group and, and a score of 0 was the negative TGF-β group. The positive signals of TGF-β expression stained yellow or brown, primarily in the cytoplasm. TGF-β1 expression was generally homogeneous in each sample.
2.7. Statistical Analysis
Data were analyzed using the Stat View J-4.5 software program (Abacus Concepts, Inc., Berkeley, CA). The relations between categorical variables were tested using a chi-squared analysis and Fisher’s exact probability test. Patient survival was calculated using the Kaplan-Meier method, and comparisons of survival curves were made using the log-rank test. Risk factors for early recurrence were evaluated by multivariate Cox regression analysis. p-values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. COX-2 and TGF-β1 Expression by Immunohistochemical Staining
In 60 HCC specimens, COX-2 staining was high in 16 (27%) samples and low in 44 (73%) samples. In the nonHCC specimens, COX-2 staining was high in 20 (33%) samples and low in 40 (67%) samples.
TGF-β1 expression of HCC sections was positive in 51 (85%) and negative in 9 (15%) samples. In the nonHCC sections, TGF-β1 expression was positive in 55 (92%) and negative in 5 (8%) samples.
Table 1 shows the relationship between COX-2 expression and clinicopathological factors among the 60 samples of HCC and nonHCC sections. There was no relationship between COX-2 expression and underlying liver disease (HBs antigen, HCV antibody) and blood examinations (total bilirubin, serum albumin, prothrombin time, AST and ALT). On the other hand, pathologically negative vascular invasion and a tumor size of less than 5
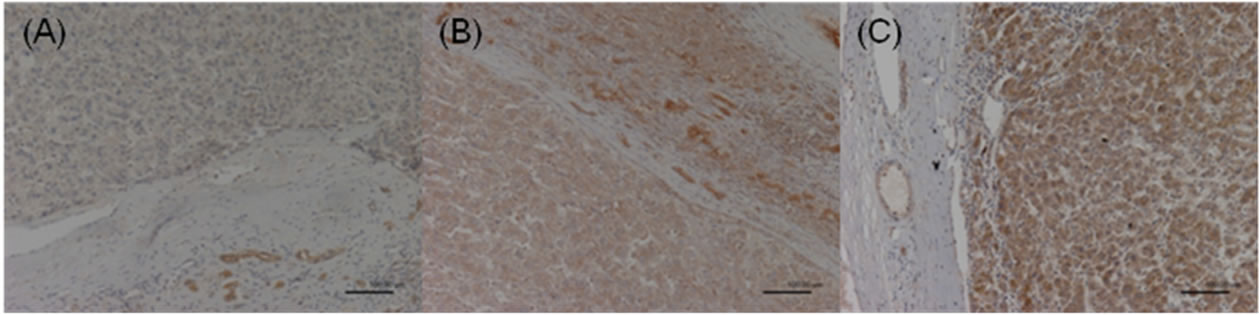
Figure 1. COX-2 expression in the HCC sections. Negative staining was defined as score 0 (A); Moderate staining was defined as score 1 (B); Strong staining was defined as score 2 (C).
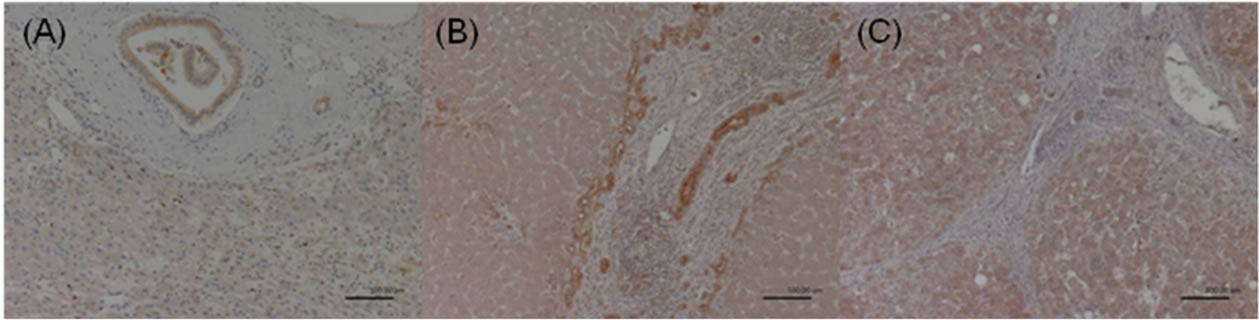
Figure 2. COX-2 expression in the adjacent nonHCC sections. Negative staining was defined as score 0 (A); Moderate staining was defined as score 1 (B); Strong staining was defined as score 2 (C).
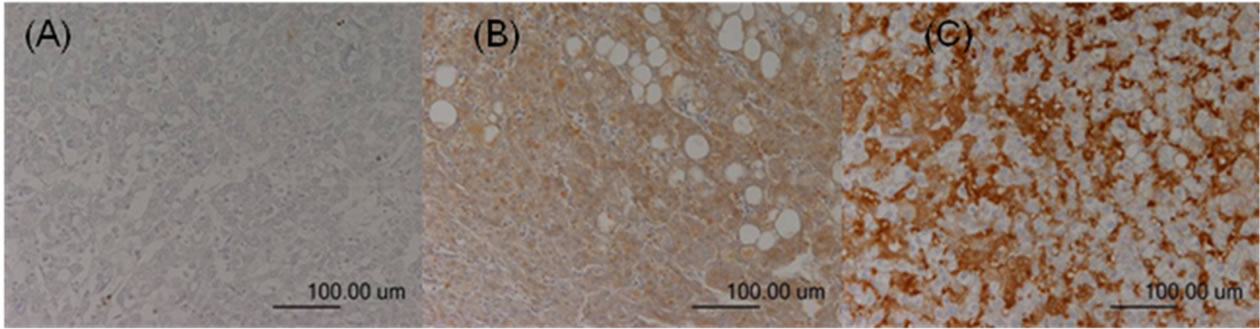
Figure 3. TGF-β expression in the HCC sections. Negative staining was defined as score 0 (A); Moderate staining was defined as score 1 (B); Strong staining was defined as score 2 (C).
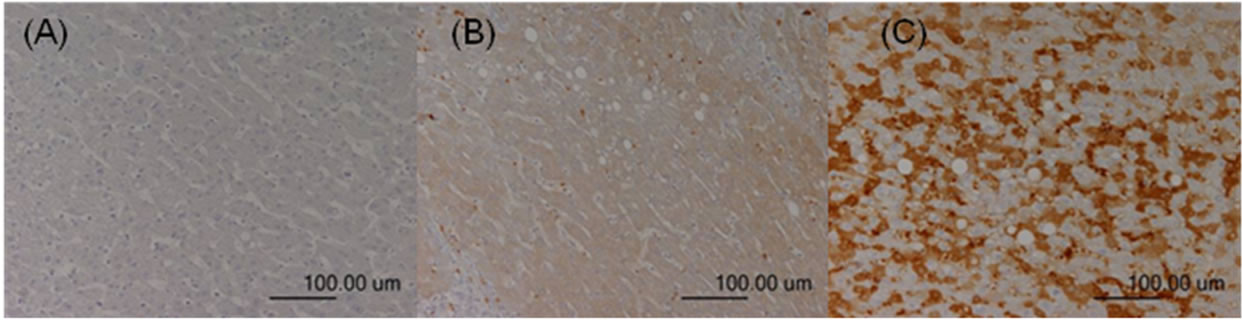
Figure 4. TGF-β expression in the adjacent nonHCC sections. Negative staining was defined as score 0 (A); Moderate staining was defined as score 1 (B); Strong staining was defined as score 2 (C).
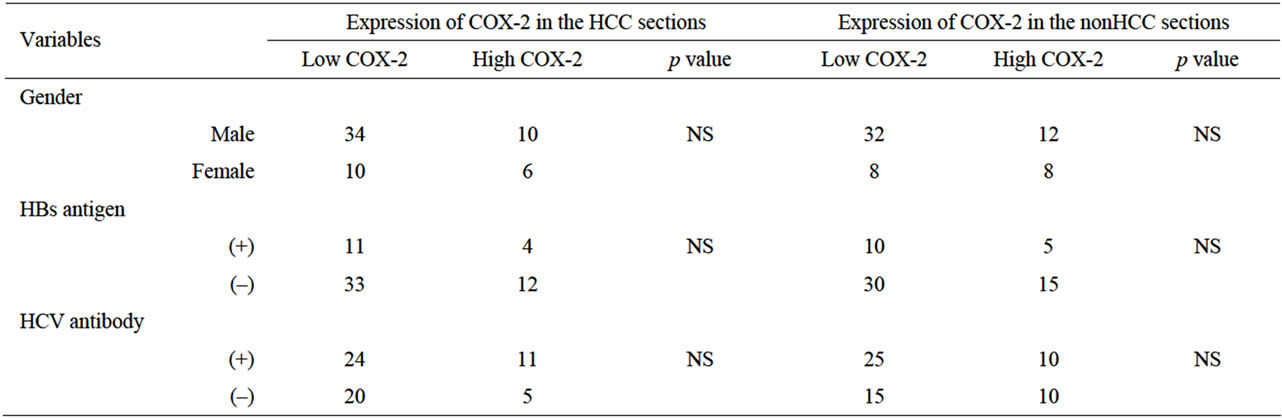
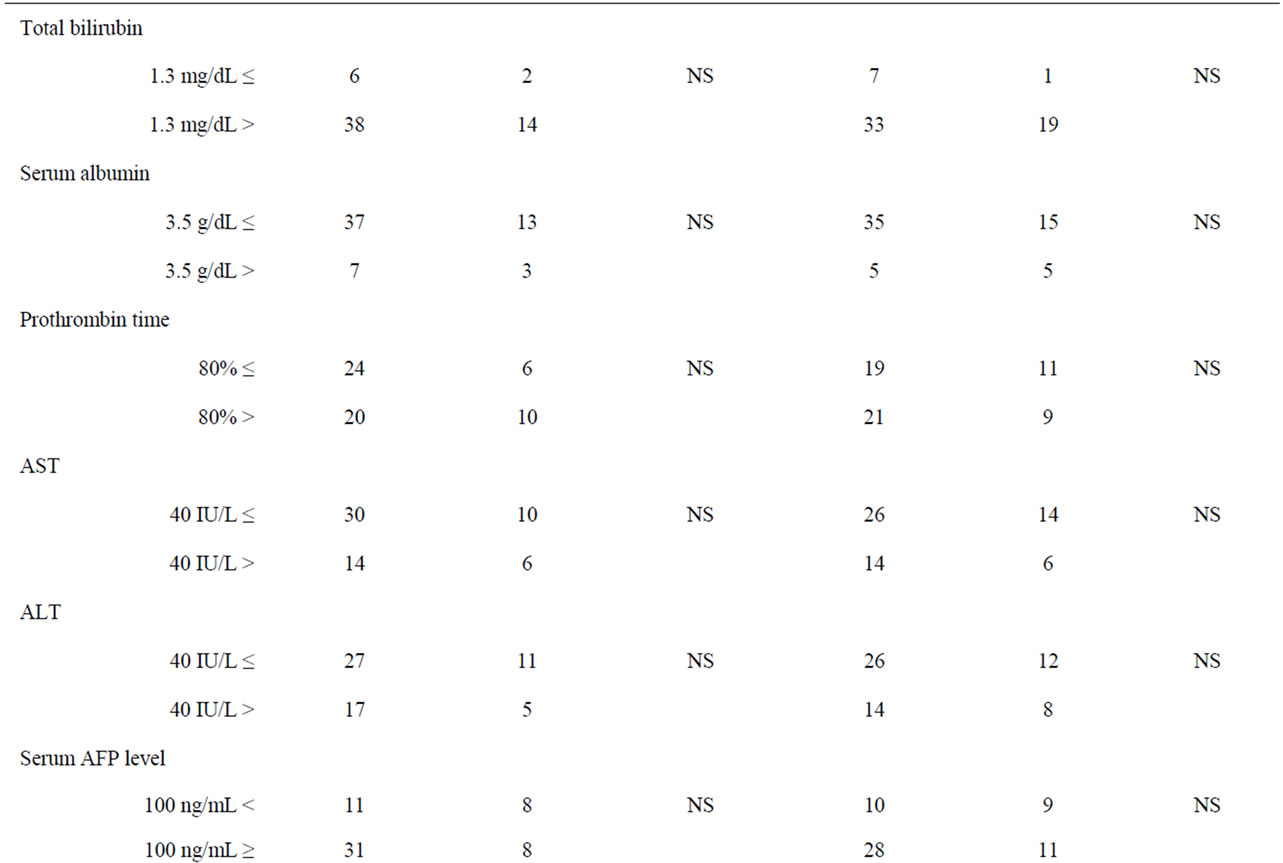
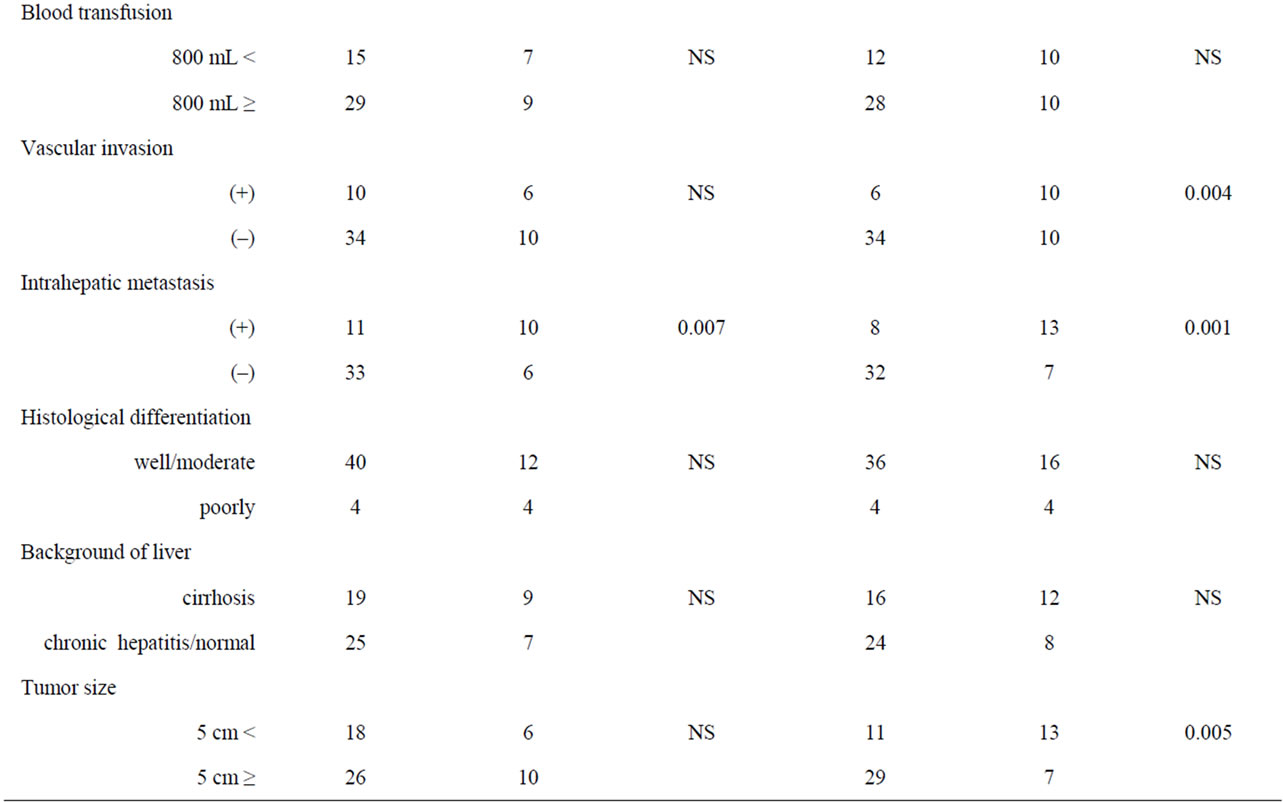
Table 1. The relationships between COX-2 expression and clinicopathological factors.
cm demonstrated significantly low COX-2 expression in the tissues of nonHCC sections (p = 0.004, p = 0.005, respectively). Also patients without intrahepatic metastasis showed low COX-2 expression in the tissues of nonHCC sections (p = 0.001) and of HCC sections (p = 0.007).
3.2. Survival and Early Recurrence Factor
Among these 60 patients, 31 patients suffered early recurrence within 2 years from surgery, and 14 patients suffered recurrences after 2 years from surgery. The cumulative overall survival rates at 3 and 5 years were 73.2 and 55.0%, respectively. The disease-free survival rates at 3 and 5 years were 47.6% and 28.7%, respectively.
Risk factors influencing early recurrence were univariately analyzed and summarized in Table 2. There were 7 potent risk factors as follows; high serum α-fetoprotein (AFP) levels more than 100 ng/mL (p < 0.025), a blood transfusion of a greater volume than 800 ml (p = 0.005), the presence of pathological vascular invasion (p < 0.001), intrahepatic metastasis (p = 0.031), a tumor size greater than 5 cm (p = 0.021), and high COX-2 expression in the nonHCC sections (p < 0.001) and in the HCC sections (p = 0.001). These factors were entered into multivariate analyses and following three factors were revealed to be independent risk factors for early recurrence; blood transfusion of greater than 800 ml (hazard ratio: 4.0, p = 0.003), presence of pathological vascular invasion (hazard ratio: 3.7, p = 0.025), and high COX-2 expression in the nonHCC sections (hazard ratio: 3.7, p = 0.029) (Table 3).
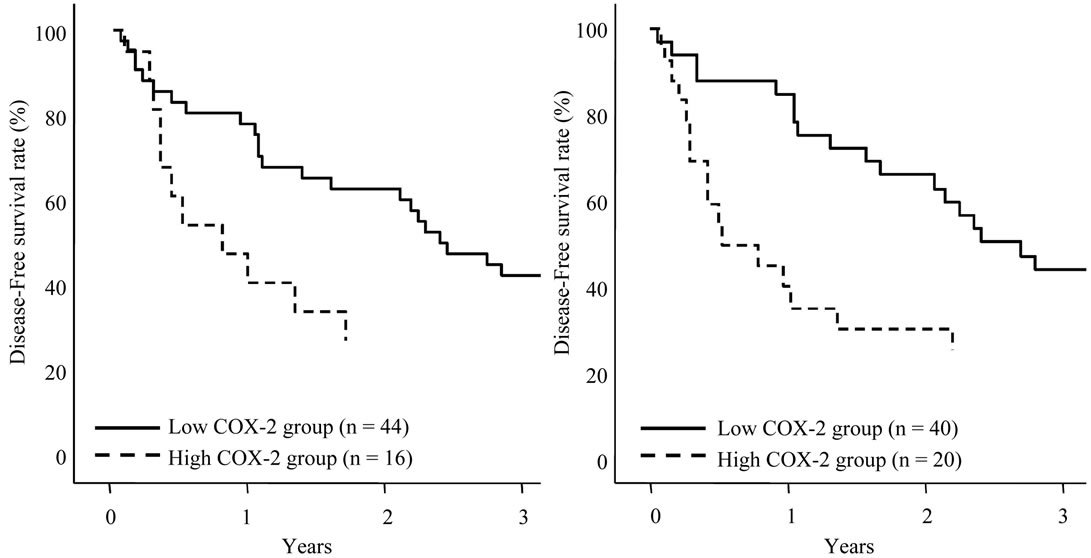
The disease-free survival curves based on the COX-2 expression in HCC sections and adjacent nonHCC sections were shown in Figures 5(a), (b). Preferable disease-free survival were observed in the patients with low COX-2 expression in the HCC sections and the adjacent nonHCC sections (p = 0.002 and p < 0.001, respectively).
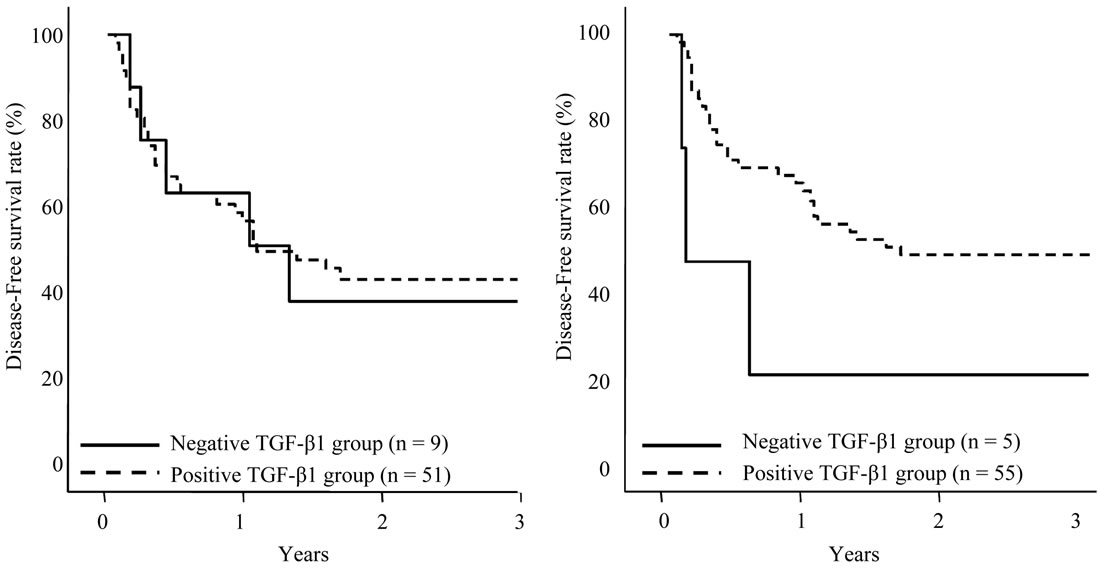
The disease-free survival curves based on the TGF-β1 expression in HCC sections and adjacent nonHCC sections were also shown in Figures 6(a), (b). Although TGF-β1 expression in the HCC sections and adjacent
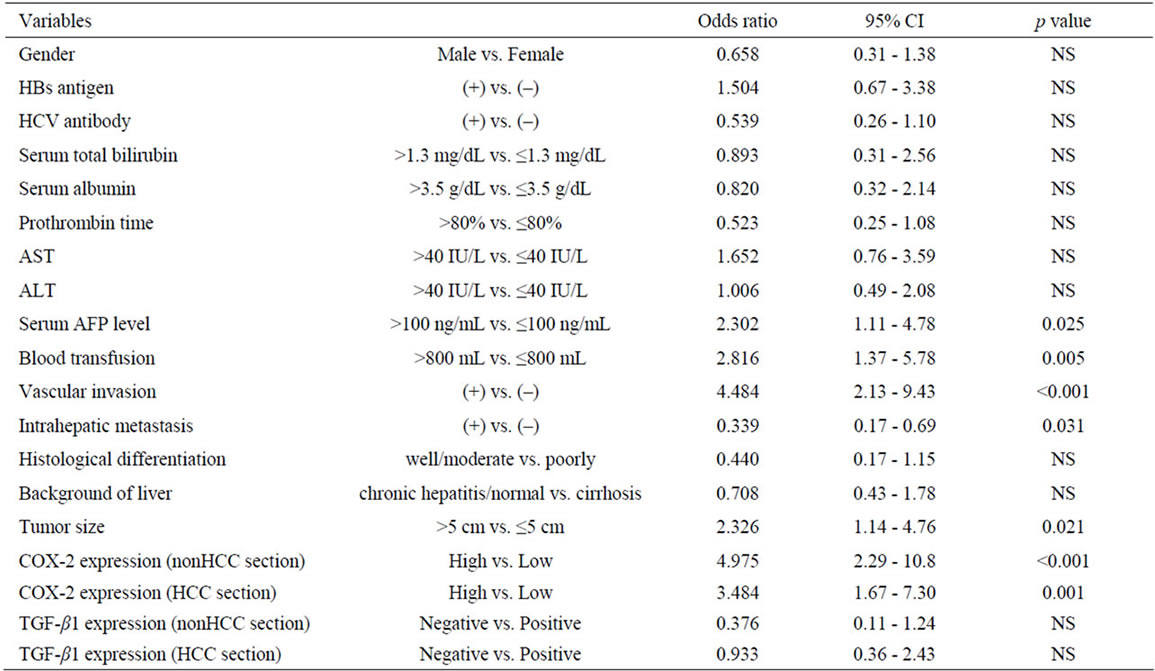
Table 2. Univariate analysis correlating the risk factors for recurrence within 2 years after hepatectomy.
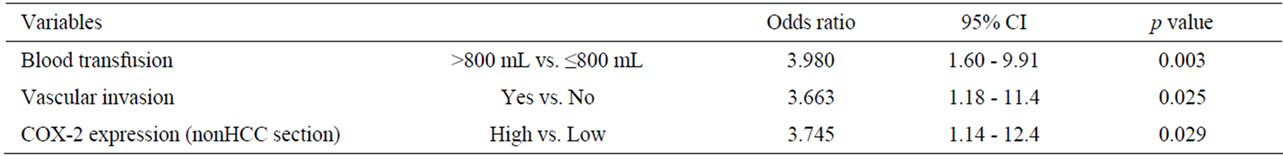
Table 3. Multivariate analysis correlating the risk factors for recurrence within 2 years after hepatectomy.
 (a) (b)
(a) (b)
Figure 5. Disease-free survival of patients with HCC following hepatectomy based on the COX-2 expression type. Solid line is low COX-2 expression group in HCC sections (a) and adjacent nonHCC sections (b). Broken line is high COX-2 expression group in HCC sections (a) and adjacent nonHCC sections (b). Patients with low COX-2 expression in HCC and adjacent nonHCC sections had a longer disease-free survival time (p = 0.002 and p < 0.001, respectively).
 (a) (b)
(a) (b)
Figure 6. Disease-free survival of patients with HCC following hepatectomy based on the TGF-β1 expression type. Solid line is negative TGF-β1 expression group in HCC sections (a) and adjacent nonHCC sections (b). Broken line is positive TGF-β1 expression group in HCC sections (a) and adjacent nonHCC sections (b). Patients with positive TGF-β1 expression in the adjacent nonHCC sections had a longer disease-free survival time (p = 0.045).
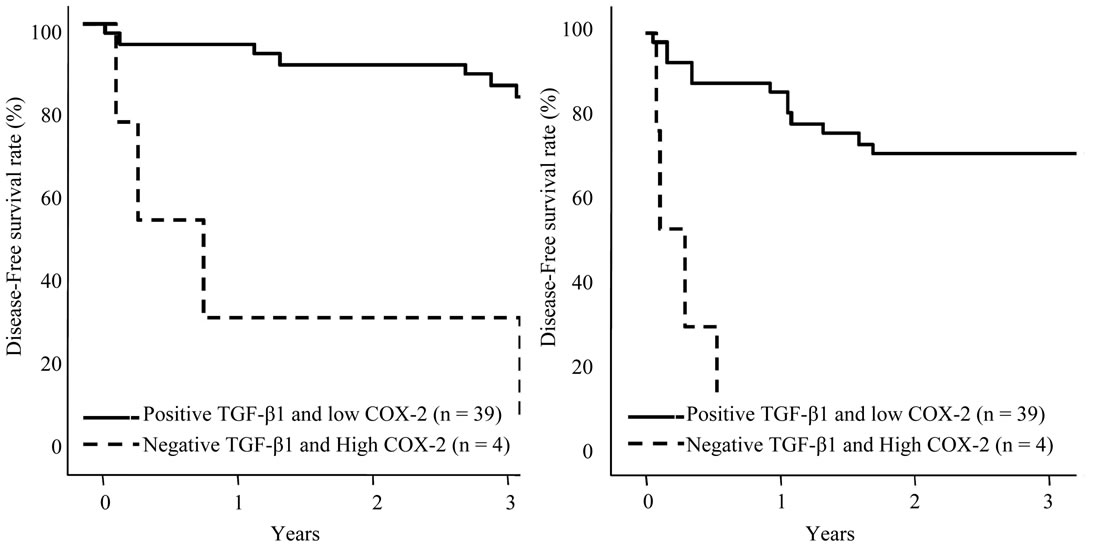
nonHCC Sections were positive in the most of the patients, patients with negative expression in adjacent nonHCC sections were significantly short disease-free interval (p = 0.045). Table 4 shows the relationship between COX-2 and TGF-β1 expression in HCC and adjacent nonHCC sections. TGF-β1 was particularly expressed in the low COX-2 expression group in the nonHCC sections (p = 0.021). The survival of the patients with HCC following hepatectomy based on the TGF-β1 and COX-2 expression patterns in the nonHCC sections were shown in Figure 7. Both the low COX-2 and positive TGF-β1 expression groups in the nonHCC sections contributed to the prolongation of overall and disease-free patient survival (p < 0.001, respectively).
 (a) (b)
(a) (b)
Figure 7. Survival curves of patients with HCC following hepatectomy based on the COX-2 and TGF-β1 expression patterns in the nonHCC sections. Solid line is the group of positive TGF-β1 and low COX-2 expression pattern in the nonHCC tissues. Broken line is the group of negative TGF-β1 and high COX-2 expression pattern in the nonHCC tissues. Patients with positive TGF-β1 and low COX-2 expression pattern in the nonHCC sections had favorable overall and disease-free survival (p < 0.001, respectively).
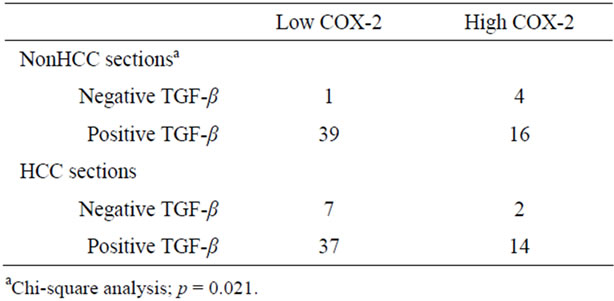
Table 4. The relationship between COX-2 and TGF-β1 expression.
4. Discussions
Hepatocellular carcinoma (HCC) is one of the most common malignant neoplasms worldwide. Although surgical resection is considered to be a potentially curative treatment, the recurrence rates of HCC are still high. The recurrence rates are in general about 50 to 60% at 2 years and 80% at 5 years. In this study, besides blood transfusion and vascular invasion, increased expression of COX-2 in the adjacent nonHCC tissue were also revealed to be one of independent risk factors for early recurrences.
The overexpression of COX-2, an inducible enzyme regulating prostaglandin release, is mechanistically linked to the development, growth, and spread of gastrointestinal cancers [12]. Previous article showed the relation between HCC’s differentiation and the tumoral COX-2 expression or the relation between the clinical course and non-tumoral COX-2 expression [14-20]. Although the association of tumoral COX-2 expression with HCC’s differentiation was not revealed in this study, patients with high COX-2 expression in the HCC tissue and adjacent nonHCC tissue were correlated with poor prognosis. Two different mechanisms may explain how COX-2 contributes to the intrahepatic recurrence of HCC in these patients. One is that COX-2 present in the background cirrhotic liver contributes to prolonged acceleration of necroinflammation and regeneration, and these pathological changes result in a relapse of the HCC [19]. The other explanation is that COX-2 in the cirrhotic liver acts as a tumor promoter by changing the tumor micro environment [20].
TGF-β was generally recognized as mitoinhibitor factor. There are five isoforms and the TGF-β1 is thought to associate with liver fibrosis via activating stellate cells in chronic liver injury [21]. The combined TGF-β1 and COX-2 expression in viral-related HCCs may suggest their synergistic action in the pathophysiology of hepatocarcinogenesis. TGF-β1 ligands are currently the only growth factors known to prevent the epithelial cell proliferation. Reduced TGF-β1 signaling was sufficient to promote tumor formation in mouse models [10]. However, in subsequent lung carcinoma studies, TGF-β1 was found to promote tumor invasion and metastasis, induce angiogenesis, and promote immune suppression [7]. Colonic adenoma and adenocarcinoma in the rat involve the increased expression of COX-2, which is localized in the tumor cells. TGF-β and COX-2 are also concurrently overexpressed in tumors, suggesting a more complex relationship between TGF-β and COX-2 in colonic carcinogenesis [22].
Concerning hepatocarcinogenesis in liver cirrhosis, TGF-β1 regulates hepatic tumor cell growth through the simultaneous activation of Smad-mediated gene transcription and phosphorylation of phospholipase A2α (PLA2α). Although Smad activation inhibits tumor cell growth, the phosphorylation of PLA2α initiates two signaling pathways that counteract Smad-mediated mitoinhibition, including the production of Prostaglandin GE2 for the activation of its G-protein coupled plasma membrane receptor, the prostaglandin E receptor 1 (EP1); and the activation of tumor cell growth [23].
In conclusion, increased COX-2 expression and decreased TGF-β1 signaling in non-tumor tissues suggested high risk of recurrence and poor survival to the HCC patients following hepatectomy. There would be some interactions with COX-2 and TGF-β1 to induce metastasis in the remnant liver.
REFERENCES
- K. Uchiyama, K. Mori, K. Tabuse, M. Ueno, S. Ozawa, T. Nakase, M. Kawai, M. Tani, H. Tanimura and H. Yamaue, “Assessment of Liver Function for Successful Hepatectomy in Patients with Hepatocellular Carcinoma with Impaired Hepatic Function,” Journal of Hepato-BiliaryPancreatic Surgery, Vol. 15, No. 6, 2008, pp. 596-602. doi:10.1007/s00534-007-1326-2
- M. Ueno, K. Uchiyama, S. Ozawa, S. Hayami, S. Kiriyama and H. Yamaue, “A New Prediction Model of Postoperative Complications after Major Hepatectomy for Hepatocellular Carcinoma,” Digestive Surgery, Vol. 26, No. 5, 2009, pp. 392-399. doi:10.1159/000229973
- S. A. Shah, S. P. Cleary, A. C. Wei, I. Yang, B. R. Taylor, A. W. Hemming, B. Langer, D. R. Grant, P. D. Greig and S. Gallinger, “Recurrence after Liver Resection for Hepatocellular Carcinoma: Risk Factors, Treatment, and Outcomes,” Surgery, Vol. 141, No. 3, 2007, pp. 330-339. doi:10.1016/j.surg.2006.06.028
- M. Ueno, K. Uchiyama, S. Ozawa, T. Nakase, N. Togo, S. Hayami and H. Yamaue, “Prognostic Impact of Treatment Modalities on Patients with Single Nodular Recurrence of Hepatocellular Carcinoma,” Surgery Today, Vol. 39, No. 8, 2009, pp. 675-681. doi:10.1007/s00595-008-3942-0
- S. Yoshioka, I. Takemasa, H. Nagano, N. Kittaka, T. Noda, H. Wada, S. Kobayashi, S. Marubashi, Y. Takeda, K. Umeshita, K. Dono, K. Matsubara and M. Monden, “Molecular Prediction of Early Recurrence after Resection of Hepatocellular Carcinoma,” European Journal of Cancer, Vol. 45, No. 5, 2009, pp. 881-889. doi:10.1016/j.ejca.2008.12.019
- M. E. Herceg, A. C. Tsiatis, J. L. Halpern, G. E. Holt, H. S. Schwartz, V. L. Keedy and J. M. Cates, “Cyclooxygenase 2 Expression in Soft Tissue Leiomyosarcoma,” Anticancer Research, Vol. 29, No. 8, 2009, pp. 2913-2917.
- C. J. Liu, T. C. Hsia, R. F. Wang, C. W. Tsai, C. C. Chu, L. W. Hang, C. H. Wang, H. Z. Lee, R. Y. Tsai and D. T. Bau, “Interaction of Cyclooxygenase 2 Genotype and Smoking Habit in Taiwanese Lung Cancer Patients,” Anticancer Research, Vol. 30, No. 4, 2010, pp. 1195-1199.
- J. G. Kim, Y. S. Chae, S. K. Sohn, J. H. Moon, H. M. Ryoo, S. H. Bae, Y. Kum, S. W. Jeon, K. H. Lim, B. M. Kang, I. J. Park, G. S. Choi and S. H. Jun, “Prostaglandin Synthase 2/Cyclooxygenase 2 (PTGS2/COX2) 8473T>C Polymorphism Associated with Prognosis for Patients with Colorectal Cancer Treated with Capecitabine and Oxaliplatin,” Cancer Chemotherapy and Pharmacology, Vol. 64, No. 5, 2009, pp. 953-960. doi:10.1007/s00280-009-0947-3
- A. T. Panagopoulos, C. L. Lancellotti, J. C. Veiga, P. H. de Aguiar and A. Colquhoun, “Expression of Cell Adhesion Proteins and Proteins Related to Angiogenesis and Fatty Acid Metabolism in Benign, Atypical, and Anaplastic Meningiomas,” Journal of Neuro-Oncology, Vol. 89, No. 1, 2008, pp. 73-87. doi:10.1007/s11060-008-9588-3
- M. G. Alexandrow and H. L. Moses, “Transforming Growth Factor Beta and Cell Cycle Regulation,” Cancer Research, Vol. 55, No. 7, 1995, pp. 1452-1457.
- H. You, W. Ding and C. B. Rountree, “Epigenetic Regulation of Cancer Stem Cell Marker CD133 by Transforming Growth Factor-Beta,” Hepatology, Vol. 51, No. 5, 2010, pp. 1635-1644. doi:10.1002/hep.23544
- Y. S. Guo, Z. Chen, X. D. Wen, T. C. Ko, C. M. Townsend Jr. and M. R. Hellmich, “Synergistic Regulation of COX-2 Expression by Bombesin and Transforming Growth Factor-Beta,” Digestive Diseases and Sciences, Vol. 53, No. 8, 2008, pp. 2045-2052. doi:10.1007/s10620-007-0122-9
- D. Saha, P. K. Datta, H. Sheng, J. D. Morrow, M. Wada, H. L. Moses and R. D. Beauchamp, “Synergistic Induction of Cyclooxygenase-2 by Transforming Growth Factor-Beta1 and Epidermal Growth Factor Inhibits Apoptosis in Epithelial Cells,” Neoplasia, Vol. 1, No. 6, 1999, pp. 508-517. doi:10.1038/sj.neo.7900051
- H. Koga, S. Sakisaka, M. Ohishi, T. Kawaguchi, E. Taniguchi, K. Sasatomi, M. Harada, T. Kusaba, M. Tanaka, R. Kimura, Y. Nakashima, O. Nakashima, M. Kojiro, T. Kurohiji and M. Sata, “Expression of Cyclooxygenase-2 in Human Hepatocellular Carcinoma: Relevance to Tumor Dedifferentiation,” Hepatology, Vol. 29, No. 3, 1999, pp. 688-696 doi:10.1002/hep.510290355
- A. S. Cheng, H. L. Chan, W. K. Leung, K. F. To, M. Y. Go, J. Y. Chan, C. T. Liew and J. J. Sung, “Expression of HBx and COX-2 in Chronic Hepatitis B, Cirrhosis and Hepatocellular Carcinoma: Implication of HBx in Upregulation of COX-2,” Modern Pathology, Vol. 17, 2004, pp. 1169-1179. doi:10.1038/modpathol.3800196
- N. Nishida, T. Nagasaka, T. Nishimura, I. Ikai, C. R. Boland and A. Goel, “Aberrant Methylation of Multiple Tumor Suppressor Genes in Aging Liver, Chronic Hepatitis, and Hepatocellular Carcinoma,” Hepatology, Vol. 47, No. 3, 2008, pp. 908-918. doi:10.1002/hep.22110
- A. J. Dannenberg and K. Subbaramaiah, “Targeting Cyclooxygenase-2 in Human Neoplasia: Rationale and Promise,” Cancer Cell, Vol. 4, No. 6, 2003, pp. 431-436. doi:10.1016/S1535-6108(03)00310-6
- M. Kondo, H. Yamamoto, H. Nagano, J. Okami, Y. Ito, J. Shimizu, H. Eguchi, A. Miyamoto, K. Dono, K. Umeshita, N. Matsuura, K. Wakasa, S. Nakamori, M. Sakon and M. Monden, “Increased Expression of COX-2 in Nontumor Liver Tissue Is Associated with Shorter Disease-Free Survival in Patients with Hepatocellular Carcinoma,” Clinical Cancer Research, Vol. 5, No. 12, 1999, pp. 4005-4012.
- Y. F. He, J. Jin, W. Wei, Y. Chang, B. Hu, C. S. Ji, W. D. Jia, X. Q. Wang, K. Chen and J. Chen, “Overexpression of Cyclooxygenase-2 in Noncancerous Liver Tissue Increases the Postoperative Recurrence of Hepatocellular Carcinoma in Patients with Hepatitis B Virus-Related Cirrhosis,” Canadian Journal of Gastroenterology, Vol. 24, No. 7, 2010, pp. 435-440.
- S. Morinaga, K. Tarao, Y. Yamamoto, Y. Nakamura, Y. Rino, K. Miyakawa, S. Ohkawa, M. Akaike, Y. Sugimasa and S. Takemiya, “Overexpressed Cyclo-oxygenase-2 in the Background Liver Is Associated with the Clinical Course of Hepatitis C Virus-Related Cirrhosis Patients after Curative Surgery for Hepatocellular Carcinoma,” Journal of Gastroenterology and Hepatology, Vol. 22, No. 8, 2007, pp. 1249-1255. doi:10.1111/j.1440-1746.2006.04367.x
- S. Ozawa, K. Uchiyama, M. Nakamori, K. Ueda, M. Iwahashi, H. Ueno, Y. Muragaki, A. Ooshima and H. Yamaue, “Combination Gene Therapy of HGF and Truncated Type II TGF-beta Receptor for Rat Liver Cirrhosis after Partial Hepatectomy,” Surgery, Vol. 139, No. 4, 2006, pp. 563-573. doi:10.1016/j.surg.2005.10.003
- J. Shao, H. Sheng, R. Aramandla, M. A. Pereira, R. A. Lubet, E. Hawk, L. Grogan, I. R. Kirsch, M. K. Washington, R. D. Beauchamp and R. N. DuBois, “Coordinate Regulation of Cyclooxygenase-2 and TGF-beta1 in Replication Error-Positive Colon Cancer and Azoxymethane-Induced Rat Colonic Tumors,” Carcinogenesis, Vol. 20, No. 2, 1999, pp. 185-191. doi:10.1093/carcin/20.2.185
- C. Han, A. J. Demetris, Y. Liu, J. H. Shelhamer and T. Wu, “Transforming Growth Factor-Beta (TGF-beta) Activates Cytosolic Phospholipase A2alpha (cPLA2alpha)- Mediated Prostaglandin E2 (PGE)2/EP1 and Peroxisome Proliferator-Activated Receptor-Gamma (PPAR-gamma)/ Smad Signaling Pathways in Human Liver Cancer Cells. A Novel Mechanism for Subversion of TGF-beta-induced Mitoinhibition,” Journal of Biological Chemistry, Vol. 279, No. 43, 2004, pp. 44344-44354. doi:10.1074/jbc.M404852200
NOTES
*Corresponding author.

