Surgical Science
Vol.3 No.2(2012), Article ID:17459,3 pages DOI:10.4236/ss.2012.32018
Chylothorax after Repair of Congenital Diaphragmatic Hernia in a Neonate: Usefulness of Conservative Management*
1Department of Pediatric Surgery, Hokkaido University Hospital, Sapporo, Japan
2Department of Gastroenterological Surgery I, Graduate School of Medicine, Hokkaido University, Sapporo, Japan
Email: #okadata@med.hokudai.ac.jp
Received October 20, 2011; revised December 15, 2011; accepted December 29, 2011
Keywords: Chylothorax; Congenital Diaphragmatic Hernia; Chest Drainage; Algorithm
ABSTRACT
Aim: Chylothorax is a recognized complication after surgery for congenital diaphragmatic hernia (CDH) in a neonate. Management strategies for chylothorax include cessation of enteral feedings, repeated aspiration, chest drainage, and total parenteral nutrition. It is important to determine which is the better plan for treatment of chylothorax after repair of CDH. The authors report successful management by use of the MCT diet for a neonate with chylothorax after repair of CDH. Case: A male infant weighing 3.0 kg was delivered by cesarean section at 38 weeks of gestation and intubated immediately after birth. Prenatal ultrasonography had disclosed left-sided posterolateral diaphragmatic hernia. After stabilization, surgery was performed via a left-sided transverse supra-abdominal incision. The unfixed colon, small bowel, stomach, and spleen were reduced from the chest with little difficulty. A hernia sac was not present and the left-sided posterolateral diaphragm showed a defect 3.5 cm × 2.0 cm in width. The child was fed via a nasogastric tube starting on postoperative day 4 and dyspnea disappeared. Plain chest X-ray on postoperative day 7 showed left pleural radioopacity. A specimen of the chest drainage examined on postoperative day 10 was typical of lymph, with a triglyceride level of 328 mg/dl. The chest drainage was dark yellowish, and a medium-chain triglyceride formula was used until postoperative day 30, by which time the effusion has disappeared. Discussion: Chylothorax after repair of CDH may be a transient disorder that will resolve after a period of diminished flow through the thoracic lymphatics.
1. Introduction
Postoperative ipsilateral pleural effusion has been reported to occur in approximately one-third of cases of congenital diaphragmatic hernia (CDH) [1]. Chylothoraces have rarely been reported after CDH repair. Such chylothorax results from trauma to the diaphragmatic lymphatics during surgery and is classified as the acquired chylothorax. On the other hand, congenital chylothorax can be diagnosed by prenatal magnetic resonance imaging [2]. If appropriate parenteral nutrition and dietary supplemention is not given, chronic loss of chylous fluid may result in hypoproteinemia, hypoalubuminemia, electrolyte depletion, immunodeficiency, and poor weight gain [3].
Management strategies include cessation of enteral feedings, repeated aspiration, chest drainage, and total parenteral nutrition. It is important to determine which is the better plan for treatment of chylothorax after repair of CDH: conservative treatment or surgical intervention. In the literature, most cases of chylothorax after repair of CDH did not require surgical interventions [1,2,4-6]. We describe herein a neonate who has treated conservatively for chylothorax after repair of CDH. After reviewing other cases, we discuss the clinical characteristics of chylothorax after repair of CDH, especially with regard to the usefulness of conservative management.
2. Case Report
A male infant weighing 3.0 kg was delivered by cesarean section at 38 weeks of gestation and intubated immediately after birth. Prenatal ultrasonography had disclosed left-sided posterolateral diaphragmatic hernia. After stabilization, surgery was performed via a left-sided transverse supraabdominal incision. The unfixed colon, small bowel, stomach, and spleen were reduced from the chest with little difficulty. A hernia sac was not present and the left-sided posterolateral diaphragm showed a defect 3.5 × 2.0 cm in width. The lung could be seen and was hypoplastic. The esophageal hiatus was intact and the hernia was repaired using interrupted 3-0 nonabsorbable EchibondTM sutures.
The child’s condition improved rapidly and the postoperative course was smooth. The child was fed via a nasogastric tube starting on postoperative day 4 and dyspnea disappeared. Plain chest X-ray on postoperative day 7 showed left pleural radioopacity (Figure 1). As the chest ultrasonography revealed left pleural effusion, chylothorax was suspected. The chest drainage was dark yellowish, and a medium-chain triglyceride (MCT) formula was used until postoperative day 30, by which time the effusion has disappeared. A specimen of the chest drainage examined on postoperative day 10 was typical of lymph, with a triglyceride level of 328 mg/dl and a cholesterol level of 45 mg/dl, which was lower than the plasma level of 112 mg/dl. The postoperative course is shown in Figure 2. The child did not require supplementary oxygen and was not fed orally between the 11th and 14th postoperative day. We replaced the chest drainage fluid with fresh frozen plasma. Drainage ceased by the 32nd postoperative day, and the child has been well for 2 years.
3. Discussion
Chylothorax is an accumulation of chyle in the thoracic cavity. It has been reported after almost every thoracic procedure in children, but is most common after cardiovascular operations. Its incidence is unclear because pediatric surgeons do not routinely use chest drainage for postoperative pleural effusions [5]. Kavvadia et al. [1] reported 6 (17 %) cases of chylothorax in 35 infants with CDH and Naik et al. [7] described 3 (20 %) cases of chylothorax among 15 patients who underwent repair of CDH. In Kavvadia’s report [1], drainage was first required at a median of 10.5 days and loss of fluid per day ranged between 3 and 70 ml/kg. Thoracentesis or continuous drainage was required for a median of 10.5 days. More than 50 % of thoracic postoperative chylothorax cases in children require surgical intervention using thoracic duct mass ligation [8]. On the other hand, it has been reported that most cases of chylothorax after repair of CDH did not require surgical intervention. Furthermore, the outcome of chylothorax after repair of CDH is generally good [1]. All 6 infants described by Kavvadia et al. [1] with chylothorax after repair of CDH survived with conservative management, and our patient was alive at 1 year.
The diagnosis of chylothorax is made for an unfed infant if more than 90% of the leucocytes in the fluid are lymphocytes [9]. If the infant had been fed, the diagnosis is confirmed if the fluid appears chylous, and if the triglceride level is higher and the cholesterol level lower than that of plasma [3,10]. We diagnosed the present case as chylothorax based on the findings that the triglyceride level was higher than the normal range and the cholesterol level was lower than that of plasma.
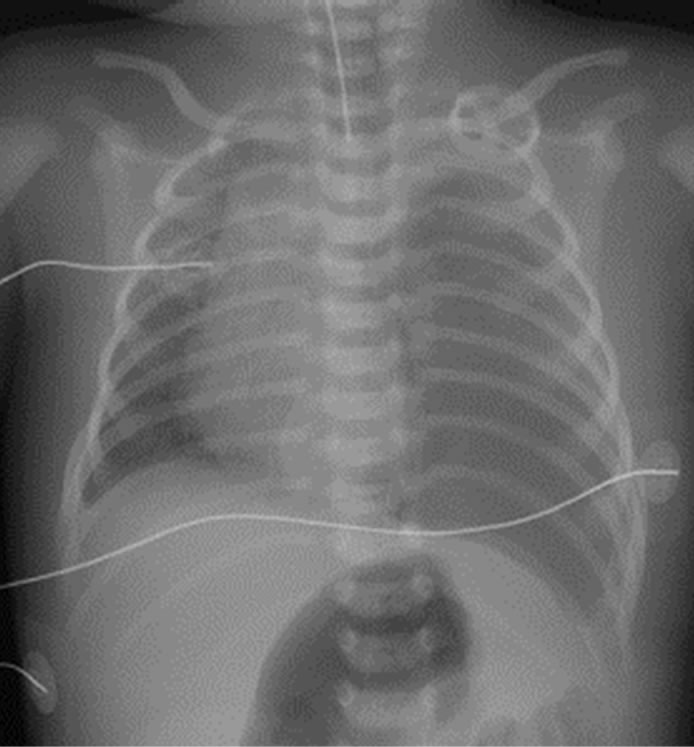
Figure 1. Chest X-ray on postoperative day 7 shows a moderate left-sided pleural effusion (white arrow).
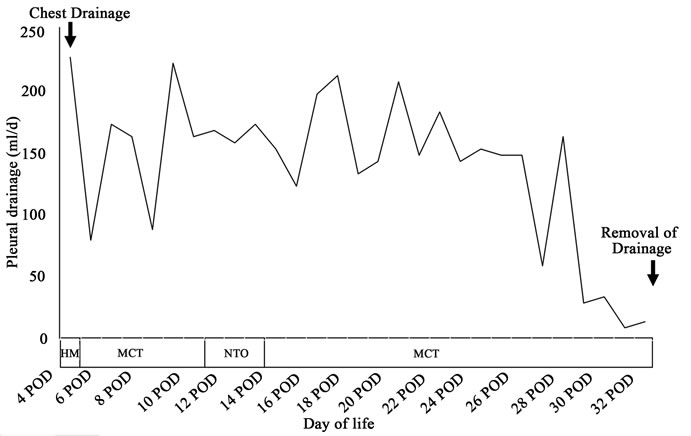
Figure 2. Daily chest drain output. HM: human milk; MCT: medium-chain triglyceride; NTO: no taking orally.
Regarding the causes of chylothorax after the repair of CDH, the presence of a sac (seen in 10% of cases of CDH) and the side of the hernia are important factors. Mercer [6] described that all 5 cases of chylothorax after repair of CDH were left-sided, and a sac was present in each case and was excised before repair. Chylothorax is presumably a result of division of the lymphatic vessels within the sac itself [1]. Chylothorax does not usually occur on the right side where the cisterna chyli and thoracic duct are normally situated. It is likely that lymph vessels related to the diaphragm and to the hernial sac were injured at surgery, rather than the main pathways of chylous drainage of the intestine. The lymph passes from the peritoneal to the pleural space through connecting retroperitoneal lymphatics, and these are mainly present on the left side [6]. It is hard to see how the posterior bronchomediastinal duct could be injured in posterolateral diaphragmatic herniorrhaphy. Since the major chylous drainage pathways are probably not injured, spontaneous cure is more likely to occur [6]. It is probable that diaphragmatic lymph channels are injured at the hernial repair. However, comparison of the infants with and without a chylothorax did not demonstrate significant differences in the occurrence of possible risk factors, such as gestational age, birth weight, age at repair, or side of hernia [1].
The appropriate treatment for chylothorax after repair of CDH remains controversial, and it remains unknown whether conservative management or surgical intervention is preferable [5]. Nutritional management is important, such as using MCT or intravenous alimentation. In Kavvadia’s reports [1], 6 of 8 infants diagnosed with chylothorax after repair of CDH were placed on MCT formula for a median of 80 days. On the other hand, surgical procedures that have been used include ligation of the leakage site, ligation of the thoracic duct above the diaphragm, partial pleurectomy, application of tissue adhesive (pleurodesis) such as somatostatin [4,5] or minocycline hydrochloride, and pleuroperitoneal shunting. These surgical procedures have been advocated for refractory chylothorax [5]. Where recorded, when the chylous leakage was above the level of 15 ml/kg/d, it was accompanied by high morbidity, which usually necessitated mass ligation of the thoracic duct in children [6]. Others have suggested that 100 ml/k/d should be used as the criterion for surgery [10]. However, it is difficult to precisely locate the site of the leakage. Injury to either thoracic duct usually results in persistent chylous leaking, causing nutritional and immunologic problems, and surgical intervention is usually required [6]. However, cessation of chylothorax can be expected between 6 and 8 weeks after its onset [7]. In our patient, resolution occurred without operative intervention within this time period.
4. Conclusions
In conclusion, the aim of our report is to demonstrate the effectiveness of conservative treatment for chylothorax after repair of CDH. We recommend conservative management for the first-line treatment for chylothorax after repair of CDH. Chylothorax after repair of CDH may be a transient disorder that will resolve after a period of diminished flow through the thoracic lymphatics.
REFERENCES
- V. Kavvadia, A. Greenough, M. Davenport, J. Karani and H. Nicolaidesk, “Chylothorax after Repair of Congenital Diaphragmatic Hernia—Risk Factors and Morbidity.” Journal of Pediatric Surgery, Vol. 33, No. 3, 1998, pp. 500-502. doi:10.1016/S0022-3468(98)90097-5
- X. Liu, M. Ashtari, J. C. Leonidas and Y. Chan, “Magnetic Resonance Imaging of the Fetus in Congenital Intrathoracic Disprders: Preliminary Observations,” Pediatric Radiology, Vol. 31, No. 6, 2001, pp. 435-439. doi:10.1007/s002470100450
- H. L. M. Van Straaten, L. J. Gerards and T. G. Krediet, “Chylothorax in the Neonatal Period,” European Journal of Pediatrics, Vol. 152, No. 1, 1993, pp. 2-5. doi:10.1007/BF02072505
- A. Macy, R. W. Thomas and E. F. Robert, “Successful Use of Somatostatin in a Case of Neonatal Chylothorax,” Journal of Pediatric Surgery, Vol. 38, No. 7, 2003, pp. 1106-1107. doi:10.1016/S0022-3468(03)00205-7
- A. Goyal, N. P. Smith, E. C. Jesudason, S. Kerr and P. D. Losty, “Octreotide for Treatment of Chylothorax after Repair of Congenital Diaphragmatic Hernia,” Journal of Pediatric Surgery, Vol. 38, No. 8, 2003, pp. E19-E20. doi:10.1016/S0022-3468(03)00294-X
- S. Mercer, “Factors Involved in Chylothorax Following Repair of Congenital Posterolateral Diaphragmatic Hernia,” Journal of Pediatric Surgery, Vol. 21, No. 9, 1986, pp. 809-811. doi:10.1016/S0022-3468(86)80374-8
- S. Naik, A. Greenough, Y. Zhang and M. Davenport, “Prediction of Morbidity during Infancy after Repair of Congenital Diaphragmatic Hernia,” Journal of Pediaticr Surgery, Vol. 31, No. 12, 1996, pp. 1651-1654. doi:10.1016/S0022-3468(96)90040-8
- G. Stringel, S. Mercer and J. Bass, “Surgical Management of Persistent Chylothorax in Children,” Canadian Journal of Surgery, Vol. 27, No. 6, 1984, pp. 543-546.
- M. T. Longaker, J. M. Laberge, J. Dansereau, J. C. Lanfer, T. M. Crombleholme, P. W. Callen, M. S. Golbus and M. R. Harrison, “Primary Fetal Hydrothorax: Natural History and Management,” Journal of Pediatric Surgery, Vol. 27, No. 6, 1989, pp. 573-576. doi:10.1016/S0022-3468(89)80509-3
- M. L. Paes and H. Powell, “Chylothorax: An Update,” British Journal of Hospital Medicine, Vol. 51, No. 9, 1994, pp. 482-490.
NOTES
*The authors received no funding for this report and declare no conflict of interest.
#Corresponding author.

