Advances in Microbiology
Vol.3 No.8A(2013), Article ID:41294,5 pages DOI:10.4236/aim.2013.38A008
Utility of Miconazole Therapy for Trichosporon Fungemia in Patients with Acute Leukemia
1Cancer Center, Mie University Hospital, Tsu, Japan
2Department of Hematology and Oncology, Mie University Graduate School of Medicine, Tsu, Japan
3Fourth Department of Internal Medicine, Hiroshima Red Cross and Atomic-Bomb Survivors Hospital, Hiroshima, Japan
Email: k2nakase@clin.medic.mie-u.ac.jp
Copyright © 2013 Kazunori Nakase et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 15, 2013; revised November 15, 2013; accepted November 21, 2013
Keywords: Miconazole; Trichosporon; Fungemia; Acute Leukemia
ABSTRACT
Invasive trichosporonosis is an extremely rare mycosis, but Trichosporon fungemia (TF) in patients with hematologic malignancies has been increasingly recognized to be a fulminant and highly lethal infection. Although the utility of azole therapy has been demonstrated in several observations, little is known about the efficacy of one of azoles, miconazole (MCZ). To assess its therapeutic role, we retrospectively investigated 6 cases of TF in patients with acute leukemia receiving MCZ containing regimens. Successful outcome was obtained in 4 patients [MCZ + amphotericin B (AmB) in 2, MCZ only and MCZ + fluconazole (FLCZ) + AmB in one each], but not in 2 (MCZ + FLCZ + AmB and MCZ + FLCZ in one each). Although MCZ and AmB exhibited good in vitro activities against isolates from all patients, FLCZ had such finding from only one patient. Considering the reportedly limited utility of AmB, MCZ seemed to play a critical role even in the combination therapies for TF. Despite the release of newer azoles and other classes of antifungals, the use of MCZ remains a potential therapeutic approach for TF in patients with acute leukemia.
1. Introduction
Trichosporon is a yeast-like fungus usually found in soil and fresh water, and it may colonize the skin, upper respiratory tract, and gastrointestinal tract of humans [1]. In tropical and subtropical regions, this fungus causes white piedra and summer-type hypersensitivity pneumonitis in immunocompetent individuals [1,2]. On the other hand, invasive trichosporonosis is extremely unusual, but it has been increasingly noted worldwide in immunocompromised patients over the past 3 decades. Among patients with hematologic malignancies, this type of mycosis has been described to be a life-threatening infection with the mortality rate of approximately 80% [1,3-7]. In particular, those with acute leukemia are by far the most common victims to be affected by Trichosporon fungemia (TF) during the period of neutropenia after chemotherapy [3,6,7]. Although the optimal treatment of TF in such patients has not yet been established, several reports describe the usefulness of azole therapy [8-10].
Miconazole (MCZ) belongs to the azole antifungals and has been widely used for more than 3 decades. Unlike other azoles, MCZ is known to have additional fungicidal activities other than the inhibition of ergosterol synthesis [11,12]. However, the therapeutic role of MCZ for trichosporonosis has not been well understood [6,8]. Here, we retrospectively investigated 6 cases of TF in patients with acute leukemia treated with MCZ containing regimens, and evaluated the utility of this azole in the treatment of TF for such patients.
2. Patients and Methods
In this study, we retrospectively reviewed 6 consecutive cases (>16 years of age) of TF in patients with acute leukemia at our collaborating 2 hospitals [Mie university hospital (MUH) and Hiroshima Red Cross and AtomicBomb Survivors Hospital] from 1992 to 2012. Patients were included in this study if they were treated with MCZ containing regimens for more than 5 days, and the in vitro activity tests of the antifungal agents used against isolates were performed. These data were collected and analyzed at the MUH, and the study was approved by the institutional review board at MUH. All but one patients were given prophylactic antibiotics (oral fluoroquinolone) and antifungals when neutropenia (<0.5 × 109/L) developed. For antifungal prophylaxis, patients received oral fluconazole (FLCZ; 0.2 g/day) or itraconazole (ITCZ; 0.1 - 0.2 g/day) with or without amphotericin B (AmB; 1.2 - 2.4 g/day), and were housed in rooms equipped with a high-efficiency particulate air filtration system. For patients with febrile neutropenia, broad-spectrum antibiotics (β-lactum occasionally combined with glycopeptides) were given as an empirical antibacterial therapy. In cases with persistent fever despite antibiotic use for 3 to 5 days, intravenous (IV) AmB (50 mg/day), or micafungin (MF; 0.1 - 0.3 g/day) was given as an empirical antifungal therapy. After the diagnosis of TF, patients were treated with IV AmB (50 mg/day), MCZ (0.8 - 1.2 g/day), FLCZ (0.4 g/day) or a combination of these agents. A patient was considered to have TF if at least one Trichosporon species was detected in blood culture, and we detected T. beigelii in all patients. Blood cultures were processed using an automated Bactec 9240 system (Becton Dickinson, Franklin Lakes, NJ) in the microbiology laboratory of each hospital. The Trichosporon species were identified on the basis of morphological characteristics, which were determined from slide cultures, and biochemical features, which were assessed using the Vitek Yeast Biochemical Card (bioMerieux Vitek Inc., Hazelwood, MO) with or without API 20C Aux (bioMerieux, Marcy l’Etoile, France). An antifungal susceptibility test was performed according to the methods prescribed by the National Committee for Clinical Laboratory Standards (NCCLS; M-27 microdilution method) [13]. Minimal inhibitory concentrations (MICs) of AmB, MCZFLCZ and MF were evaluated for the available strains isolated from patients. A part of the MIC data were already described in our previous report [7].
3. Results
3.1. Patient Characteristics
Patient characteristics are shown in Table 1. Age ranged from 33 to 71 years (median, 57 years), and all patients were male. Underlying diseases included acute myeloid leukemia in 5 patients, and acute lymphoblastic leukemia in one. All patients had undergone cytotoxic chemotherapy for acute leukemia, and were neutropenic (<0.5 × 109/L) at the onset of TF. TF developed as a breakthrough infection in 5 patients while receiving prophylactic or empirical antifungal agents (4 were receiving MF, and one did AmB). Neutrophil recovery (≥0.5 × 109/L) was observed in 5 patients. Central venous line was inserted in all patients during the clinical course, and it was exchanged in only one patient (Patient 5). Hyperglycemia (≥200 mg/dL) was observed in only one patient (Patient 6), and his blood glucose level elevated to 630 mg/dL before death.
3.2. The Dose and Therapeutic Duration of MCZ
The dose and therapeutic duration of MCZ are shown in Table 2. In 4 patients with therapeutic success, one was treated with MCZ alone (patient 1, 0.8 - 1.2 g/day for 35 days), 2 with a combination of MCZ (Patients 2 and 3, 1 g/day for 55 - 70 days) with AmB, 1 with MCZ (Patient 4, 1 g/day for 7 days) followed by that of AmB with FLCZ. In 2 patients with therapeutic failure, one was treated with a combination of MCZ (Patient 5, 1 g/day for 27 days) with FLCZ plus AmB, and 1 with that of MCZ (Patient 6, 0.8 g/day for 7 days) with FLCZ.
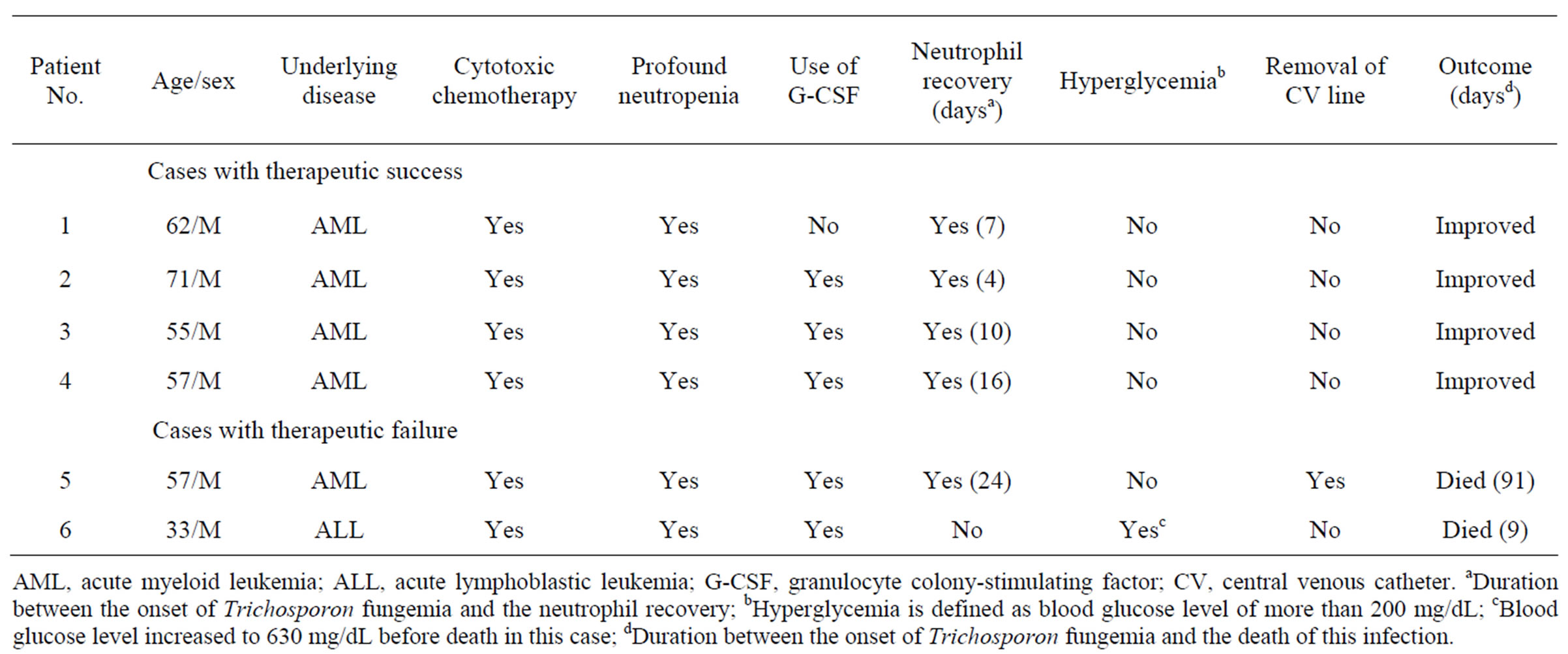
Table 1. Clinical characteristics of patients with Trichosporon fungemia.
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; G-CSF, granulocyte colony-stimulating factor; CV, central venous catheter. aDuration between the onset of Trichosporon fungemia and the neutrophil recovery; bHyperglycemia is defined as blood glucose level of more than 200 mg/dL; cBlood glucose level increased to 630 mg/dL before death in this case; dDuration between the onset of Trichosporon fungemia and the death of this infection.
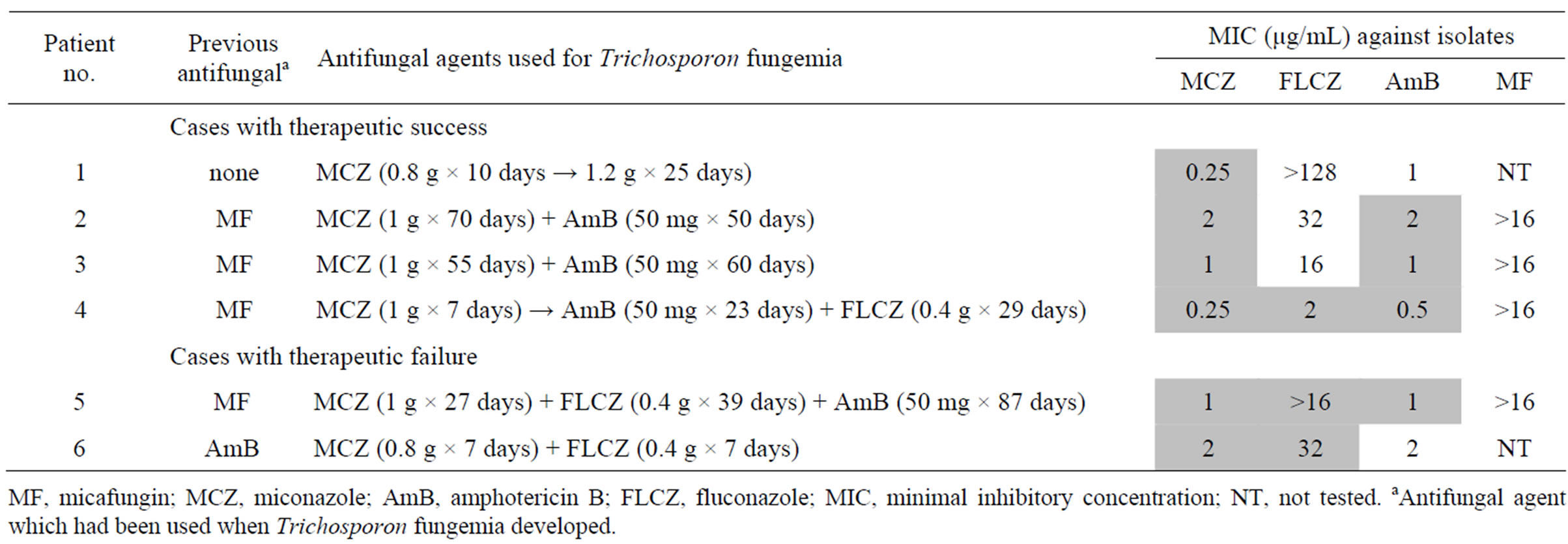
Table 2. Anitifungal therapy for Trichosporon fungemia.
MF, micafungin; MCZ, miconazole; AmB, amphotericin B; FLCZ, fluconazole; MIC, minimal inhibitory concentration; NT, not tested. aAntifungal agent which had been used when Trichosporon fungemia developed.
3.3. In Vitro Susceptibilities of Antifungals against Isolates
In vitro susceptibilities of antifungals against isolates are shown in Table 2. The MICs of MCZ and AmB were relatively low (0.25 - 2 μg/mL and 0.5 - 2 μg/mL, respectively) in every case. Whereas the MICs of FLCZ varied in patient to patient (2 - 32 μg/mL). In 2 patients with therapeutic failure, the MICs of FLCZ used were high (Patients 5 and 6, ≥16 μg/mL) unlike that in a patient with therapeutic success (Patient 4, 2 μg/mL).
3.4. Clinical Courses of Patients
Clinical courses of patients are shown in Table 1. Four patients (Patients 1 - 4) recovered from the infection. The remaining 2 patients (Patients 5, 6) died with this infection. In Patient 5, Trichosporon was cleared from the bloodstream, but he finally died of meningitis 91 days after the onset of TF. Patient 6 died 9 days after the onset of TF.
4. Discussion
Despite the development of various antifungal agents, the treatment of patients with invasive trichosporonosis remains a challenge. There are increasing data that suggest limited clinical usefulness of AmB for such mycosis irrespective of its good in vitro activity against Trichosporon species. On the other hand, there are several reports that describe the clinical utility of certain azoles such as voriconazole (VCZ) or FLCZ [8-10]. VCZ is especially considered to represent a promising therapy for this infection.
Unlike other azoles, MCZ have multiple fungicidal actions in addition to the inhibition of ergosterol synthesis. Such actions include direct membrane damage, and cytoplasmic accumulation of reactive oxygen species [11,12]. These MCZ actions are noted to explain the extremely rare development of fungal resistance to this agent [11].
However, little information is available about the responsiveness of MCZ for trichosporonosis so far. Although Girmenia et al. [6] demonstrated that this azole was useful in 5 of 9 (56%) patients with hematologic diseases and disseminated trichosporonosis, its dose, the duration of therapy, and the in vitro activities were not described.
In this study, 4 of 6 (67%) patients recovered from this infection: MCZ was given at a dose of more than 1 g/day, and the total therapeutic durations ranged from 7 to 70 days. In agreement with most previous reports [3,14], MCZ showed good activities against isolates from all patients, showing a contrast finding with FLCZ in this study. Patient 1 was treated with MCZ alone: both its low MIC (0.25 μg/mL) and its relatively high dose (1.2 g/day) appeared to result in successful outcome. In patient 4, the initial mono-therapy with MCZ having a low MIC (0.25 μg/mL) might contribute to finally achieve the satisfactory result. In this case, however, the following therapy with a combination of AmB and FLCZ was also likely to play a role to overcome this infection because of their low MICs against isolates. In patients 2 and 3, they were treated with a combination of MCZ and AmB. Although the effects of AmB and azole are known to be antagonistic [15], there are some studies that the activity of this combined therapy showed superior to that of each agent alone [16,17]. Further analyses will be required to better understand the usefulness of such therapy. Considering the reportedly limited clinical utility of AmB [18,19], above mentioned excellent actions of MCZ might play a critical role even in this combination therapy.
Therapeutic failures in 2 patients (patients 5 and 6) were likely to be ascribed to their difficult situations. In patient 5, fungemia improved and he showed a relatively prolonged survival (91 days), but finally died of meningitis. As the tissue availability of MCZ is reported to be low, suffering from this central nervous system disease seemed to be related to a lower MCZ delivery to the brain. In patient 6, the possibility that hyperglycemia [7]
as well as the insufficient dose of MCZ (0.8 g/day) affected clinical outcome could not be denied. In these 2 cases, we could not also expect the effect of another azole, FLCZ because of its poor activities against isolates.
The subsidence of fungal infection can not be achieved despite antifungal therapy unless neutropenia resolve [7,8]. In our study, neutropenia improved in 5 patients, but one of them died of infection. In all the patients with treatment success, neutropenia had persisted when antifungal therapy was started, indicating that the antifungal treatments might suppress the progression of infection until neutrophil recovery.
5. Conclusion
Taken together, despite the release of newer azoles and other classes of antifungals [19], our data suggest that the use of MCZ remains a potential therapeutic approach for invasive trichosporonosis in patients with acute leukemia and neutropenia.
6. Acknowledgements
The authors thank T. Kohara for providing important information.
REFERENCES
- T. J. Walsh, “Trichosporonosis,” Infectious Disease Clinics of North America, Vol. 3, No. 1, 1989, pp. 43-52.
- K. Shimazu, M. Ando, T. Sakata, K. Yoshida and S. Araki, “Hypersensitivity Pneumonitis Induced by Trichosporon cutaneum,” American Review of Respiratory Disease, Vol. 130, No. 3, 1984, pp. 409-411.
- S. Kataoka-Nishimura, H. Akiyama, K. Saku, M. Kashiwa, S. Mori, S. Tanikawa, H. Sakamaki and Y. Onozawa, “Invasive Infection Due to Trichosporon cutaneum in Patients with Hematologic Malignancies,” Cancer, Vol. 82, No. 3, 1998, pp. 484-487. http://dx.doi.org/10.1002/(SICI)1097-0142(19980201)82:3<484::AID-CNCR9>3.0.CO;2-P
- V. Kremery Jr., F. Mateicka, A. Kunova, S. Spanik, J. Gyarfas, Z. Sycova and J. Trupl, “Hematogenous Trichosporonosis in Cancer Patients: Report of 12 Cases Including 5 during Prophylaxis with Itraconazole,” Support Care Cancer, Vol. 7, No. 1, 1999, pp. 39-43. http://dx.doi.org/10.1007/s005200050221
- D. P. Kontoyiannis, H. A. Torres, M. Chagua, R. Hachem, J. J. Tarrand, G. P. Bodey and I. I. Raad, “Trichosporonosis in a Tertiary Care Cancer Center: Risk Factors, Changing Spectrum and Determinants of Outcome,” Scandinavian Journal of Infectious Disease, Vol. 36, No. 8, 2004, pp. 564-569.
- C. Girmenia, L. Pagano, B. Martino, D. D’Antonio, R. Fanci, G. Specchia, L. Melillo, M. Buelli, G. Pizzarelli, M. Venditti and P. Martino, “Invasive Infections Caused by Trichosporon Species and Geotrichum capitatum in Patients with Hematological Malignancies: A Retrospective Multicenter Study from Italy and Review of the Literature,” Journal of Clinical Microbiology, Vol. 43, No. 4, 2005, pp. 1818-1828. http://dx.doi.org/10.1128/JCM.43.4.1818-1828.2005
- K. Suzuki, K. Nakase, T. Kyo, T. Kohara, Y. Sugawara, T. Shibazaki, K. Oka, T. Tsukada and N. Katayama, “Fatal Trichosporon Fungemia in Patients with Hematological Malignancies,” European Journal of Haematology, Vol. 84, No. 5, 2010, pp. 441-447. http://dx.doi.org/10.1111/j.1600-0609.2010.01410.x
- E. Anaissie, A. Gokaslan, R. Hachem, R. Rubin, G. Griffin, R. Robinson, J. Sobel and G. Bodey, “Azole Therapy for Trichosporonosis: Clinical Evaluation of Eight Patients, Experimental Therapy for Murine Infection, and Review,” Clinical Infectious Disease, Vol. 15, No. 5, 1992, pp. 781-787. http://dx.doi.org/10.1093/clind/15.5.781
- S. Fournier, W. Pavageau, M. Feuillhade, S. Deplus, A. M. Zagdanski, O. Verola, H. Dombret and J. M. Molina, “Use of Voriconazole to Successfully Treat Disseminated Trichosporon asahii Infection in a Patient with Acute Myeloid Leukemia,” European Journal of Clinical Microbiology & Infectious Disease, Vol. 21, No. 12, 2002, pp. 892-896.
- R. Falk, D. G. Wolf, M. Shaprio and I. Polacheck, “Multidrug Resistant Trichosporon asahii Isolates Are Susceptible to Voriconazole,” Journal of Clinical Microbiology, Vol. 41, No. 2, 2003, p. 911. http://dx.doi.org/10.1128/JCM.41.2.911.2003
- I. J. Sud and D. S. Feingold, “Heterogeneity of Action Mechanisms among Antimycotic Imidazoles,” Antimicrobial Agents and Chemotherapy, Vol. 20, No. 1, 1981, pp. 171-174. http://dx.doi.org/10.1128/AAC.20.1.71
- D. Kobayashi, K. Kondo, N. Uehara, S. Otokozawa, N. Tsuji, A. Yagihashi and N. Watanabe, “Endogenous Reactive Oxygen Species Is an Important Mediator of Miconazole Antifungal Effect,” Antimicrobial Agents and Chemotherapy, Vol. 46, No. 10, 2002, pp. 3113-3117. http://dx.doi.org/10.1128/AAC.46.10.3113-3117.2002
- National Committee for Clinical Laboratory Standards, “Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast; Approved Standard,” NCCLS document M27-A, National Committee for Clinical Laboratory Standards, Wayne, 1997.
- K. Perparim, H. Nagai, A. Hashimoto, Y. Goto, T. Tashiro and M. Nasu, “In Vitro Susceptibility of Trichosporon beigelii to Antifungal Agents,” Journal of Chemotherapy, Vol. 8, No. 6, 1996, pp. 445-448.
- K. Marr, “Combination Antifungal Therapy: Where Are We Now, and Where Are We Going?” Oncology (Williston Park), Vol. 18, No. 13, 2004, pp. 24-29.
- H. Senati, C. F. Ramos, A. S. Bayer and M. A. Ghannoum, “Combination Therapy with Amphotericin B and Fluconazole against Invasive Candidiasis in NeutropenicMouse and Infective-Endocarditis Rabbit Models,” Antimicrobial Agents and Chemotherapy, Vol. 41, No. 6, 1997, pp. 1345-1348.
- A. I. Popp, M. H. White, T. Quadri, L. Walshe and D. Armastrong, “Amphotericin B with and without Itraconazole for Invasive Aspergillosis: A Three-Year Retrospective Study,” International Journal of Infectious Disease, Vol. 3, No. 3, 1999, pp. 157-160. http://dx.doi.org/10.1016/S1201-9712(99)90038-3
- T. J. Walsh, G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelly, J. Lee, D. Callender, M. Rubin and P. A. Pizzo, “Trichosporon beigeli, an Emerging Pathogen Resistant to Amphotericin B,” Journal of Clinical Microbiology, Vol. 28, No. 7, 1990, pp. 1616-1622.
- A. L. Colombo, A. C. B. Padovan and G. M. Chaves, “Current Knowledge of Trichosporon spp. and Trichosporonosis,” Clinical Microbiology Reviews, Vol. 24, No. 4, 2011, pp. 682-700. http://dx.doi.org/10.1128/CMR.00003-11
Abbreviations
TF: Trichosporon fungemia
MCZ: miconazole
MUH: Mie university hospital
FLCZ: fluconazole
ITCZ: itraconazole
AmB: amphotericin B
IV: intravenous
MF: micafungin
MIC: minimal inhibitory concentration
AML: acute myeloid leukemia
ALL: acute lymphoblastic leukemia
G-CSF: granulocyte colony-stimulating factor
CV: central venous catheter
NOTES
*Corresponding author.

