Open Journal of Medical Microbiology
Vol.3 No.4(2013), Article ID:41028,6 pages DOI:10.4236/ojmm.2013.34038
Synergistic Combination of Carbapenems and Colistin against P. aeruginosa and A. baumannii
1Clinical Microbiology Laboratory, Faculty of Medicine, University of Balamand, Tripoli, Lebanon
2Infectious Diseases Department, Centre Hospitalier du Nord, Zgharta, Lebanon
Email: *ziad.daoud@balamand.edu.lb
Copyright © 2013 Ziad Daoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Ziad Daoud et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian.
Received October 24, 2013; revised November 22, 2013; accepted November 29, 2013
Keywords: Antibiotic Combination; Antibiotic Synergy; MIC; FIC; Acinetobacter baumannii; Pseudomonas aeruginosa; Colistin; Meropenem; Imipenem
ABSTRACT
Background: Intubated patients are particularly at risk of developing infections caused by these pathogens, specifically, P. aeruginosa and A. baumannii. In the past fifteen years, Carbapenems were known to be the drugs of choice for these bacteria. With the increase in the use and misuse of antibiotics, these bacteria became highly resistant, and almost all available antibiotics, including Carbapenems, became inefficient. Synergistic combination therapy may be a useful strategy in slowing as well as overcoming the emergence of resistance. The aim of this study was to evaluate the antibacterial activity on P. aeruginosa and A. baumannii of the combination of two antibiotics: Colistin and a Carbapenem (Meropenem or Imipenem). Methods: The antibacterial activity was assessed by determining the MIC. Then, the effect of combining the antibiotics was studied using the Checkerboard Technique described by White et al., 1996. The Fractional Inhibitory Concentration (FIC) for each strain was then calculated and classified as synergy, additive, indifference or antagonism. 11 strains of A. baumannii and 11 strains of P. aeruginosa were tested in the presence of Meropenem combined with Colistin or Imipenem combined with Colistin. Results: For the combination of Meropenem and Colistin, 6 strains of A. baumannii and 3 strains of P. aeruginosa showed synergy while 5 strains of A. baumannii and 7 strains of P. aeruginosa showed additive effect, only 1 strain of P. aeruginosa showed antagonism. For Imipenem and Colistin, only 1 strain of A. baumannii and 3 strains of Pseudomonas showed synergy while 8 strains of Acinetobacter and 8 strains of Pseudomonas showed additive effect. Conclusion: The “in vitro” combination Colistin-Carbapenem is associated with an improvement in MIC. In the majority of the cases, this improvement suggests a synergistic combination or an additive effect.
1. Introduction
Nosocomial infections are mostly encountered in patients admitted to the intensive care units [1]. Among these, catheterized or critically ill patients are considered as number one group developing hospital acquired infections [2]. Gram positive as well as many gram negative bacteria can be responsible for such infections [3,4]. P. aeruginosa and A. baumannii are opportunistic pathogens that mostly cause hospital acquired pneumonia (HAP) and ventilated associated pneumonia (VAP) [5]. They are usually more resistant than community acquired pathogens due to extensive exposure to antibiotics in hospitals.
Due to the lack or inefficiency of infection control programs in many hospitals, random/extensive use of antibiotics and many others reasons, resistance highly emerged within these pathogens and they became known as highly resistant microorganisms [6]. Carbapenem resistant P. aeruginosa and A. baumannii are nowadays widely spread. By nature, these pathogens are more resistant than other Gram negative organisms because of their outer membrane that is less permeable [7] and their ability to form biofilm [8]. Furthermore, the main mechanisms of resistance acquired by these pathogens can be summarized by the decrease/modification in the porin channels that is usually coupled by efflux pumps [9] and by the hydrolytic enzymes such as Metallo Beta Lactamases (MBL) and Oxacillinases. For example, a decrease in OprD causes increased level of resistance to Carbapenems since they resemble amino acids that usually gain entry via these channels [10,11]. In a recent study, carbapenems resistant P. aeruginosa (CRPA) isolated in Russia produced MBL [12]. Furthermore, 73% and 95% of carbapenems resistant Acinetobacter baumanii (CRAB) isolated in 2010 and 2011 respectively from Greece and Brazil were due to OXA-23 acquisition [13].
Similarly to the worldwide scenario, resistance in the Middle East in general and specifically in Lebanon is increasing and many strains were found to be carbapenem resistant [14-16]. In a study including hospitals from different Middle Eastern countries, it was found that CRAB isolated from a Lebanese hospital carried the oxa-58, oxa-23 and oxa-72 genes conferring the carbapenems resistance [17].
Through history, these pathogens were treated using different anti-pseudomonal and anti-Acinetobacter agents such as aztreonam, aminoglycosides and fluoroquinolones [18]. Carbapenems were considered as the drugs of extreme cases of resistance. With the increase in resistance against carbapenems, most of the available antimicrobial agents are becoming useless [17]. Physicians have limited solution for the treatment of such infections. The old antibiotic Colistin, in spite of its toxicity and side effects, is considered nowadays as the last resource when these multi-drug organisms are observed [18,19]. The idea of combining Colistin with other antibiotics seeking a synergistic activity, and probably a less toxicity, looks promising. Combination therapy limits and suppresses bacterial resistance, decreases antibiotic toxicity, covers a broad range of pathogens with greater efficacy and most importantly leads to synergy [20,21].
The aim of this study was to evaluate the antibacterial activity on P. aeruginosa and A. baumannii of the combination of two antibiotics: Colistin and a Carbapenem (Meropenem or Imipenem) using Checkerboard technique.
2. Materials and Methods
Bacterial strains: 11 strains of A. baumannii and 11 strains of P. aeruginosa of different susceptibility profiles were isolated from patients of Centre Hospitalier du Nord (CHN), North Lebanon. Most of the strains were resistant or intermediate to Carbapenems and susceptible to Colistin.
Inoculum preparation: Strains were incubated overnight at 37˚C. Two to 3 colonies were added to Muller Hinton Broth and then the turbidity was standardized to 0.5 McFarland (108 CFU/mL). Dilutions were done in order to match a final concentration of 5 × 105 CFU/mL that was used for MIC determination and Checkerboard technique.
Antibiotic preparation: Imipenem (Merck Sharp & Dohme B/V, Netherlands), Meropenem (AstraZeneca UK Limited, United Kingdom) and Colistin (Forest laboratories, United Kingdom) were obtained as powder. Aliquots were prepared and stored at −20˚C.
MIC determination: The MICs of Imipenem, Meropenem and Colistin for the 22 strains were determined by broth macrodilution method as described by Clinical and Laboratory Standard Institute-CLSI [23]. For each strain, duplicate sets of 16 tubes for serial dilutions each were prepared. The MIC was defined as the lowest concentration of antibiotic that inhibits the visual growth of bacteria after 18 hours of incubation. The final inoculum was 5 × 105 CFU/mL. The tubes were incubated for 16 h - 20 h at 37˚C and then interpreted.
Synergy testing by Checkerboard technique: The combinations tested for each strain of each microorganism were Meropenem plus Colistin and Imipenem plus Colistin. The concentration of Colistin ranged from 1/32 × MIC to 32 × MIC while that of the carbapenem (Imipenem or Merpenem) ranged from 1/8 × MIC to 8 × MIC. The checkerboard technique consists of the following steps: In panel B, MHB was added in all wells except row A and H. 100 μL of Imipenem or Meropenem was then added into the raw G (G1 ® G12) and raw H (H1 ® H11; except H12) to give a final concentration of 8MIC. Serial dilution from G to B was performed. In panel A: 50 μL of MHB was added in all the wells except column 1 and 12. Then, 50 μL of Colistin was added to the column 11 (A11 ® H11) and column 12 (A12 ® G12; except H12) to give a final concentration of 32 MIC. Serial dilution from column 11 to 2 was performed. After serially diluting panel A and Panel B, 50 μL was taken from each well of panel B and dispensed into the corresponding well of panel A. Then the bacterial inoculum was prepared and added into the wells. The plates were incubated for 18 - 24 h at 37˚C and then interpreted.
FIC calculation: To evaluate the antibacterial effect of each combination, the Σ FIC was calculated.
 .
.
The results were then classified as: synergy for Σ FIC ≤ 0.5; additive for Σ FIC between 0.5 and 1.5; and indifference for values of Σ FIC between 1.5 and 2; Antagonism was linked to values above 2 [22,24,25].
3. Results
Minimum Inhibitory Concentrations: The MICs obtained for each antibiotic are shown in Table 1 and 2. All P. aeruginosa strains used were sensitive to Colistin. 4 strains were resistant to Meropenem and Imipenem. All the other strains were intermediate for both carbapenems. P21 had the lowest MIC to Colistin while P24 had the highest MIC to Colistin. For Meropenem, P27 had the lowest MIC while P28 had the highest MIC. For Imipenem, P11, P21 and P25 had the lowest MIC while P30 had the highest MIC. For A. baumannii, 10 of the 11 strains used were sensitive to Colistin. 5 strains were resistant to Meropenem and Imipenem. A2, A4, A5, A8, A9, A10, A12 and A13 had the lowest MIC to Colistin while A100 had the highest MIC to Colistin. For Meropenem, A8 had the lowest MIC while A2, A5 and A6 had the highest MIC. For Imipenem, A9 had the lowest MIC while A5, A6, A12 and A100 had the highest MIC.
3.1. Checkerboard Results
Table 3 shows the FICs calculated for all the Acinetobacter strains using the 2 combinations of antibiotics; while table 4 shows the FICs calculated for all the Pseudomonas strains using the 2 combinations of antibiotics.
For the combination of Meropenem and Colistin on A. baumannii, 6 of the 11 strains showed synergy while 5 strains showed additive results. The average of the “Mean of Σ FIC” for the 11 strains of A. baumannii is 0.5393 ± 0.180 with Meropenem combined to Colistin. For the combination of Imipenem and Colistin on A. baumannii, 1 of the 11 strains showed synergy while 8 strains showed additive results and 2 strains showed antagonism. The average of the “Mean of Σ FIC” was 1.2019 ± 0.942 with Imipenem combined to Colistin.
For the combination of Meropenem and Colistin on P.aeruginosa, 3 of the 11 strains showed synergy while 7 strains of 11 showed additive results. Antagonistic result
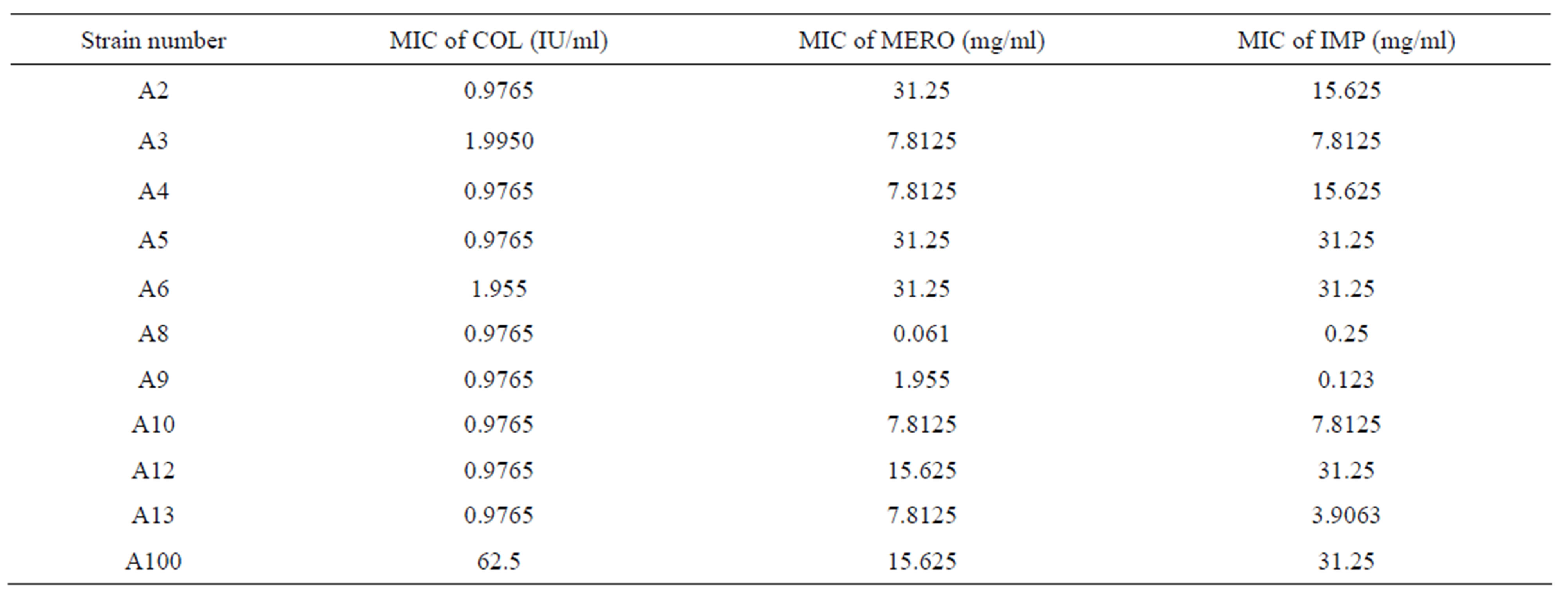
Table 1. MICs results by broth macrodilution of Colistin (COL), Meropenem (MERO), and Imipenem (IMP) for 11 A. baumannii strains.
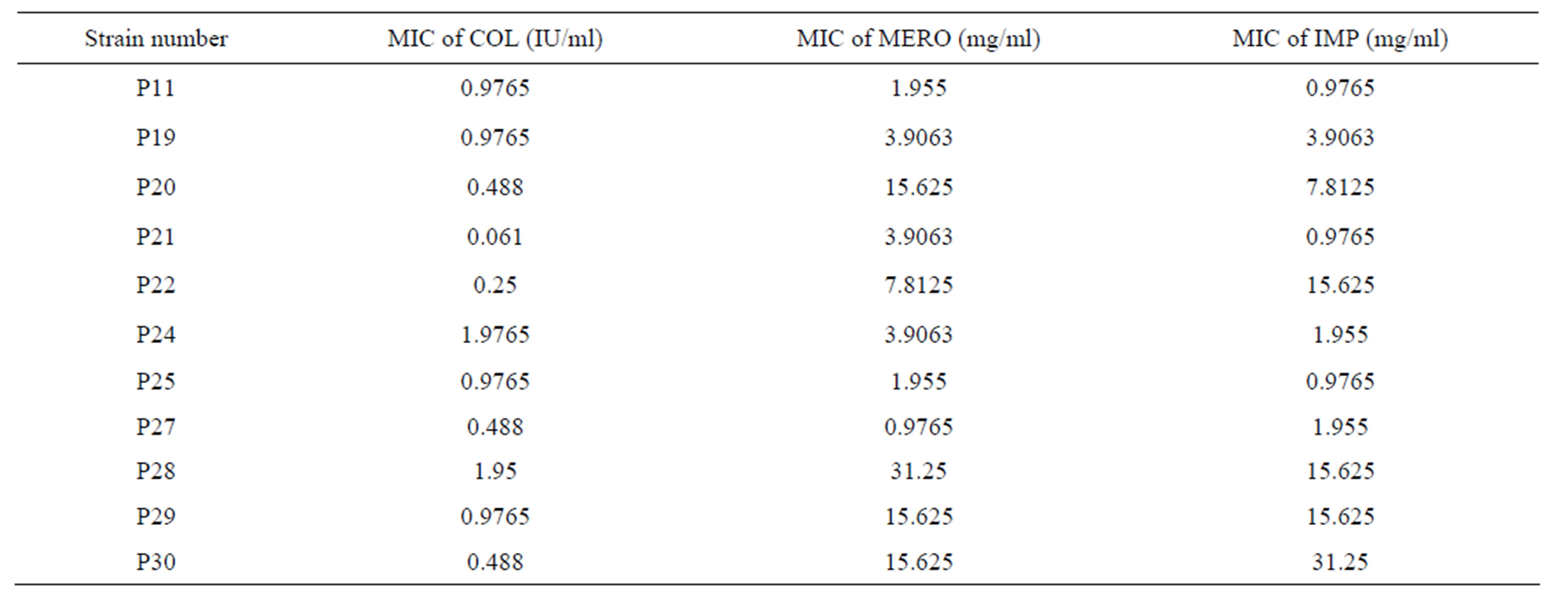
Table 2. MICs of Colisitn (COL), Meropenem (MERO), and Imipenem (IMP) against 11 strains of P. aeruginosa.
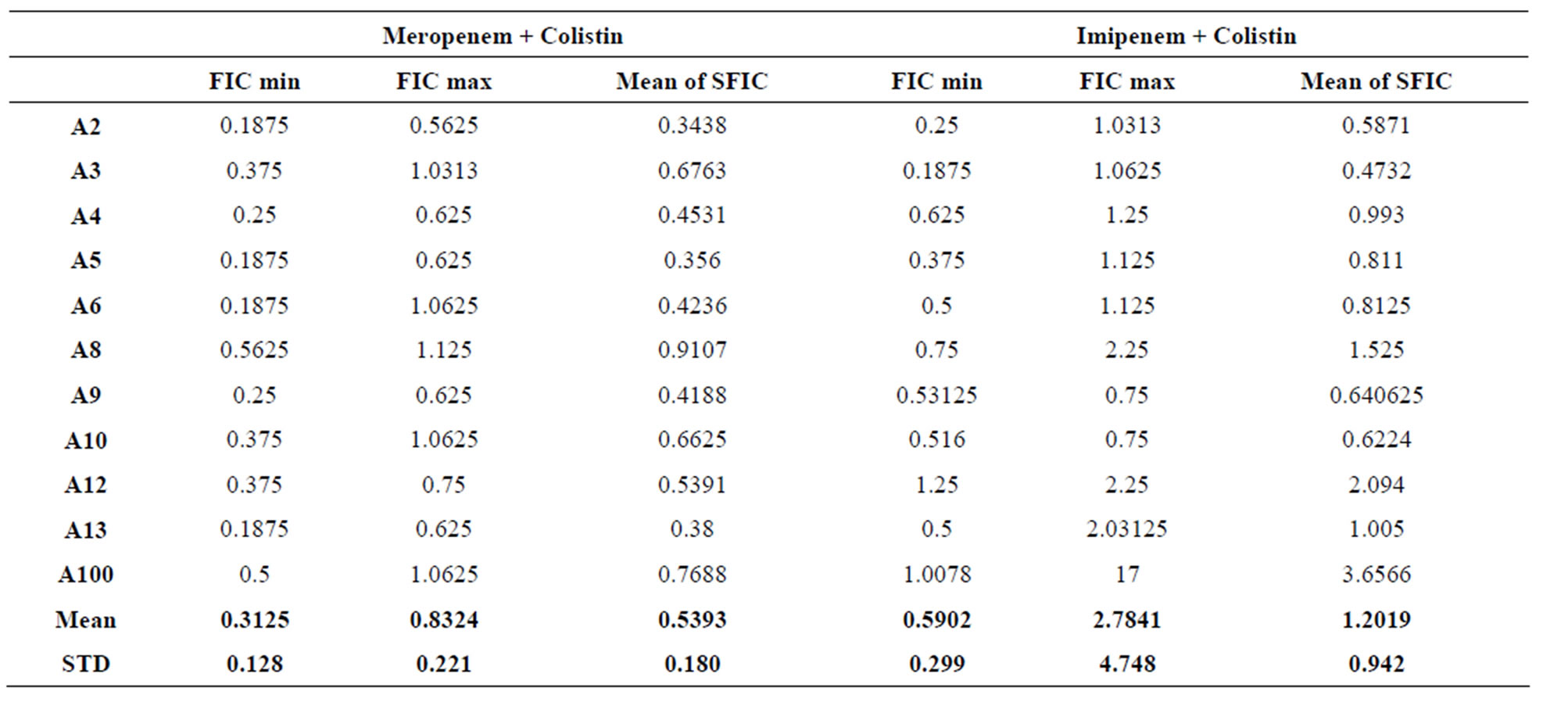
Table 3. FICs calculated for Acinetobacter baumannii in Meropenem + Colistin and Imipenem + Colistin combinations.
was seen with the strain P25. The average of the “Mean of Σ FIC” for the 11 strains of P. aeruginosa is 0.9342 ± 0.572 with the combination of Meropenem and Colistin.
For the combination of Imipenem and Colistin on P. aeruginosa, 3 stains showed synergy while 7 strains of 11 showed additive results and 1 showed indifference. The best synergy was detected with the strain P30. The average of the “Mean of Σ FIC” for the 11 strains of P. aeruginosa is 0.9619 ± 0.431 with the combination of Imipenem and Colistin.
The antibacterial effect of both combinations on both A. baumannii and P. aeruginosa showed mostly synergistic or additive results. To a significant extent Meropenem and Colistin showed a better synergy when compared to Imipenem and Colistin.
4. Discussion
Most P. aeruginosa and A. baumannii are becoming multidrug resistant, the major issue being resistance to Carbapenems [26]. All the strains used in this study were resistant or intermediate to Carbapenems however susceptible to Colistin except the strain A100 that was highly resistant to Colistin (Tables 1 and 2). The only differences in susceptibility to Carbapenems were seen with the following strains P22, P20 and A4. Strain P22 showed intermediate resistance to Meropenem but full resistance to Imipenem. It has been described that Meropenem has greater in vitro efficacy than Imipenem against P. aeruginosa [23]. P20 strain is the only intermediate strain for Imipenem but resistant for Meropenem. Such profile may be mostly related to an upregulation in the efflux system MexAB/OprM resulting in increased resistance to Meropenem as compared to Imipenem [25]. Strain A4 showed intermediate resistance for Meropenem but fully resistance for Imipenem and this might be explained by a down-regulation in the OprD porin channel which will cause an increase in the resistance to Imipenem as compared to Meropenem [26].
On the other hand, the combination of Meropenem and Colistin can be considered as a better combination for the treatment of A. baumannii and P. aeruginosa when compared to the combination Imipenem and Colistin because the means of FICs for Meropenem/Colistin were 0.5393 ± 0.180 and 0.9342 ± 0.572 respectively while those of Imipenem/Colistin were 1.2019 ± 0.942 and 0.9619 ± 0.431 respectively.
The best synergy rate and subsequent highest antibacterial activity were noticed for the combination of Meropenem and Colistin. This suggests that the combination could be a good alternative for the treatment of Acinetobacter and Pseudomonas infections until the successful development of a better antibiotic agent. The reason for the increased synergy with Meropenem than Imipenem might be that most OXA and MBLs carbapenemases target with greater affinity Imipenem as compared to Meropenem [28,29]. In addition, the synergistic or additive effect might be influenced by the ability of Colistin to disrupt the bacterial outer membrane and increase its permeability for Carbapenems [30-32] and therefore stop the cross linking of the new synthesized polymers.
Another advantage for combination therapy is the delay in emergence of bacterial resistance and specifically the rapidly developing resistance and heteroresistance toward Colistin [6]. It must be mentioned that not only synergy is considered as an advantage for the therapy but also additive result is by itself beneficial, because even a miniature raise in the antibacterial activity using the combination therapy may help clinical success and recovery.
Our study offers data in support for the combination therapy to treat infections caused by multi-drug-resistant Acinetobacter and Pseudomonas. However, in order to be successfully implemented, such a decision requires more pharmacokinetic and pharmacodynamic studies mainly to specify the doses that need to be administered in order to maintain an acceptable profile of toxicity of these combinations.
5. Acknowledgements
We thank the Lebanese National Research Council for their financial support.
REFERENCES
- C. R. Kiffer and A. C. Pignatari, “Pharmacodynamic Evaluation of Commonly Prescribed Oral Antibiotics against Respiratory Bacterial Pathogens,” BMC Infectious Diseases, Vol. 11, 2011, p. 286.
- G. Capellier, H. Mockly, C.Charpentier, D. Annane, G. Blasco, T. Desmettre and L. Papazian, “Early-Onset Ventilator-Associated Pneumonia in Adults Randomized Clinical Trial: Comparison of 8 versus 15 Days of Antibiotic Treatment,” PloS One, Vol. 7, No. 8, 2012, Article ID: e41290. http://dx.doi.org/10.1371/journal.pone.0041290
- A. C. Breier, C. Brandt, D. Sohr, C. Geffers and P. Gastmeier, “Laminar Airflow Ceiling Size: No Impact on Infection Rates Following Hip and Knee Prosthesis,” Infection Control and Hospital Epidemiology: The Official Journal of the Society of Hospital Epidemiologists of America, Vol. 32, No. 11, 2011, pp. 1097-1102.
- S. Micek, M. T. Johnson, R. Reichley and M. H. Kollef, “An Institutional Perspective on the Impact of Recent Antibiotic Exposure on Length of Stay and Hospital Costs for Patients with Gram-Negative Sepsis,” BMC Infectious Diseases, Vol. 12, 2012, p. 56.
- M. H. Kollef, G. Sherman, S. Ward and V. J. Fraser, “Inadequate Antimicrobial Treatment of Infections: A Risk Factor for Hospital Mortality among Critically Ill Patients,” Chest, Vol. 115, No. 2, 1999, pp. 462-474. http://dx.doi.org/10.1378/chest.115.2.462
- M. E. Falagas and D. E. Karageorgopoulos, “Pandrug Resistance (PDR), Extensive Drug Resistance (XDR), and Multidrug Resistance (MDR) among Gram-Negative Bacilli: Need for International Harmonization in Terminology,” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, Vol. 46, No. 7, 2008, pp. 1121-1122.
- B. L. Angus, A. M. Carey, D. A. Caron, A. M. Kropinski and R. E. Hancock, “Outer Membrane Permeability in Pseudomonas aeruginosa: Comparison of a Wild-Type with an Antibiotic-Supersusceptible Mutant,” Antimicrobial Agents and Chemotherapy, Vol. 21, No. 2, 1982, pp. 299-309. http://dx.doi.org/10.1128/AAC.21.2.299
- W. Hengzhuang, H. Wu, O. Ciofu, Z. Song and N. Hoiby, “Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem on Mucoid and Nonmucoid Pseudomonas aeruginosa Biofilms,” Antimicrobial Agents and Chemotherapy, Vol. 55, No. 9, 2011, pp. 4469-4474. http://dx.doi.org/10.1128/AAC.00126-11
- I. Ziha-Zarifi, C. Llanes, T. Kohler, J. C. Pechere and P. Plesiat, “In Vivo Emergence of Multidrug-Resistant Mutants of Pseudomonas aeruginosa Overexpressing the Active Efflux System MexA-MexB-OprM,” Antimicrobial Agents and Chemotherapy, Vol. 43, No. 2, 1999, pp. 287-291.
- E. Yoshihara and T. Nakae, “Separation of Gateand Channel-Forming Domains in the Pore-Forming Protein of the Outer Membrane of Pseudomonas aeruginosa,” FEBS Letters, Vol. 306, No. 1, 1992, pp. 5-8. http://dx.doi.org/10.1016/0014-5793(92)80826-3
- K. H. Buscher, W. Cullmann, W. Dick and W. Opferkuch, “Imipenem Resistance in Pseudomonas aeruginosa Resulting from Diminished Expression of an Outer Membrane Protein,” Antimicrobial Agents and Chemotherapy, Vol. 31, No. 5, 1987, pp. 703-708. http://dx.doi.org/10.1128/AAC.31.5.703
- D. V. Ivanov and A. M. Egorov, “Spreading and Mechanisms of Antimicrobial Resistance of Microorganisms, Producing Beta-Lactamases. Phenotypical Screening for MBL Producers (carbapenemases B1) among strains of Pseudomonas genus, Isolated in Cases of Nosocomial Infections,” Biomeditsinskaia Khimiia, Vol. 53, No. 6, 2007, pp. 653-661.
- L. L. Correa, L. A. Botelho, L. C. Barbosa, C. S. Mattos, J. M. Carballido, C. L. de Castro, C. R. de MendoncaSouza, “Detection of bla(OXA-23) in Acinetobacter spp. Isolated from Patients of a university Hospital,” The Brazilian Journal of Infectious Diseases: An Official Publication of the Brazilian Society of Infectious Diseases, Vol. 16, No. 6, 2012, pp. 521-526. http://dx.doi.org/10.1016/j.bjid.2012.10.003
- R. Zarrilli, D. Vitale, A. Di Popolo, M. Bagattini, Z. Daoud, A. U. Khan, C. Afif and M. Triassi, “A Plasmid-Borne blaOXA-58 Gene Confers Imipenem Resistance to Acinetobacter baumannii Isolates from a Lebanese Hospital,” Antimicrobial Agents and Chemotherapy, Vol. 52, No. 11, 2008, pp. 4115-4120. http://dx.doi.org/10.1128/AAC.00366-08
- M. Giannouli, F. Tomasone, A. Agodi, H. Vahaboglu, Z. Daoud, M. Triassi, A. Tsakris and R. Zarrilli, “Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Strains in Intensive Care Units of Multiple Mediterranean Hospitals,” Journal of Antimicrobial Chemotherapy, Vol. 63, No. 4, 2009, pp. 828-830. http://dx.doi.org/10.1093/jac/dkp032
- A. Di Popolo, M. Giannouli, M. Triassi, S. Brisse and R. Zarrilli, “Molecular Epidemiological Investigation of Multidrug-Resistant Acinetobacter baumannii Strains in Four Mediterranean Countries with a Multilocus Sequence Typing Scheme,” Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, Vol. 17, No. 2, 2011, pp. 197-201. http://dx.doi.org/10.1111/j.1469-0691.2010.03254.x
- G. F. Araj, A. Z. Avedissian, N. S. Ayyash, H. A. Bey, R. G. El Asmar, R. Z. Hammoud and S. A. Sabai, “A Reflection on Bacterial Resistance to Antimicrobial Agents at a Major Tertiary Care Center in Lebanon over a Decade,” The Lebanese Medical Journal, Vol. 60, No. 3, 2012, pp. 125-135.
- G. M. Rossolini and E. Mantengoli, “Treatment and Control of Severe Infections Caused by Multiresistant Pseudomonas aeruginosa,” Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, Vol. 11, Suppl. 4, 2005, pp. 17-32. http://dx.doi.org/10.1111/j.1469-0691.2005.01161.x
- W. S. Choi, S. H. Kim, E. G. Jeon, M. H. Son, Y. K. Yoon, J. Y. Kim and D. W. Park, “Nosocomial Outbreak of Carbapenem-Resistant Acinetobacter baumannii in Intensive Care Units and Successful Outbreak Control Program,” Journal of Korean Medical Science, Vol. 25, No. 7, 2010, pp. 999-1004. http://dx.doi.org/10.3346/jkms.2010.25.7.999
- J. Li, R. L. Nation, R. W. Milne, J. D. Turnidge and K. Coulthard, “Evaluation of Colistin as an Agent against Multi-Resistant Gram-Negative Bacteria,” International Journal of Antimicrobial Agents, Vol. 25, No. 1, 2005, pp. 11-25. http://dx.doi.org/10.1016/j.ijantimicag.2004.10.001
- K. Lee, D. Yong, S. H. Jeong and Y. Chong, “Multidrug-Resistant Acinetobacter spp.: Increasingly Problematic Nosocomial Pathogens,” Yonsei Medical Journal, Vol. 52, No. 6, 2011, pp. 879-891. http://dx.doi.org/10.3349/ymj.2011.52.6.879
- G. M. Eliopoulos and R. C. Moellering Jr., “Antibiotic Synergism and Antimicrobial Combinations in Clinical Infections,” Reviews of Infectious Diseases, Vol. 4, No. 2, 1982, pp. 282-293. http://dx.doi.org/10.1093/clinids/4.2.282
- Clinical and Laboratory Standards Institute, “Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement,” Clinical and Laboratory Standards Institute, 2011.
- D. K. Joung, H. Joung, D. W. Yang, D. Y. Kwon, J. G. Choi, S. Woo and D. W. Shin, “Synergistic Effect of Rhein in Combination with Ampicillin or Oxacillin against Methicillin-Resistant staphylococcus aureus,” Experimental and Therapeutic Medicine, Vol. 3, No. 4, 2012, pp. 608- 612.
- R. L. White, D. S. Burgess, M. Manduru and J. A. Bosso, “Comparison of Three Different in Vitro Methods of Detecting Synergy: Time-kill, Checkerboard, and E Test,” Antimicrobial Agents and Chemotherapy, Vol. 40, No. 8, 1996, pp. 1914-1918.
- A. B. Teixeira, A. F. Martins, J. Barin, D. M. Hermes, C. Pormann, A. L. Barth, “First Report of Carbapenem-Resistant Acinetobacter nosocomialis Isolates Harboring ISAba1-blaOXA-23 Genes in Latin America,” Journal of Clinical Microbiology, Vol. 51, No. 8, 2013, pp. 2739- 3741.
- N. Masuda, E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto and T. Nishino, “Substrate Specificities of MexABOprM, MexCD-OprJ, and MexXY-oprM Efflux pupms in Pseudomonas aeruginosa,” Antimicrobial Agents and Chemotherapy, Vol. 44, No. 12, 2000, pp. 3322-3327.
- G. Santella, S. Pollini, J. D. Docquier, M. Almuzara, G. Gutkind, G. M. Rossolini and M. Radice, “Carbapenem Resistance in Pseudomonas aeruginosa Isolates: An Example of Interaction between Different Mechanisms,”
- A. Oliver, B. R. Levin, C. Juan, F. Baquero and J. Blazquez, “Hypermutation and the Preexistence of Antibiotic-Resistant Pseudomonas aeruginosa Mutants: Implications for Susceptibility Testing and Treatment of Chronic Infections,” Antimicrobial Agents and Chemotherapy, Vol. 48, No. 11, 2004, pp. 4226-4233. http://dx.doi.org/10.1128/AAC.48.11.4226-4233.2004
- T. R. Walsh, M. A. Toleman, L. Poirel and P. Nordmann, “Metallo Beta-Lactamses: The Clam before the storm?” Clinical Microbiology Reviews, Vol. 18, No. 2, 2005, pp. 306-325. http://dx.doi.org/10.1128/CMR.18.2.306-325.2005
- A. Y. Peleg, H. Seifert and D. L. Paterson, “Acinetobacter baumannii: Emergence of a Successful Pathogen,” Clinical Microbiology Reviews, Vol. 21, No. 3, 2008, pp. 538- 582.
- J. P. Zhang, W. Zhu, S. F. Tian, Y. Z. Chu and B. Y. Chen, “Molecular Characteristics and Resistant Mechanisms of Imipenem-Resistant Acinetobacter baumannii Isolates in Shenyang, China,” Journal of Microbiology, Vol. 48, No. 5, 2010, pp. 689-694. http://dx.doi.org/10.1007/s12275-010-0137-3
NOTES
*Corresponding author.

