American Journal of Analytical Chemistry
Vol.07 No.04(2016), Article ID:65783,15 pages
10.4236/ajac.2016.74037
Okra Leaves―Agricultural Waste for the Removal of Cr(III) and Cr(VI) from Contaminated Water
Mazhar Iqbal Khaskheli1, Saima Qayyum Memon1, Zaheer Ahmed Chandio1, Wahid Bux Jatoi2, Mohammad Tariq Mahar1, Faiz Mohammad Khokhar1
1M. A. Kazi Institute of Chemistry, University of Sindh, Jamshoro, Pakistan
2Institute of Chemistry, Shah Abdul Latif University Khairpur, Sindh, Pakistan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 February 2016; accepted 19 April 2016; published 22 April 2016
ABSTRACT
The present study demonstrates the sorptive efficiency of okra leaves for the Cr(III) and Cr(VI) in aqueous system. The batch dynamic sorption method was carried out as the function of the pH of the solution, sorbent doze, initial bulk concentration, shaking speed and contact time. The experimental data were examined by Langmuir and Freundlich isotherms. Cr(III) at pH-4.0 and Cr(VI) pH-2.0 showed a maximum sorption capacities 221.17 mg/g and 81.94 mg/g respectively. Kinetically experimental data of both chromium species were well fitted to pseudo second order rate, Elovich, Morris-Weber and Richenberg equations. Positive values of enthalpy change and negative values of Gibbs free energy indicated the endothermic and spontaneous nature of sorption reaction respectively. The developed method was applied to real ground water samples (affected by industrial wastewater). Up to 92.15% of chromium metal was removed from the real water samples.
Keywords:
Okra Leaves, Chromium, Pseudo Second Order

1. Introduction
Increasing industrial development causes environmental problems, especially in developing countries. Water pollution by heavy metals through industrial effluents or any other source is one of the hottest issues of the world. Generally, contamination sources are the effluents from batteries, refractory, wielding, atomic power plants, mining, metallurgy and industries (leather tanning, electroplating, textile, dyes, pigment, glass, steel, wood preservative, paint and cement etc.) [1] - [4] . It is estimated that per liter of industrial effluent contains 0.5 - 270,000 mg of chromium [5] .
The metals having a density equal to or greater than 6.0 g/cm3 are referred to as “heavy metals” [6] . According to the definition, chromium is a heavy metal and has density 7.19 g/cm3 [7] . Chromium belongs to group A, carcinogen declared by USEPA and 16th toxic metal in the list of 20 toxic metals [8] - [10] . Metallic chromium is harmless, but ionic chromium (+III and +VI) has enormous toxicity [11] . Chromium exists in nine oxidation states (−2, −1, 0, +1, +2, +3, +4, +5, +6) [12] . In aqueous medium two stable oxidation states―trivalent chromium (i.e., Cr3+, Cr(OH)2+ or , etc.) and hexavalent chromium (i.e.,
, etc.) and hexavalent chromium (i.e., 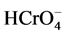 ,
, 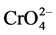 or
or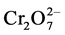 , etc.) are found [13] . Recommended safe limits of chromium in drinking water are WHO 0.05 mg/L, Japan Cr(VI) 0.5 mg/L, USEPA [Cr(III) 0.1 mg/L & Cr(VI) 0.05 mg/L], Pakistan and most Asian developing countries ≤0.05 mg/L [11] [14] [15] .
, etc.) are found [13] . Recommended safe limits of chromium in drinking water are WHO 0.05 mg/L, Japan Cr(VI) 0.5 mg/L, USEPA [Cr(III) 0.1 mg/L & Cr(VI) 0.05 mg/L], Pakistan and most Asian developing countries ≤0.05 mg/L [11] [14] [15] .
Cr(III) is considered as an essential trace element for the regulation of some metabolic chemical reactions such as regulation of sugar, and fat in living organisms (plants and animals) [16] . An estimated safe and adequate daily intake of Cr(III) for adults ranges from 50 to 200 µg [17] . Cr(III) is harmful when its use exceeds the permissible level. It interferes with essential body metabolism, acts as a gastric irritant and causes skin ulcers, bronchitis and dermatitis [18] .
Most compounds of Cr(VI) are detrimental to the health of flora and fauna tissues. Cr(VI) is soluble in almost the whole range of pH [19] . It is 500 times more toxic and highly mobile than Cr(III) [20] . It has teratogenic characteristics [12] . USEPA declared as carcinogenic and mutagenic pollutant [21] . Cr(VI) is a strong oxidizing agent capable of being penetrated rapidly through soils, aquatic environment and skin [22] . It badly affects the organisms by oxidizing DNA and some proteins. Toxicity of chromium (VI) causes vomiting, severe diarrhea, hemorrhage, asthma, pulmonary congestion, affects digestive organs, dermatitis, ulcer (skin, hands and forearms), cancer (digestive tract and lungs) and damage to liver, kidney, circulatory tissues and nerve tissues [23] - [27] .
A wide range of physio-chemical processes such as precipitation, ion-exchange, reverse osmosis, solvent extraction, coagulation, adsorption etc. are available for the removal of toxic metals from the wastewater [28] - [31] . Except adsorption all methods have various drawbacks like cost intensive (require large quantities of chemical reagents), remove metals incompletely and input high cost of energy for the disposal or treatment of generated toxic sludge [32] - [33] . Adsorption, a potential alternative is simple, versatile, cost effective and non-hazardous technique [34] - [36] . It is most favorable because of its high efficiency and complete removal of metals from contaminated water even at low concentration [11] [37] . It is an eco-friendly and has excellent performance [12] . Recovery of metals from sorbent is an easy and sorbent can be reutilized. Toxic waste from an adsorption process can easily be treated and disposed of [38] [39] .
Various natural sorbents such as coconut husk, coconut shell, rice husk, hazelnut shell, husk of Bengal gram, sugarcane bagasse, rice bran, wheat residue, wheat bran, sunflower stalks, eucalyptus bark, maize bran, waste tea, tea factory waste, maple sawdust, walnut husk, peanut husk, bael fruit shell, orange waste, apple waste, orange peel, carrot residue have potentially been used for the removal of toxic metals [4] [6] [11] [13] [32] [40] - [43] . Biosorbents are cheapest, most abundant and environmentally friendly [44] [45] . They provide a large surface area and adsorption on them is possible under the broad range of environmental conditions. Agricultural waste materials contain proteins, polysaccharides and lignin, which are associated with functional groups responsible for metal ion adsorption [46] [47] . The presence of large amounts of various natural biosorbents provides a good alternative to expensive synthetic adsorbents. Metals are attached with sorbent either by physically (London-Van der Waals forces) or by chemically (ionic or covalent bonds).
The purpose of the present work is to use okra leaves (an agricultural waste) for the sorption of Cr(III) and Cr(VI). The efficiency of okra leaves for the sorption of chromium ions was evaluated by optimizing the different parameters in batch method. The developed method was applied to the chromium contaminated real water samples.
2. Materials and Method
2.1. Preparation of the Sorbent
The leaves were washed with tap and distill water. The washed leaves were dried in air and then into the oven at 60˚C for 24 hours. The leaves were ground in blender and sieved to obtain desired particle sizes in the range 0.125 - 0.42 mm. For the sorption of Cr(III) and Cr(VI), 1 g of sorbent of optimized particle size (0.149 mm) was treated with 20 mL of 0.5 M HCl by maintaining the conditions 250 rpm, 45 min and 25˚C temperature. Then acid treated okra sorbent was washed with double distilled water till washing became colorless and pH became neutral. Finally washed sorbent was again dried in oven at 60˚C for 20 hours.
2.2. Preparation of Stock Solution
All chemicals, used in various experiments, were of analytical grade. Stock solutions (1000 mg/L) of Cr(III) and Cr(VI) were prepared by dissolving Cr(NO3)3∙9H2O and K2Cr2O7 respectively in de-ionized water. Desired concentrations were freshly prepared from the stock solutions when required for the experiments. For adjusting the pH, acetic acid, sodium acetate, potassium chloride, hydrochloric acid and sodium hydroxide buffers were used.
2.3. Equipments Used
All pH measurements were carried out by Thermo Scientific Orion 5 Star (pH.ISE. Cond. DO Benchtop, 8102- BNUWP; (Made in USA) PH-meter. Shaking Incubator Model 1-40000 Irmeco GmbH (Geesthacht/Germany) was used for batch adsorption experiments. For EDX analysis, BRUKER X-FLASH 4010 133ev (Made in Germany) and IR study, Smart iTR Thermo Fisher Scientific, 5225 Verona Rd, Madison, WI. 53711, IEC/EN 60825-1:2007, Model: NICOLET iS 10 (Made in USA) were used. Atomic Absorption Spectrometer (Analyst 800, Perkin Elmer, Singapore) equipped with a hallow cathode lamp having current 25 mA, wavelength 357.9 nm and energy 83 was used to measure concentration of chromium.
2.4. Collection and Analysis of Chromium Contaminated Real Water Samples
Chromium contaminated drinking water samples were collected in plastic bottles from different areas of Pakistan. First sample (S1) hand pump water collected from Sarwar Mirani, village Wango 500 m away from evaporation ponds of the Fuji Fertilizer Company (FFC) Mirpur Mathelo, District Dharki, Sindh, Pakistan. Second sample (S2) hand pump water collected from Waheed Ahmed Mirani, village Wango 700 m away from evaporation ponds of the Fuji Fertilizer Company (FFC) Mirpur Mathelo, District Dharki, Sindh, Pakistan. Samples were filtered and initial chromium concentrations were determined using Atomic Absorption. A suitable amount of contaminated water was spiked with 10 mg/L of chromium. Sorption experiments were carried out as stated at optimum conditions. Removal efficiency for both spiked and un-spiked water samples were calculated.
2.5. Procedure for the Sorption Studies of Chromium Ions
The sorptive capacity of okra leaves for Cr(III) and Cr(VI) was investigated by performing various batch experiments. For the sorption of both species of chromium except pH-4.0, 35˚C for Cr(III) and pH-2.0, 30˚C for Cr(VI) all other parameters (amount of adsorbent 0.1 g, agitation time 30 min, volume 20 mL, shaking speed 200 rpm) were kept constant. The treated samples were filtered and chromium ion concentrations of both species were analyzed by flame technique of Atomic Absorption.
For the calculation of sorption efficiency (%) of okra leaves sorbent and the amount of chromium ions sorption at equilibrium (qe, mg/g) following equations were used.
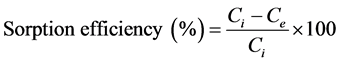 (1)
(1)
where Ci and Ce are initial and equilibrium concentrations (mg/L) chromium ions respectively.
3. Results and Discussion
3.1. pH Dependence of Chromium Removal
The influence of hydrogen ion concentration on the sorption of Cr(III) and Cr(VI) was examined by varying the pH of metal ion solutions in the range 1 - 9 (Figure 1). 0.1 mL of stock solutions of Cr(III) and Cr(VI) were added to two sets of 20 mL volumetric flasks containing de-ionized water to prepare a 5 mg/L solutions. The pH was adjusted by using 1 M HCl and 1 M NaOH. At a lower pH, sorption efficiency of sorbent was poor. Rise in sorption was observed when pH was increased. Maximum sorption of Cr(III) at pH 4 (0.64 mg/g) and Cr(VI) at
Figure 1. Effect of pH on the removal of Cr(III) and Cr(VI) on okra leaves.
pH 2 (0.44 mg/g) was noticed. With the removal of Cr(VI) by hazelnut shell [48] it was reported that at lower pH the oxo-groups of the adsorbent attach largely hydrogen protons (Equation (2)) and at moderate pH carbonaceous adsorbent release hydroxyl ions (Equation (3)) into solution.
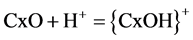 (2)
(2)
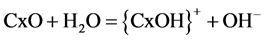 (3)
(3)
At pH-2.0, Cr(VI) exists in the form of oxy-anions (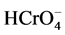 ,
, 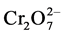 ,
,  , etc.) and sorbent surface becomes cationicin nature due to the presence of excessive hydrogen protons (H+) [13] . As a result strong attraction is created between Cr(VI) and the surface of the sorbent. High pH decreases positive charges on the surface of sorbent, which in turn decreases sorption. At pH-4.0, excess of negatively charged groups on the surface of okra sorbent are responsible for the bonding between Cr(III) and sorbent. This causes the maximum sorption of Cr(III) on okra sorbent. Asimilar trend of maximum sorption of Cr(III) and Cr(VI) with respect to pH was observed with cross-linked chitosan, ectodermis of Opuntia, hazelnut shelland rubber wood saw dust (Table 1). Further experiments were carried out by keeping the constant pH-4.0 for Cr(III) and pH-2.0 for Cr(VI) sorp- tion.
, etc.) and sorbent surface becomes cationicin nature due to the presence of excessive hydrogen protons (H+) [13] . As a result strong attraction is created between Cr(VI) and the surface of the sorbent. High pH decreases positive charges on the surface of sorbent, which in turn decreases sorption. At pH-4.0, excess of negatively charged groups on the surface of okra sorbent are responsible for the bonding between Cr(III) and sorbent. This causes the maximum sorption of Cr(III) on okra sorbent. Asimilar trend of maximum sorption of Cr(III) and Cr(VI) with respect to pH was observed with cross-linked chitosan, ectodermis of Opuntia, hazelnut shelland rubber wood saw dust (Table 1). Further experiments were carried out by keeping the constant pH-4.0 for Cr(III) and pH-2.0 for Cr(VI) sorp- tion.
3.2. Effect of Shaking Speed
Generally shaking speed play an important role in sorption process. The trend of sorption of both chromium species remained similar. Up to 200 rpm sorption was increased (~20%) and then decreased (Table 2). Decrease in sorption at higher shaking speed may be explained on the basis of desorption of chromium was taken place at higher energy.
3.3. Effect of Sorbent Doze
In sorption process the dosage of sorbent is considered to be an effective and basic parameter to develop an efficient method. Figure 2 shows a similar trend of sorption of Cr(III) and Cr(VI) on okra leaves. An increase of ~20% was observed as the amount of sorbent increased from 50 mg to 100 mg. The increase in sorption was due to the availability of the more active sites for the sorption of Cr(III) and Cr(VI) ions [49] . As the amount of sorbent was raised from 100 mg to onward, the percent sorption became constant because instead of increasing active sites, sorbent particles were attached with each other. Thus, for further study 100 mg were selected.
3.4. Effect of Metal Ion Concentration
The effect of metal ion concentration on the percent sorption was studied in the range of 0.5 - 200 mg/L. As metal ion concentration was increased the percent sorption continuously increased up to 200 mg/L. Figure 3 shows the nearly similar trend of per cent sorption of Cr(III) and Cr(VI) as was observed with Cu(II) and Cr(III) by using rose waste biomass [50] .
Table 1. Comparison of adsorption capacity (mg/g) of Cr(III) and Cr(VI) for various natural sorbents.
Table 2. Effect of the shaking speed on the sorption of Cr(III) and Cr(VI).
Figure 2. Cr(III) and Cr(VI) sorption with respect to sorbent doze.
4. Effect of Contact Time and Kinetic Study
By maintaining the optimum pH, shaking speed, volume and dosage of sorbent, time was varied in the range of 0 - 90 minutes. Figure 4 shows that in both cases ((Cr(III) and Cr(VI)) as reaction time increased, per cent sorption increased up to 30 minutes then sorption was nearly constant till 90 minutes. Comparatively, the sorption of okra sorbent for Cr(III) was greater than Cr(VI). Initial increase in sorption could be attributed to the large empty active sites available on the surface of sorbent. Here the shape of the graph is similar to those were reported in the literature [55] - [57] .
4.1. Pseudo-Second Order Rate Equation
In kinetic study, first experimental data were applied to pseudo-first order rate equation, but the data did not fit into the equation. So the experimental data were applied to the following linear form of pseudo-second order rate equation (Equation (4)).
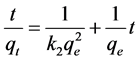 (4)
(4)
where k is the rate constant of pseudo-second order rate equation (g/mg・min); qt the amount of chromium metal
Figure 3. Uptake of Cr(III) and Cr(VI) on okra leaves as a function of metal ions concentration.
Figure 4. Uptake of Cr(III) and Cr(VI) on okra leaves as a function of time.
ion sorbed on okra leaves sorbent at a time (mg/g) and qe is the metal ion sorbed at equilibrium (mg/g). The constants of pseudo-second order rate equation were calculated by plotting t/qt versus t. The high value of correlation co-efficient (0.99) in Table 3 along with straight lines on graph (Figure 5) of Cr(III) and Cr(VI) show that experimental data are in good agreement with the equation. This assumes the sorption process of Cr(III) and Cr(VI) may be chemisorption (mono-layer) may involve valence forces through the exchange or sharing of electrons or complex formation between sorbent and chromium metal ions [55] . Further, this equation also states that heterogeneous energetic active sites are present on the surface of okra leaves sorbent.
4.2. Elovich Equation

where qt is th sorption capacity of metal ions at time t (mg/g), α initial sorption rate (mg/g∙min) and β desorption constant (mg/g・min). Elovich constants α and β were calculated by plotting qt versus lnt. In Table 3 correlation co-efficient values 0.99 and 0.95 for Cr(III) and Cr(VI) respectively show that observed experimental data are well fitted to Elovich equation. This equation supports the pseudo-second order rate equation. The large value of α (11.7) for Cr(VI) illustrates that there is a better attraction of sorbate sites for Cr(VI) ions than Cr(III) ions. This equation also describes that system contains highly heterogeneous sorbing surface on okra leaves sorbent.
4.3. Intra Particle Diffusion (Morris-Weber Equation)
Intra-particle diffusion model (Equation (6)) is used to verify the influence of mass transfer resistance on the binding of metal ions to the sorbent.

where qt is the sorption capacity of metal ions on sorbent at time t (mg/g), kid intra-particle diffusion rate constant (mg/g×min0.5) and C intercept which gives the idea of the thickness of the boundary layer. The graph was
Table 3. Kinetic parameters for the sorption of Cr(III) and Cr(VI) on okra leaves at various temperatures.
Figure 5. Richenberg plot for the sorption of Cr(III) and Cr(VI) on okra leaves.
plotted between qt versus t1/2. According to Table 3, Cr(III) and Cr(VI) experimental data follow equation up to 30 min (with =1.47 mg/g×min0.5) and 90 min with kid (0.35 mg/g×min0.5) respectively and then deviates. Further, a linear portion of the plot which is not passing through origin indicates that intra-particle diffusion is not only the rate limiting step [56] . It supports the idea that sorption mechanism is complex; intra-particle diffusion as well as surface sorption occurs. A similar trend was also observed with Cr(VI) adsorption on oak sawdust and maize bran [41] [42] .
4.4. Richenberg Equation

where Q = qt/qm, Bt = π2Di/ɤ2, qt is the adsorption capacity of metal ions at time t, qm the maximum sorption capacity of the sorbent, Di an effective diffusion co-efficient of ions exchanging inside the sorbent particle. The value of Bt (a mathematical function of Q) can be evaluated for each value of Q using the following the following equation.
In Figure 5 the plot of Bt versus t of Cr(III) is linear from 0 to 15 min with a regression co-efficient of 0.97 and Cr(VI) 0 to 75 min with regression coefficient 0.98. Figure 5 shows that the regression lines of the plot of Cr(III) and Cr(VI) are not passing through origin, it means that the thin metal film is formed on the surface of sorbent [57] .
5. Sorption Isotherm Study
Isotherm study deals sorbent surface properties, affinities for ions at fixed temperature and pressure. Experimental data were applied to following sorption isotherm models.
5.1. Langmuir Adsorption Model

where qe is amount of metal ions sorbed on the surface of sorbent (mg/g), Ce concentration of metal ions in the liquid phase at equilibrium (mg/L), b monolayer sorption capacity of sorbent and KL Langmuir constant is related to binding sorption energy. Regression coefficient values of both Cr(III) and Cr(VI) mentioned in Table 4 indicate that observed experimental data of both species of chromium follow the Langmuir isotherm. Results show that sorbent surface possesses energetically equivalent active sites. KL values for Cr(III) and Cr(VI)
Table 4. Regression parameters for the Langmuir and Fruendlich adsorption isotherms by using okra leaves as sorbent at different temperatures.
increase with the increase of temperature, this supports the endothermic type of reaction.

The separation factor (RL) is used to determine the favorability and the tendency of the isotherm calculated by the equation (Equation (9)). The range of RL values (Table 4) for Cr(III) is 0.024 - 0.91 and Cr(VI) 0.125 - 0.98 and indicates that sorption of chromium species on okra leaves is favorable [9] .
5.2. Freundlich Adsorption Isotherm Model
For the sorption equilibrium, like other most widely used equations Freundlich equation is also used for the adsorption of a variety of organic and inorganic compounds on the surface various adsorbents (natural and synthetic). The experimental data were applied to the following linear form of the equation [58] .

where qe is amount of metal ions sorbed per unit mass of sorbent, Ce the amount of metal ions in the liquid phase at equilibrium, KF (Freundlich Constant) relative sorption capacity and n is sorption intensity. 1/n < 1 suggest the presence of convex isotherm while 1/n >1 concave isotherm [59] . Logqe is plotted against logce. The fit of the experimental data indicates the formation of multilayer of the metal ions on the heterogeneous surface (non- equivalent binding sites) of the sorbent. The values of KF and n are determined from intercept and slope respectively. Here the calculated values of nf (Table 4) are less than 1 (1/n > 1) supports the concave isotherm [59] . In such type of isotherm, marginal sorption energy increases with the increase of metal ions sorption. Similar values of nf were also observed in sorption of Cr(VI) on fresh and spent Spirulina platensis, Chromium(VI) on Hevea brasilinesis saw dust activated carbon, Fe(II) and As(V) on waste tea fungal biomass and Pb(II), Cr(III) and Cu(II) on peanut husk [43] [60] - [62] . Such type of observation may be attributed to the complex nature of sorbent having multiple active sites for the sorption of metal ions.
6. Thermodynamic Study
The thermodynamic parameters such as Gibbs free energy, enthalpy change and entropy change are used to estimate the feasibility and the nature of the sorption process [50] . These parameters were calculated from the following equations (Equation (11) & Equation (12)) [41] .

where ∆G is Gibbs free energy, R the general gas constant (8.3143 J/mol∙K), T solution temperature (K) and b sorption capacity (from Langmuir Isotherm).

The values of enthalpy change (∆H) and entropy change (∆S) were calculated from the slope and intercept respectively from the plot lnb versus 1/T. Table 5 shows that Gibbs free energy (∆G) values of both Cr(III) and Cr(VI) are negative, means the sorption reaction of metal ions on okra leaves sorbent was spontaneous (i.e sorption forces were quite strong to overcome the potential barrier). Further, an increase values with the increase of temperature favored the sorption process. Positive values of entropy (∆S) for Cr(III) and Cr(VI) show that there was randomness of metal ions on the surface of sorbent during the process. Positive values of enthalpy change (∆H) for both species of chromium indicate that the sorption process was an endothermic. A similar trend of Gibbs free energy and entropy change was observed with heavy metal adsorption on oak sawdust and adsorption of Cr(III) and Cr(VI) on Agave Lechuguilla biomass [41] [63] .
7. Desorption Study
For the regeneration of sorbent and recovery of metal ions, different mineral acids and bases were employed. The efficiency of leaching agents was evaluated with different concentrations (0.1 - 1 M) and volumes (1 - 5 mL). It was observed that 5 mL of 1 M HCl recovered more than 96% of metal ions from the chromium sorbed okra leaves.
Table 5. Thermodynamic constants for the sorption of Cr(III) and Cr(VI) on the okra leaves at different temperatures.
Figure 6. FT-IR spectra of (a) plain okra leaves and (b) Cr(III)-loaded okra leaves.
Figure 7. FT-IR spectra of (a) plain okra leaves and (b) Cr(VI)-loaded okra leaves.
Figure 8. Effect of electrolytes on the removal of Cr(III) by using okra leaves.
Figure 9. Effect of electrolytes on the removal of Cr(VI) by using okra leaves.
Table 6. FT-IR absorption bands and assignments for okra leaves and chromium loaded okra leaves.
Table 7. Removal of chromium from industrial waste contaminated ground water samples.
8. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
FT-IR is a spectroscopic method used to collect the information about a possible interaction between the chromium ions and the functional groups present in the okra sorbent. The spectra of unloaded and chromium ions loaded okra leaves sorbent are shown in Figure 6 and Figure 7. Table 6 shows the vibration frequencies of peaks and their possible assignments. In case of Cr(III) (Figure 6), the shifting of frequency and change in intensity was occurred in the stretching bands at (3260, 2933 - 2839, 1613, 1233) cm−1 and bending vibrations at (1417 and 1311) cm−1. This indicated the involvement of O-H, C=N (oximes), C-O (carboxylic acid), O-H and -N=O (nitro) groups respectively in sorption process.
The peaks of unloaded and Cr(VI) loaded okra leaves sorbent are given in Figure 7. The change in vibrational frequencies was observed at 2921 - 2855 cm−1 (H-C-H symmetric and asymmetric stretching), 1621 cm−1 (-N-H bending) and 1233 - 1025 cm−1 (C-O stretching). A new peak appeared at 1405 cm−1 (C-H in plane bending) two peaks at 1527 cm−1 (N=O stretching) and 1388 cm−1 (N=O bending) disappeared in the spectra of Cr(VI) loaded sorbent demonstrate the engagement of the functional groups in the metal binding process.
9. Interference Study
The effect of some common electrolytes (ratios of 1:10 and 1:100) on the removal efficiency of okra leaves sorbent for Cr(III) and Cr(VI) was examined. According to histogram Figure 8, in the presence of KCl, the sorption of Cr(III) was increased up to 2%. Similarly, 6% increase in the sorption of Cr(VI) was observed with Na2C2O4 (Figure 9). This increase in sorption may be due the presence of anions.
10. Applications
The developed method was applied for sorption of chromium ions from ground water samples affected by fertilizer factory. Removal of around 92.15% was achieved from chromium spiked real samples (Table 7).
11. Conclusion
From the whole study and comparative capacities of various sorbents mentioned in Table 1, it is concluded that okra leaves sorbent for the removal of chromium ions from the industrial waste contaminated ground water can effectively be used. At pH 4.0 and pH 2.0, Cr(III) and Cr(VI) okra leaves sorbent showed maximum sorption capacities 221.17 mg/g and 81.94 mg/g respectively. Kinetically, pseudo second order rate equation supported the monolayer formation of metal ions on the surface of sorbent. While thermodynamic parameters Gibbs free energy and enthalpy change values indicated that the sorption reaction was spontaneous and exothermic in nature respectively. By employing the developed method, maximum 92.15% of chromium metal ions successfully were removed from the real water samples (affected by industrial waste water).
Cite this paper
Mazhar Iqbal Khaskheli,Saima Qayyum Memon,Zaheer Ahmed Chandio,Wahid Bux Jatoi,Mohammad Tariq Mahar,Faiz Mohammad Khokhar, (2016) Okra Leaves—Agricultural Waste for the Removal of Cr(III) and Cr(VI) from Contaminated Water. American Journal of Analytical Chemistry,07,395-409. doi: 10.4236/ajac.2016.74037
References
- 1. Miretzky, P. and Fernandezn, A.C. (2010) Cr(VI) and Cr(III) Removal from Aqueous Solution by Raw and Modified Lignocellulosic Materials: A Review. Journal of Hazardous Materials, 180, 1-19.
http://dx.doi.org/10.1016/j.jhazmat.2010.04.060 - 2. Bansal, M., Grag, U., Singh, D. and Grag, V.K. (2009) Removal of Cr(VI) from Aqueous Solutions Using Pre-Consumer Processing Agricultural Waste: A Case Study of Rice Husk. Journal of Hazardous Materials, 162, 312-320.
http://dx.doi.org/10.1016/j.jhazmat.2008.05.037 - 3. Dubey, S.P. and Gopal, K. (2007) Adsorption of Chromium(VI) on Low Cost Adsorbent Derived from Agricultural Waste Material: A Comparative Study. Journal of Hazardous Materials, 145, 465-470.
http://dx.doi.org/10.1016/j.jhazmat.2006.11.041 - 4. Jain, M., Garg, S.P. and Kadirvelu, K. (2009) Chromium(VI) Removal from Aqueous System Using Helianthus annuus (Sunflower) Stem Waste. Journal of Hazardous Materials, 162, 365-372.
http://dx.doi.org/10.1016/j.jhazmat.2008.05.048 - 5. Kobya, M. (2004) Removal of Cr(VI) from Aqueous Solutions by Adsorption onto Hazelnut Shell Activated Carbon: Kinetic and Equilibrium Studies. Biosource Technology, 91, 317-321.
http://dx.doi.org/10.1016/j.biortech.2003.07.001 - 6. Barrera, H., Urena-Nunez, F., Bilyeu, B. and Barrera-Diaz, C. (2006) Removal of Chromium and Toxic Ions Present in Mine Drainage by Ectodermis of Opuntia. Journal of Hazardous Materials, B136, 846-853.
http://dx.doi.org/10.1016/j.jhazmat.2006.01.021 - 7. Wright, J. (2003) Heavy Metals and Pollution of the Lithosphere. Taylor and Francis Group, Routledge, New York.
- 8. Chirwa, E.M. and Wang, Y.T. (2001) Simultaneous Chromium(VI) Reduction and Phenol Degradation in a Fixed-Film Coculture and Bioreactor: Reactor Performance. Water Research, 35, 1921-1932.
http://dx.doi.org/10.1016/S0043-1354(00)00472-3 - 9. Nameni, M., Alavi Moghadam, M.R. and Arami, M. (2008) Adsorption of Hexavalent Chromium from Aqueous Solutions by Wheat Bran. International Journal of Environmental Science and Technology, 5, 161-168.
http://dx.doi.org/10.1007/BF03326009 - 10. Kortenkamp, A., Casdevall, M., Faux, S.P., Jenner, A., Shayer, R.O.J., Woodbridge, N. and Brien, P.O. (1996) A Role for Molecular Oxygen in the Formation of DNA Damage during the Reduction of the Carcinogen Chromium(VI) by Glutathione. Archives of Biochemistry and Biophysics, 329, 199-208.
http://dx.doi.org/10.1006/abbi.1996.0209 - 11. Chand, R., Narimura, K., Kawakita, H. and Ohto, K. (2009) Grape Waste as a Biosorbent for Removing Cr(VI) from Aqueous Solution. Journal of Hazardous Materials, 163, 245-250.
http://dx.doi.org/10.1016/j.jhazmat.2008.06.084 - 12. Gupta, V.K. and Rastogi, A. (209) Biosorption of Hexavalent Chromium by Raw and Acid-Treated Green Alga Oedogonium hatei from Aqueous Solutions. Journal of Hazardous Materials, 163, 396-402.
http://dx.doi.org/10.1016/j.jhazmat.2008.06.104 - 13. Kumar, J.A. and Mandal, B. (2009) Removal of Cr(VI) from Aqueous Solution Using Bael Fruit (Aeglemarmelos correa) Shell as an Adsorbent. Journal of Hazardous Materials, 168, 633-640.
http://dx.doi.org/10.1016/j.jhazmat.2009.02.136 - 14. Dil, A.S., Qazi, I.A., Baig, M.A., Khan, E.A. and Tahir, A. (2008) National Standards for Drinking Water quality (NSDWQ). Pakistan Environment Protection Agency, Ministry of Environment, Government of Pakistan, Mingora.
- 15. Kurniawan, T.A., Chan, G.Y.S., Lo, W.-H. and Babel, S. (2006) Comparison of Low Cost Adsorbents for Treating Waste Waters Laden with Heavy Metals. Science of the Total Environment, 366, 409-426.
http://dx.doi.org/10.1016/j.scitotenv.2005.10.001 - 16. Kotas, J. and Stasicka, Z. (2000) Chromium Occurrence in the Environment and Methods of Its Speciation. Environmental Pollution, 107, 263-283.
http://dx.doi.org/10.1016/S0269-7491(99)00168-2 - 17. Memon, S.Q., Bhangar, M.I. and Khuhawar, M. Y. (2005) Preconcentration and Separation of Cr(III) and Cr(VI) Using Sawdust as a Sorbent. Analytical and Bioanalytical Chemistry, 383, 619-624.
http://dx.doi.org/10.1007/s00216-005-3391-1 - 18. Abia, A.A. and Asquo, E.D. (2007) Kinetics of Cd2+ and Cr3+ Sorption from Aqueous Solutions Using Mercaptoacetic Acid Modified and Unmodified Oil Palm Fruit Fibre (Elaeis guineensis) Adsorbents. Tsinghua Science and Technology, 12, 485-492.
http://dx.doi.org/10.1016/S1007-0214(07)70072-X - 19. Ramos, R.L., Azuara, A.J., Dias-Flores, P.E., Gurrero-Coronado, R.M., Mendoza-Barron, J. and Berber-Mendoza, M.S. (2008) Adsorption of Chromium(VI) from an Aqueous Solution on a Surfactant-Modified Zeolite. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 330, 35-41.
http://dx.doi.org/10.1016/j.colsurfa.2008.07.025 - 20. Sarin, V. and Pant, K.K. (2006) Removal of Chromium from Industrial Waste by Using Eucalyptus Bark. Bioresource Technology, 97, 15-20.
http://dx.doi.org/10.1016/j.biortech.2005.02.010 - 21. Wang, X.S., Li, Z.Z. and Tao, S.R. (2009) Removal of Chromium(VI) from Aqueous Solution Using Walnut Hull. Journal of Environmental Management, 90, 721-729.
http://dx.doi.org/10.1016/j.jenvman.2008.01.011 - 22. Park, S. and Jung, W.Y. (2001) Removal of Chromium by Activated Carbon Fibers Plated with Copper Metal. Carbon Science, 2, 15-21.
- 23. Klaassen, C.D. (2001) Casarett and Doull’s Toxicology: The Basic Science of Poisons. McGraw-Hill, New York.
- 24. Cieslak-Golonka, M. (1996) Toxic and Mutagenic Effects of Chromium(VI). Polyhedron, 15, 3667-3918.
http://dx.doi.org/10.1016/0277-5387(96)00141-6 - 25. Malkoc, E. and Nuhoglu, Y. (2006) Fixed Bed Studies for the Sorption of Chromium(VI) onto Tea Factory Waste. Chemical Engineering Science, 61, 4363-4372.
http://dx.doi.org/10.1016/j.ces.2006.02.005 - 26. Rengaraj, S., Joo, C.K., Kim, Y. and Yi, J. (2003) Kinetics of Removal of Chromium from Water and Electronic Process Waste Water by Ion Exchange Resins: 1200H, 1500H and IRN97H. Journal of Hazardous Materials, 102, 257-275.
http://dx.doi.org/10.1016/S0304-3894(03)00209-7 - 27. Travanti, G., Petruzzeli, D. and Passino, R. (1997) Pretreatment of Tannery Wastewater by an Ion Exchange Process for Cr(III) Removal and Recovery. Water Science and Technology, 36, 197-207.
http://dx.doi.org/10.1016/S0273-1223(97)00388-0 - 28. Linda, A., Maris, K., Maruta, J. and Artus, V. (2014) Biomass Sorbents for Metalloid Removal. Adsorption, 20, 275-286.
http://dx.doi.org/10.1007/s10450-013-9594-4 - 29. Juang, R.S. and Shiau, R.C. (2000) Metal Removal from Aqueous Solutions Using Chitosan-Enhanced Membrane Filtration. Journal of Membrane Science, 165, 159-167.
http://dx.doi.org/10.1016/S0376-7388(99)00235-5 - 30. Vaghetti, J.C.P., Lima, E.C., Royer, B., Brasil, J.L., da Cunha, B.M., Simon, N.M., Cardoso, N.F. and Norena, C.P.Z. (2008) Application of Brazilian-Pine Fruit Coat as a Biosorbent to Removal of Cr(VI)from Aqueous Solution-Kinetics and Equilibrium Study. Biochemical Engineering Journal, 42, 67-76.
http://dx.doi.org/10.1016/j.bej.2008.05.021 - 31. Yan, G. and Viraraghvan, T. (2001) Heavy Metal Removal in a Biosorption Column by Immobilized M. rouxii Biomass. Bioresource Technology, 78, 243-249.
http://dx.doi.org/10.1016/S0960-8524(01)00020-7 - 32. Bhatti, I., Qureshi, K., Kqzi, R.A. and Ansari, A.K. (2007) Preparation and Characterization of Chemically Activated Almond Shells by Optimization of Adsorption Parameters for Removal of Chromium(VI) from Aqueous Solutions. World Academy of Science Engineering and Technology, 34, 199-204.
- 33. Mane, P.C. and Bhosle, A.B. (2012) Bioremoval of some Metals by Living Algae Spirogyra sp. and Spirullina sp. from Aqueous Solution. International Journal of Environmental Research, 6, 571-576.
- 34. Jesus, M.A., Jose, A., Amaya, A. and Maria, S.L.G. (2014) Aqueous Mercury Adsorption in a Fixed Bed Column of Thiol Functionalized Mesoporous Silica. Adsorption, 20, 311-319.
http://dx.doi.org/10.1007/s10450-013-9586-4 - 35. Sharma, D.C. and Forster, C.F. (1994) A Preliminary Examination into the Adsorption of Hexavalent Chromium Using Low-Cost Adsorbents. Bioresource Technology, 47, 257-264.
http://dx.doi.org/10.1016/0960-8524(94)90189-9 - 36. Uysal, M. and Ar, I. (2007) Removal of Cr(VI) from Industrial Wastewaters by Adsorption Part1: Determination of Optimum Conditions. Journal of Hazardous Materials, 149, 482-491.
http://dx.doi.org/10.1016/j.jhazmat.2007.04.019 - 37. Elangovan, R., Philip, L. and Chandraraj, K. (2008) Biosorption of Hexavalent and Trivalent Chromium by Palm Flower (Borassus aethiopum). Chemical Engineering Journal, 141, 99-111.
http://dx.doi.org/10.1016/j.cej.2007.10.026 - 38. Basha, S. and Murthy, Z.V.P. (2007) Kinetic and Equilibrium Models for the Biosorption of Cr(VI) on Chemically Modified Seaweed, Cystoseira indica. Process Biochemistry, 42, 1521-1529.
http://dx.doi.org/10.1016/j.procbio.2007.08.004 - 39. Seki, H., Suzuki, A. and Maruyama, H. (2005) Biosorption of Chromium(VI) and Arsenic(V) onto Methylated Yeast Biomass. Journal of Colloid and Interface Science, 281, 261-266.
http://dx.doi.org/10.1016/j.jcis.2004.08.167 - 40. Khaskheli, M.I., Memon, S.Q., Siyal, A.N. and Khuhawar, M.Y. (2011) Use of Orange Peel Waste for Arsenic Remediation of Drinking Water. Waste Biomass Valorization, 2, 423-433.
http://dx.doi.org/10.1007/s12649-011-9081-7 - 41. Argun, M.E., Dursun, S., Ozdemir, C. and Karatas, M. (2007) Heavy Metal Adsorption by Modified Oak Sawdust: Thermodynamics and Kinetics. Journal of Hazardous Materials, 141, 77-85.
http://dx.doi.org/10.1016/j.jhazmat.2006.06.095 - 42. Hasan, S.H., Singh, K.K., Prakash, O., Talat, M. and Ho, Y.S. (2008) Removal of Cr(VI) from Aqueous Solutions Using Agricultural Waste Maize Bran. Journal of Hazardous Materials, 152, 356-365.
http://dx.doi.org/10.1016/j.jhazmat.2007.07.006 - 43. Li, Q., Zhai, J., Zhang, W., Wang, M. and Zhou, J. (2007) Kinetic Studies of Adsorption of Pb(II), Cr(III) and Cu(III) from Aqueous Solution by Sawdust and Modified Peanut Husk. Journal of Hazardous Materials, 141, 163-167.
http://dx.doi.org/10.1016/j.jhazmat.2006.06.109 - 44. Cabatingan, L.K., Agapay, R.C., Rakhels, J.L.L., Ottens, M. and Vander Wielen, L.A.M. (2001) Potential for the Recovery of Chromate in Industrial Wastewater. Industrial and Engineering Chemistry Research, 40, 2302-2309.
http://dx.doi.org/10.1021/ie0008575 - 45. Nasernejad, B., EsslamZadeh, T., Bonakadar Pour, B., Esmaail Bygi, M. and Zamani, A. (2005) Comparasion of Biosorption Modeling by Heavy Metals Cr(III), Cu(II), Zn(II) Adsorption from Wastewater by Carrot Residues. Process Biochemistry, 40, 1319-1322.
http://dx.doi.org/10.1016/j.procbio.2004.06.010 - 46. Bulut, Y. and Tez, Z. (2007) Adsorption Studies on Ground Shells of Hazelnut and Almond. Journal of Hazardous Materials, 149, 35-41.
http://dx.doi.org/10.1016/j.jhazmat.2007.03.044 - 47. Hasar, H. (2003) Adsorption of Nickel(II) from Aqueous Solution onto Activated Carbon Prepared from Almond Husk. Journal of Hazardous Materials, 97, 49-57.
http://dx.doi.org/10.1016/S0304-3894(02)00237-6 - 48. Cimino, G., Passerini, A. and Toscano, G. (2000) Removal of Toxic Cations and Cr(VI) from Aqueous Solution by Hazelnut Shell. Water Research, 34, 2955-2962.
http://dx.doi.org/10.1016/S0043-1354(00)00048-8 - 49. Rojas, G., Silva, J., Flores, J.A., Rodriguez, A., Ly, M. and Maldonado, H. (2005) Adsorption of Chromium onto Cross-Linked Chitosan. Separation and Purification Technology, 44, 31-36.
http://dx.doi.org/10.1016/j.seppur.2004.11.013 - 50. Iftikhar, A.R., Bhatti, H.N., Hanif, M.A. and Nadeem, R. (2009) Kinetic and Thermodynamic Aspects of Cu(II) and Cr(III) Removal from Aqueous Solutions Using Rose Waste Biomass. Journal of Hazardous Materials, 161, 941-947.
http://dx.doi.org/10.1016/j.jhazmat.2008.04.040 - 51. Oliveira, E.A., Montanher, S.F., Andrade, A.D., Nobrega, J.A. and Rollemberg, M.C. (2005) Equilibrium Studies for the Sorption of Chromium and Nickel from Aqueous Solutions Using Raw Rice Bran. Process Biochemistry, 40, 3485-3490.
http://dx.doi.org/10.1016/j.procbio.2005.02.026 - 52. Sawalha, M.F., Peralta-Videa, J.R., Romero-González, J. and Gardea-Torresdey, J.L. (2006) Biosorption of Cd(II), Cr(III), and Cr(VI) by Saltbush (Atriplex canescens) Biomass: Thermodynamic and Isotherm Studies. Journal of Colloid and Interface Science, 300, 100-104.
http://dx.doi.org/10.1016/j.jcis.2006.03.029 - 53. Lalvani, S.B., Hubner, A. and Witowski, T.S. (2009) Chromium Adsorption by Lignin. Energy Sources, 22, 45-56.
- 54. Zakaria, Z.A., Suratmam, M., Mohammad, N. and Ahmad, W.A. (2009) Chromium(VI) Removal from Aqueous Solution by Untreated Rubber Wood Sawdust. Desalination, 244, 109-121.
http://dx.doi.org/10.1016/j.desal.2008.05.018 - 55. Sag, Y. and Abtay, Y. (2002) Kinetic Studies of Cr(VI) and Cu(II) Ions by Chitin, Chitosan and Rhizopus arrhius: Application of the Adsorption Models. Hydrometallurgy, 50, 297-314.
- 56. Chen, S., Yue, Q., Gao, B. and Xu, X. (2010) Equilibrium and Kinetic Adsorption Study of the Adsorptive Removal of Cr(VI) Using Modified Wheat Residue. Journal of Colloid and Interface Science, 349, 256-264.
http://dx.doi.org/10.1016/j.jcis.2010.05.057 - 57. Richenberg, D. (1953) Properties of Ion-Exchange Resins in Relation to Their Structure, III: Kinetics of Exchange. Journal of American Chemical Society, 75, 589-597.
http://dx.doi.org/10.1021/ja01099a022 - 58. Ekmekyapar, F., Aslan, A., Bayhon, Y.K. and Cakici, A. (2012) Biosorption of Pb(II) by Non-Living Lichen Biomass of Cladonia rangiformis Hoffm. International Journal of Environmental Research, 6, 417-424.
- 59. Febrianto, J., Kosasih, A.N., Sunarso, J., Ju, Y.H., Indraswati, N. and Ismadji, S. (2009) Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. Journal of Hazardous Materials, 162, 616-645.
http://dx.doi.org/10.1016/j.jhazmat.2008.06.042 - 60. Gokhale, S.V., Jyoti, K.K. and Lele, S.S. (2008) Kinetic and Equilibrium Modeling of Chromium (VI) Biosorption of Fresh and Spent Spirulina platensis/Chlorella vulgaris Biomass. Bioresource Technology, 99, 3600-3608.
http://dx.doi.org/10.1016/j.biortech.2007.07.039 - 61. Karthikeyan, T., Rajgopal, S. and Miranda, L.R. (2005) Chromium(VI) Adsorption from Aqueous Solution by Hevea Brasilinesis Sawdust Activated Carbon. Journal of Hazardous Materials, 124, 192-199.
http://dx.doi.org/10.1016/j.jhazmat.2005.05.003 - 62. Murugesan, G.S., Sathishkumar, M. and Swaminathan, K. (2006) Arsenic Removal from Ground Water by Pretreated Waste Tea Fungal Biomass. Bioresource Technology, 97, 483-487.
http://dx.doi.org/10.1016/j.biortech.2005.03.008 - 63. Romero-Gonzalez, J., Gardea-Torresdey, J.L., Peralta-Videa, J.R. and Rodriguez, E. (2005) Determination of Equilibrium and Kinetic Parameters of the Adsorption of Cr(III) and Cr(VI) from Aqueous Solutions to Agave lechuguilla Biomass. Bioinorganic Chemistry and Applications, 3, 55-68.
http://dx.doi.org/10.1155/BCA.2005.55












