American Journal of Analytical Chemistry
Vol.06 No.04(2015), Article ID:54693,8 pages
10.4236/ajac.2015.64031
Evaluation of Analytical Procedures in the Determination of Trace Metals in Heavy Crude Oils by Flame Atomic Absorption Spectrophotometry
Mahmood M. Barbooti
Applied Chemistry Department, School of Applied Sciences, University of Technology, Baghdad, Iraq
Email: brbt2m@gmail.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 January 2015; accepted 10 March 2015; published 17 March 2015
ABSTRACT
In the present work, four trace metals, V, Ni, Cu and Fe, have been determined in five crude oil samples of Eastern Baghdad area, Iraq by flame atomic absorption spectrophotometry. The crude oils are characterized with API gravity values in the range, 25 - 15. The V/Ni ratios for the studied crudes were in the range 3.7 to 5.4 and indicated a typical of the sapropelic-type organic matter. The Ni and V contents were correlated with API gravity where an increase of metal contents with the decrease in the API values could be observed. The plots of V and Ni versus sulfur indicated linear correlation. This is attributed to the increase of porphyrinic structures of the metals. The method of direct dilution (DD) with organic solvent and the standard addition (SA) were evaluated as compared with standard dry ashing-acid dissolution (DA). In most cases the DD method gave V and Ni concentrations that are higher than DA and for the heavier crude oil (API = 15) the DD results were significantly smaller than those of the DA method. The SA method can be a good solution to determine the trace metals in heavy crude oils provided that the addition must not exceed the levels of the metal concentration in the diluted samples.
Keywords:
Crude Oil Analysis, Atomic Absorption, Trace Metals, Analytical Procedures

1. Introduction
The determination of metals in crude oils and its products is important for exploration, production and refining purposes. The distribution of trace metals in crude oils may be utilized to clarify potential genetic relationships between oils obtained from reservoirs in specific geologic provinces [1] . Hitchon and Filby [2] used trace elements for the classification of crude oils from similar lithostratigraphic situations into families. The trace metal composition was often employed to distinguish crude oils of various origins in problems of marine pollution [3] . Environmental site assessment studies of locations contaminated with petroleum products are often supported by heavy metal analysis to estimate the extent of pollution [4] . The heavy metal contents of crude oils are often correlated with percentages of light, middle, heavy and residual fractions of crude oils [5] . For heavy crude oil upgrading, the high metals levels necessitate the processing to be costly, either through the high costs of hydrogen addition or high yield loss due to carbon rejection [6] . This process in turn requires the availability of reliable method for metal determination.
Trace metal contents of crude oils can be determined by various methods including pulse polarography [3] , neutron activation [1] [7] , proton induced X-ray emission [8] , high performance liquid chromatography, [9] and atomic absorption spectrophotometry, AAS [10] . Induced coupled plasma emission, ICP allows simultaneous determination of many elements but suffers from severe matrix interferences due to plasma interactions [11] . Moreover, the cost of the system is relatively high and the requirements may not be available in many laboratories. Many methods were introduced to overcome such problems encountered with the organic injection by improving the nebulization system [12] , using electrothermal vaporization [13] and improvement of sample treatment [14] .
The AAS is the most widely used method regarding simplicity and cost of the instrumentation requirements. Bettinelli and Tittarelli, [15] , carried out a comparison between flame atomic absorption with other instrumental techniques for the determination of metals in fuel oil and concluded that the precision, repeatability and reproducibility of the flame direct method showed improvement on comparison with the precision data given by the Institute of Petroleum (IP) 288 method. Several procedures were developed for the introduction of crude oil samples to the atomization system [10] [16] -[18] . Brandao, et al., [19] used palladium with triton ?x-100 as a modifier to enable the direct introduction of petroleum into electrothermal atomization, ETA, for the determination of copper, iron and vanadium and the limits of detection were 10, 200 and 800 pg, respectively.
Barbooti et al., [20] , used silica gel as a stabilizer for the used lubricating oils during the acid digestion and reference was made to inorganic standards solution to determine wear metals. Udoh et al., [21] used p-xylene- sulphonic acid ashing procedure for the mineralization of crude oil samples to be analyzed for Ca, Cu, Fe, Mg, Na, Ni and Zn. The method prevented the loss of material during ashing. Osuji and Onojake, [18] , employed dithionite-citrate carbonate for the pre-extraction of Ni, V, Cu, Cd, and Pb and analyzed them by AAS to overcome matrix interferences. Kowalewska, [22] found that the application of Pd as a chemical modifier (xylene solution of Pd(II) acetylacetonate) seemed to be crucial in the determination of vanadium in crude oils by ETA AAS when it was introduced to a furnace together with a sample. Dittert, et al., [23] , used a continuum source radiation for the simultaneous determination of Cr and Fe in crude oil with furnace atomization atomic absorption. The sample pretreatment was simple and involved homogenization in an ultrasonic bath. Sedykh, et al., [24] employed autoclave digestion with a mixture of nitric acid and hydrogen peroxide and the method was applicable over a wide range of V and Ni contents. More recently, Ortega et al., [25] tested microwave digestion based on maximum temperature and pressure programs (ranging from 220˚C and 40 bar to ~1400˚C and 80 bar) and compared them with a digestion method in the High Pressure Asher (HPA-S) autoclave. They found that increasing the temperature and pressure by 40˚ and 20 bar, improved the digestion and allowed an increase in the sample size and therefore the detection limits.
The purpose of the present work is to determine V, Ni, Cu and Fe in some newly explorated heavy crude oils of the Eastern Baghdad area and correlation of the results with other basic characteristics of crude oils like API gravity and sulfur contents. Also, the analytical procedures employed for samples preparation in flame AAS determination of metals in crude oils are evaluated.
2. Materials and Methods
2.1. Apparatus
The AAS measurements were carried out on a Pye-Unicam SP 9 atomic absorption spectrophotometer set at the conditions specified in Table 1. The sulfur contents were determined using a LAB-X 100 of radio-isotope excited X-ray fluorescence in accordance with the standard method IP-336 method [26] . The relative density, API, gravity, following the American Petroleum Institute, were determined by the hydrometer method (PM-B-4) according to the standard method IP 160 [27] . The sulfur contents and the API gravity values are listed in Table 2.
2.2. Materials
All chemicals were of analytical grade. The AAS standard solutions, 1000 µg∙mL−1, were purchased from Merck. The five crude oil samples were supplied by the Iraqi National Oil company to represent the Eastern Baghdad region and are studied for the first time for trace metals. The i-propanol was a Merck spectroscopic grade reagent. The white spirit was purchased from a local refinery.
2.3. Procedures
2.3.1. Dry Ashing-Acid Dissolution (DA Method)
Accurately about 1-3 g of the crude oil samples were weighed in porcelain crucibles and heated on a hot plate at ~130˚C for 4-5 h. Sulfuric acid was added (1 - 3 mLs and charring was performed at 180˚C. samples were then ashed in a muffle furnace at 550˚C for about 6 h. The ash was taken with 5 mL of HCl with some warming and the material was transferred into calibrated flasks and diluted to the mark with deionized water.
2.3.2. Direct Dilution (DD Method)
The direct dilution of crude oil samples was done using an organic solvent which is a mixture of 90% petroleum spirit and 10% iso-propanol [28] .
2.3.3. Standard Addition (SA Method)
Three of the crude oils were chosen, 1, 3 and 5 to show the effect of density (API gravity, 25, 21.5 and 15). The appropriate weight of oils were diluted by the organic solvent and used as stock solutions, six equal aliquots were placed in 10-mL calibrated flasks, and to five of them different additions of V and Ni standard solutions were applied to get five different concentrations of V and Ni. The six solutions were completed to the mark with the organic solvent mixture above.
3. Results and Discussion
The trace metal contents of the crude oil samples were determined following their DA in triplicates and shown
Table 1. Operating parameters for flame atomic absorption spectrophotometer.
Table 2. API gravity and sulfur contents of crude oil samples.
in Table 3. The trace metals can be determined with acceptable accuracy and precision by flame atomic absorption. However, the values of Ni concentration were the best in accuracy among the group of metals of interest. The average values of the results will be employed as reference values of the trace metal content in the coming discussion and using Ni and V as test elements.
The crude oil samples are characterized with a dominance of V over Ni with a V/Ni ratio of 3.7 to 5.4 which is indicative of typical of the sapropelic-type organic matter generating oil [24] . Such range of V/Ni ratio is typical of Arab crude oils [29] . The V and Ni contents were correlated with the API gravity values for the studied crude oils (Figure 1). Both metals are related with API gravity with a power equation and the correlation coefficients were 0.8845 and 0.9794, respectively.
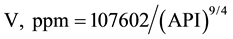 (1)
(1)
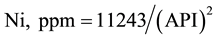 (2)
(2)
An increase of V and Ni content can be observed with the decrease of API value (Figure 1) which may be attributed to the increase of porphyrinic structures of the metals [29] . The decrease in vanadium contents reflects the maturation of the crude oil and not related to variations in source rocks [30] .

Figure 1. Correlation of API gravity with vanadium content (a) and nickel content (b) of crude oils.
Table 3. Trace metal contents of crude oils treated by DA method, µg∙mL−1.
Figure 2 shows plots of vanadium and nickel versus sulfur contents of the crudes. Both metals have linear correlation with sulfur with R2 values of 0.8154 and 0.913, respectively. The correlation with nicke was better than that of vanadium, as sulfide ions might tie-up Ni2+ ions but not the vanadyl ion, VO2+ ions) [31] .
The crude oil samples prepared by the DD method are nebulized directly into the AAS flames. The results are given in Table 4. Except for crude oil No. 5, which showed significantly smaller values than those of DA method (25% less for V and 30% less for Ni), the obtainable metal contents were somewhat higher than the DA results. The directly diluted samples were left for 48 h to allow for complete precipitation. The solutions were then nebulized without shaking in order to enable the soluble matter to find their way to the flame. The concentration of V and Ni were measured and the results were less than those previously obtained (Table 4). Thus, the absorption signals of the diluted samples were due partially to AA and due to some scattering of light, which exceeds the capability of the background corrector system employed. This was checked by switching off the deuterium lamp and measuring the AAS signals of Ni, in which they were almost double of those of the DD method with background correction. On the other hand, the crude oils under investigation were characterized by the existence of ~70% of V and ~50% of Ni in non-porphyrinic forms [32] . On this basis several atomization mechanisms will be involved in those samples, because the various compositions of the organo vanadium and nickel lead to various responses in AAS analysis [33] .
For the sake of studying the standard additions (SA) method, the absorbance values of the prepared solutions were plotted against concentration of the added standards with the untreated samples standing at zero concentration as shown in Figure 3 and Figure 4 for nickel and vanadium, respectively. The characteristics of the plots are shown in Table 4. The figures also show the calibration graphs of Ni and V. For vanadium, the slope of the SA curve was slightly higher than that of the calibration graph as regard to the high API gravity crude oil. This indicates that vanadium exists mostly in organic forms in these crude oils which make the homogenization with the calibration standard easier. Whereas, for the heaviest crude oil 5 (API 15), the SA curve showed negative deviation towards the concentration axis and lower slope as a result of less atomization efficiency. The SA curves were extrapolated and the concentration values of the solutions were estimated. The results were in reasonable agreement with those of the DA (Table 5). Meanwhile, they were smaller than those for the DD for the high API gravity crudes and higher for the heaviest crude. Thus, the heavy crude oil shows a strong matrix effect on the atomization of V compounds and resulted in low values of concentration. Also, the added V is absorbed

Figure 2. Correlation of sulfur content with vanadium content (a) and nickel content (b) of crude oils.
Table 4. Characteristics of calibration and standard additions curves for V and Ni in Heavy crude oils.


Figure 3. Standard addition of Nickel, (a) calibration graph; (b), (c) and (d) are Standard addition curves for crude oils 1, 3 and 5, respectively.
Table 5. Vanadium and nickel contents of the crude oils by different methods, µg∙mL−1.
by the heavy matrix and the atomization could be partially inhibited.
For Ni, there was a significant difference in the slopes of the calibration graph and the SA curves (Table 4). The three curves dissect the calibration graph at different concentration levels, 2.6, 2.4 and 2.0 µg∙mL−1 corresponding crude oils of API 25, 21.5 and 15 respectively. The heavier the crude oil, the less was the slope of the standard addition curve (see curve D in Figure 3). The atomization efficiency is lower for the heavy crude oils in comparison with the calibration standards. The heavier the crude oil the greater would be the suppression of the AAS signal. Thus, we have an evidence that vanadium organic fraction is more than nickel organic form in the heavy crude oils which resulted in such significant differences in metal atomization.
Standard addition was also recommended by Lienemann, et al., [34] , who used ultrasonic nebulization for the direct introduction of crude oil samples to the ICP-OES after simple dilution to overcome the petroleum matrix problems.
4. Conclusion
The problems encountered in the analysis of organic solutions of the crude oils by AAS are matrix problems caused by organic and inorganic nature of petroleum. Some authors recommended the use of metallo-organic


Figure 4. Standard addition of vanadium, (a) calibration graph; (b), (c) and (d) are Standard addition curves for crude oils 1, 3 and 5, respectively.
compounds for the preparation of standard solutions and others used asphaltine concentrate separated from heavy crude oil high in Ni and V contents. The standard addition method could be an acceptable solution to overcoming the strong matrix interference of heavy crude oils provided that the amount of the added standard is optimized not to exceed the levels of Ni and V in the diluted samples.
Acknowledgements
The author would like to thank E.B. Hassan and S.S. Baha-Udding for their help in the practical work.
References
- Elrich, J., Hilner, A. and Stark, H. (1985) Distribution of Trace Elements in Crude Oils from Southern Germany. Chemical Geology, 48, 313-323. http://dx.doi.org/10.1016/0009-2541(85)90056-7
- Hitchon, B. and Filby, R.H. (1984) Use of Trace Elements for Classification of Crude Oils into families; Example from Alberta, Canada. American Association of Petroleum Geologists (AAPG) Bulletin, 68, 838-849.
- Bakirova, S.F., Kotova, A.V., Yagyayeva, S., Fedorova, N. and Nadirov, N.K. (1984) Structural Features of Vanadyl Porphyrins of Petroleum of West Kazakhstan. Petroleum Chemistry U.S.S.R., 24, 196-202.
- Barbooti, M.M., Al-Taee, M.A. and Qasim, B.H. (2010) Electrothermal Atomic Absorption Spectrophotometric Determination of V, Ni and Pb in Hydrocarbon Polluted Soils. Engineer. Technol., 28, 17-28.
- Barbooti, M.M., Al-Madfai, S.H. and Al-Sammerrai, D.A. (1986) Thermogravimetric Characterization of Quayarah Heavy Crude Oils. Journal of Thermal Analysis, 31, 253-260. http://dx.doi.org/10.1007/BF01911056
- Reynolds, J.G. (2004) Removal of Nickel and Vanadium from Heavy Crude Oils by Exchange Reactions. Preprints of Papers-American Chemical Society, Division of Fuel Chemistry, 49, 79-80.
- Chifang, C., Zhuguo, D., Jiamo, F. and Guoying, S. (2005) Determination of Trace Elements in crude Oils and Organic Materials Extracted from Oil-Forming Source Rocks in China by INAA. Journal of Radioanalytical and Nuclear Chemistry, 151, 177-184. http://dx.doi.org/10.1007/BF02040144
- Fischbeck, H.J., Engel, M.H., Ruffel, A.V. and Weaver, B.L. (1987) Application of an External Beam PIXE Method for Determining the Distribution of Trace Metals in Degraded and Nondegraded Crude Oils. Nuclear Instruments and Methods in Physics Research, 24, 655-657. http://dx.doi.org/10.1016/S0168-583X(87)80218-5
- Tadayon, F., Massoumi, A. and Eslami, M. (1999) Determination of Vanadium, Nickel, and Iron in Crude Oil by High-Performance Liquid Chromatography. Journal of Chromatographic Science, 37, 371-4. http://dx.doi.org/10.1093/chrsci/37.10.371
- Sebor, G., Lang, I., Vavrecka, P., Sychra, V. and Weisser, O. (1975) The Determination of Metals in Petroleum Samples by Atomic Absorption Spectrometry, Part I., The determination of Vanadium. Analytica Chimica Acta, 78, 99-106. http://dx.doi.org/10.1016/S0003-2670(01)84756-5
- Botto, R.I. (1987) Matrix Interferences in the Analysis of Organic Solutions by Inductively Coupled Plasma-Atomic Emission Spectrometry. Spectrochimica Acta Part B, 42, 181-199. http://dx.doi.org/10.1016/0584-8547(87)80060-5
- Dreyfus, S., Pécheyran, C., Magnier, C., Prinzhofer, A., Lienemann, C.P. and Donard, O.F.X. (2005) Direct Trace and Ultra-Trace Metals Determination in Crude Oil and Fractions by Inductively Coupled Plasma Mass Spectrometry. Journal of ASTM International, 2, 1-8. http://dx.doi.org/10.1520/JAI12969
- Saint’Pierre, T., Dias, L.F., Pozebon, D., Aucelio, R.Q., Curtius, A.J. and Welz, B. (2002) Determination of Cu, Mn, Ni and Sn in Gasoline by Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry, and Emulsion Sample Introduction. Spectrochimica Acta Part B, 57, 1991-2001. http://dx.doi.org/10.1016/S0584-8547(02)00202-1
- De Souza, R.M., Da Silveira, C.L.P. and Aucelio, R.Q. (2004) Determination of Refractory Elements in Used Lubricating Oil by ICP-OES Employing Emulsified Sample Introduction and Calibration with Inorganic Standards. Analytical Sciences, 20, 351-355. http://dx.doi.org/10.2116/analsci.20.351
- Bettinelli, M. and Tittarelli, P. (1994) Evaluation and Validation of Instrumental Procedures for the Determination of Nickel and Vanadium in Fuel Oils. Journal of Analytical Atomic Spectrometry, 9, 805-812. http://dx.doi.org/10.1039/ja9940900805
- Barbooti, M.M. and Jasim, F. (1982) Electrothermal Atomic Absorption Spectrometric Determination of Vanadium. Talanta, 29, 107-111. http://dx.doi.org/10.1016/0039-9140(82)80029-5
- Fabec, J.L. and Rushak, M.L. (1985) Determination of Nickel, Vanadium, and Sulfur in Crudes and Heavy Crude Fractions by Inductively Coupled Argon Plasma/Atomic Emission Spectrometry and Flame Photometry. Analytical Chemistry, 57, 1853-1863. http://dx.doi.org/10.1021/ac00286a015
- Osuji, L.C. and Onojake, C.M. (2004) Trace Heavy Metals Associated with Crude Oil: A Case Study of Ebocha-8 Oil-Spill-Polluted Site in Niger Delta, Nigeria. Chemistry & Biodiversity, 1, 1708-1715. http://dx.doi.org/10.1002/cbdv.200490129
- Brandao, G.P., De Campos, R.C., De Castro, E.V.R. and De Jesus, H.C. (2007) Determination of Copper, Iron and Vanadium in Petroleum by Direct Sampling Electrothermal Atomic Absorption Spectrpmetry. Spectrochimica Acta Part B, 62, 962-969. http://dx.doi.org/10.1016/j.sab.2007.05.001
- Barbooti, M.M., Zaki, N.S., Baha-Uddin, S.S. and Hassan, E.B. (1990) Use of Silica Gel in the Preparation of Used Lubricating Oils for the Determination of Wear Metals by Flame Atomic Absorption Spectrophotometry. Analyst (London), 115, 1059-1061. http://dx.doi.org/10.1039/an9901501059
- Udoh, A.P., Thomas, S.A. and Ekanem, E.J. (1992) Application of p-Xylenesulphonic Acid as Ashing Reagent in the Determination of Trace Metals in Crude Oil. Talanta, 39, 1591-1595. http://dx.doi.org/10.1016/0039-9140(92)80189-K
- Kowalewska, Z. (2007) Effect of Chemical Modification on Behavior of Various Organic Vanadium Forms during Analysis by Electrothermal Atomic Absorption Spectrometry. Spectrochimica Acta Part B, 62, 273-282. http://dx.doi.org/10.1016/j.sab.2007.01.005
- Dittert, I.M., Silva, J.S.A., Araujo, R.G.O., Curtius, A.J., Welz, B. and Becker-Ros, H. (2009) Direct and Simultaneous Determination of Cr and Fe in Crude Oil Using High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry. Spectrochimica Acta Part B, 64, 537-543. http://dx.doi.org/10.1016/j.sab.2009.02.006
- Sedykh, E.M., Bannykh, L.N., Korobeinik, G.S. and Starshinova, N.P. (2011) Determination of Nickel and Vanadium in Crude Oils by Electrothermal Atomic Absorption Spectrometry and Inductively Coupled Plasma Atomic Emission Spectroscopy after Mineralization in an Autoclave. Inorganic Materials, 47, 1539-1543. http://dx.doi.org/10.1134/S0020168511140196
- Ortega, G.S., Pécheyran, C., Hudin, G., Marosits, E. and Donard, O.F.X. (2013) Different Approaches of Crude Oil Mineralisation for Trace Metal Analysis by ICPMS. Microchemical Journal, 106, 250-254. http://dx.doi.org/10.1016/j.microc.2012.07.012
- Institute of Petroleum (1989) Standard Methods of Analysis and Testing of Petroleum and Related Products. London Vol. 2, 336.
- Institute of Petroleum (1989) Standard Methods of Analysis and Testing of Petroleum and Related Products. London Vol. 1, 160.
- Zaki, N.S., Barbooti, M.M., Baha-Uddin, S.S. and Hassan, E.B. (1989) Determination of Trace Metals and Their Distribution in Heavy Crude Oil Distillates (350˚C+) by Atomic Absorption Spectrophotometry. Applied Spectroscopy, 43, 1257-1259. http://dx.doi.org/10.1366/0003702894203552
- Ali, M.F., Bukhari, A. and Saleem, M. (1983) Trace Metals in Crude Oils from Saudi Arabia. Industrial & Engineering Chemistry Product Research and Development, 22, 691-694. http://dx.doi.org/10.1021/i300012a034
- Must, R.F., Ruch, R.R. and Meents, W.F. (1973) Vanadium in Devonian, Silurian, and Ordovician Crude Oils of Illinois. Illinois State Geological Survey, Urbana, Circular 483.
- Carlson, R.M.K., Pena, M.M., Boduszynski, M.M., Rechsteiner, C.E., Shafizadeh, A.S.G. and Henshaw, P.C. (1998) Geochemical-Viscosity Correlations among Heavy Crude Oils of the San Joaquin Valley. California, Paper 1998.203, 7th UNITAR Heavy Crude and Tar Sands International Conference Proceedings.
- Barbooti, M.M., Said, E.Z., Hassan, E.B. and Abdul-Ridha, S.M. (1989) Separation and Spectrophotometric Investigations of the Distribution on Nickel and Vandium in Heavy Crude Oils. Fuel, 68, 84-87. http://dx.doi.org/10.1016/0016-2361(89)90016-1
- Šebor, G., Kubelka, V. and Weisser, O. (1979) Separation and Characterization of Vanadylporphyrins from Romashkino Petroleum. Collection of Czechoslovak Chemical Communications, 44, 551-557. http://dx.doi.org/10.1135/cccc19790551
- Lienemann, C.P., Dreyfus, S., Pécheyran, C. and Donard, O.F.X. (2007) Trace Metal Analysis in Petroleum Products: Sample Introduction Evaluation in ICP-OES and Comparison with ICP-MS Approach. Oil & Gas Science and Technology, 62, 69-77. http://dx.doi.org/10.2516/ogst:2007006








