American Journal of Analytical Chemistry
Vol.4 No.7A(2013), Article ID:34009,9 pages DOI:10.4236/ajac.2013.47A008
An Evaluation of Cotinine as an Index of Exposure to Tabacco Smoke in Children with Recurrent Respiratory Tract Infections Using HPLC Method
12nd Department of Pediatrics and Allergology, Polish Mother’s Memorial Hospital Research Institute, Lodz, Poland
2Institute of General and Ecological Chemistry, Lodz University of Technology, Lodz, Poland
Email: jkaluzna@p.lodz.pl
Copyright © 2013 Barbara Kamer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 15, 2013; revised June 15, 2013; accepted July 1, 2013
Keywords: HPLC; Cotinine; Urine; Passive Smoking; Respiratory Infections
ABSTRACT
The authors evaluated the frequency of exposure to tobacco smoke among children suffering from respiratory tract infections. The investigations comprised 141 children aged from 2 months to 6 years that were treated in the 2nd Department of Pediatric and Allergology of Polish Mother’s Memorial Hospital Research Institute in Łódź (Poland). 69 of them were exposed to tobacco smoke in their home environment. The remaining 72 children came from non-smoking families. 26 (37.7%) individuals among the passive smokers and 15 (20.83%) among the children from non-smoking families suffered from recurrent respiratory tract infections. Cotinine concentrations were evaluated in the group of 69 children using the HPLC-UV method. The determined average cotinine/creatinine index expressed as median was higher in passive smokers with recurrent respiratory infections than among passive smokers with non-recurrent respiratory infections. Moreover, it was stated that the exposure to cigarette smoke was more often among children of younger and less well educated parents as well as living in poor housing conditions. These studies clearly indicate that there is a need for extensive education on the harmful effects of passive smoking and the recurrence of infections.
1. Introduction
Recurrent respiratory tract infection among children, particularly young ones, is an important clinical problem. It is the most common cause of children’s visits to pediatricians in open medicine as well as their hospitalization [1-4]. Exposure to tobacco smoke, the so-called passive smoking is one of the factors predisposing to recurrent respiratory infections [2,4-6].
Tobacco smoke adversely affects human health, particularly in the case of young children, who are passive smokers in their home environment [7-11]. The intensity of exposure depends on the number of smokers, the cigarettes they smoke per day as well as the size of rooms and their ventilation [7,12,13]. According to studies by Florek and coworkers [14] 43% of men and 22% of women smoke in Poland. Other authors claim that approximately 4 million children in Poland are exposed to passive smoking. All over the world over a billion adults smoke tobacco and 700 million children are passive smokers [15-17]. The studies carried out among pregnant women showed that about a third of them smoke cigarettes [18-20]. It should be emphasized that nicotine has a negative influence on pregnancy and can cause miscarriages, premature births, fetal hypothrophy, congenital effects and other abnormalities [18,21-23]. The studies carried out in Łódź by Sabanty and Brózik [24] among boys attending primary schools showed that 75.1% of them were exposed to tobacco smoke in their home environment. The studies by Kardas-Sobantka et al. [25] also showed that a high percentage (65.1%) of kindergarten children were exposed to tobacco smoke. It should be emphasized that according to WHO, Poland belongs to the list of countries with high tobacco consumption [26].
Tobacco smoke contains over 4 thousand toxic substances. Among them nicotine, carbon monoxide, differentiated free radicals and polycyclic aromatic hydrocarbons are considered the most harmful to health [8,14,27]. Nicotine circulates to various body organs. The liver converts nicotine to several metabolites with the major metabolite being cotinine (70% - 80%). Approximately 10% - 15% of cotinine is excreted in the urine; the remainder is further converted to other metabolites such as trans-3’-hydroxycotinine, cotinine-N-glucuronide. While the half-life of nicotine averages only 2 - 3 h, the half-life of cotinine averages about 17 h, making cotinine a better biomarker than nicotine [28]. The level of cotinine in body fluids is dependent on age, sex, race, ethnic differences, time and duration of smoking or passive smoking [29]. Determination of nicotine and cotinine in biological fluids has become an important component of behavioral and medical studies [30-32]. Urine and saliva are more widely investigated since they can be obtained non-invasively.
Different analytical methods have been presented for the determination of cotinine in urine [30,31,33], blood serum [10], seminal fluid and saliva [33] and hairs [34]. The most reliable methods are gas chromatography with nitrogen-specific (NPD) [35] or mass spectrometric [36] detection. Application of high-performance liquid chromatography (HPLC) with ultraviolet detection is another very useful technique [37]. These methods distinguish by very lower detection limits and simpler sample preparation than gas chromatography methods [33]. In the literature there is information about the high correlations between the amounts of self reported environmental tobacco smoke (ETS) exposure and urinary cotinine levels in biological fluids [38,39].
Many authors stress the fact that exposure to tobacco smoke in children significantly increases the incidence of respiratory diseases [2,6,23,26,40]. It can also increase bronchial hyperreactivity and encourage the development of asthma and other allergic diseases [3,7,41-45]. Moreover, tobacco smoke increases the production of reactive forms of oxygen [7,46,47].
The aim of this work was the assessment of exposure to passive smoking among children with respiratory diseases according to the survey concerning children’s ages, parents’ ages and education, length of pregnancy, the infant’s weight, living conditions and number of cigarettes smoked in households and to relate and rationalize the survey results by an evaluation of urinary cotinine (expressed as the index of cotinine/creatinine) among passive smokers according to frequency of respiratory tract diseases.
2. Material and Methods
2.1. Characteristic of Patients
The object of current research was the children aged from 2 months to 6 years who were treated in 2nd Department of Pediatric and Allergology of Polish Mother’s Memorial Hospital Research Institute in Łódź in the years 2009-2010 due to respiratory tract infections. Each child was interviewed according to the survey which included data on the exposure to passive smoking and frequency of respiratory diseases in their medical history. Infants under 6 months who experience at least 2 infections and infants over 7 months of age who experience at least 3 incidents are considered to have a recurrent infection. Moreover, the data concerning the parents’ age, education, length of pregnancy and the infant’s weight were collected. Living conditions—density (number of persons per room) were also assessed. The information was obtained on the basis of an individual conversation with the child’s mother, father or guardian. The concentration of cotinine in urine expressed as the cotinine/creatinine ratio was quantified among children from smoking families, using high pressure liquid chromatography with ultraviolet detection (HPLC-UV). All procedures were carried out with the written consent of a parent. The study was approved by the Review Board of the Institute and performed in agreement with the Standards and Ethics in Biological Rhythm Research. An approval was given by Committee of Scientific Research Ethics of Polish Mother’s Memorial Hospital—Research Institute in Lodz, Poland.
2.2. Methods
2.2.1. Chemicals
Cotinine standard, creatinine standard and water for HPLC were purchased from Sigma-Aldrich (Warsaw, Poland), dichloromethane for HPLC, di-sodium hydrogen orthophosphate and sodium dihydrogen orthophosphate from POCH S.A. (Gliwice, Poland). Acetonitrile for HPLC was purchased from J.T. Baker (Deventer, Nederland) and ammonium hydrogen carbonate and sodium hydroxide from Chempur (Piekary Śląskie, Poland).
2.2.2. Measuring Apparatus
The LC system consisted of an Agilent Technologies (Palo Alto, USA) 1100 series HPLC system with degasser (model G1379A), iso pump (model G1310A), UVVIS detector (model G1314A) and injection valve (model G1375-87304). C18 column (4.6 mm × 150 mm, 2.6 μm particle size) (Kinetex, Phenomenex) was used for the separation of cotinine and C18 column (4.6 mm × 150 mm, 5 μm particle size) (Agilent Technology) for the separation of creatinine.
2.2.3. Determination of Cotinine
A simple HPLC method for the determination of levels of cotinine in the urine of children was used. Urine samples were collected in polypropylene tubes and analyzed directly or kept frozen at −20˚C until analysis. 5 ml of urine alkalized by 500 μl 1 M NaOH was added to a glass test tube, then 5 ml of dichloromethane was added. Liquid-liquid extraction supported by ultrasounds was carried out in an ultrasonic bath for 15 minutes. The organic phase was received and evaporated in a nitrogen stream. The dry residue was dissolved in 150 μl mixture of 95% 10 mmol NH4HCO3 and 5% CH3CN. The mobile phase was composed of 10 mmol NH4HCO3 (phase A) and CH3CN (phase B). The gradient elution was applied from 95% phase A and 5% phase B to 5% phase A and 95% phase B for 10 minutes, the composition of 5% phase A and 95% phase B was held for 2 minutes. The apparatus conditions were as follows: flow rate: 0.7 ml/min, wavelength: 254 nm, injection volume: 20 μl, run time: 12 minutes. The linear detector response was in the concentration range 100 - 1000 ng/ml, the correlation coefficient reached 0.997, and in the concentration range 1 - 100 μg/ml the correlation coefficient reached 0.998. Figure 1 shows a typical chromatogram of urine sample taken from a child exposed to tobacco smoke according to surveys.
2.2.4. Determination of Creatinine
The results are expressed as a ratio to the urinary creatinine concentration in μmol/mmol creatinine. Creatinine measurements in urine were carried out based on the HPLC-UV method described by Kuśmierek and co-authors [48].
2.2.5. Statistical Analysis
Data were statistically evaluated using statistical analysis package (StatSoft, Poland STATISTICA, version 9.0.).
The Shapiro-Wilk test was used to check the normal distribution of the results. The Mann-Whitney U test was used to determine differences in single variables. The level of statistical significance was defined as p < 0.05.
3. Results
According to the analysis of age there were most of all, 87, infants (61.7%), 31 children aged 3 (22%), only 23 were aged 2 (16.3%). The assessment of the frequency of exposure to the tobacco smoke activity revealed that among the examined children 69 were passive smokers (48.9%)—infants in the majority. However, the other children, 72 (51.1%) came from non-smoking families (Figure 2). It was also observed that in this group infants were in the majority.
On the basis of data involving the frequency of respiratory tract diseases it was stated that 41 children (29.1%) had recurrent infections. Amongst them, significantly more were (26 = 63.4%) exposed to tobacco smoke. The percentage of exposed children was also significantly higher in comparison with 43 exposed patients from the group of 100 children with non-recurrent respiratory infections (Figure 3).
In the next stage of our work, parents’ age and education were analyzed. They were divided into two groups: under and over 30 years of age for mothers and under and over 35 for fathers. These results were shown in Figure 4.
It was confirmed that among children exposed to tobacco smoke there were essentially more mothers under 30 and fathers under 35. Parents’ education was divided into: higher, secondary, vocational and primary. The majority of parents, 41.6% had secondary education,

Figure 1. Typical chromatogram of cotinine in the urine of the child exposed to tobacco smoke according to surveys, 1—peak of cotinine.

Figure 2. Percentage of children exposed to tobacco smoke in their home environment taking their age into account.
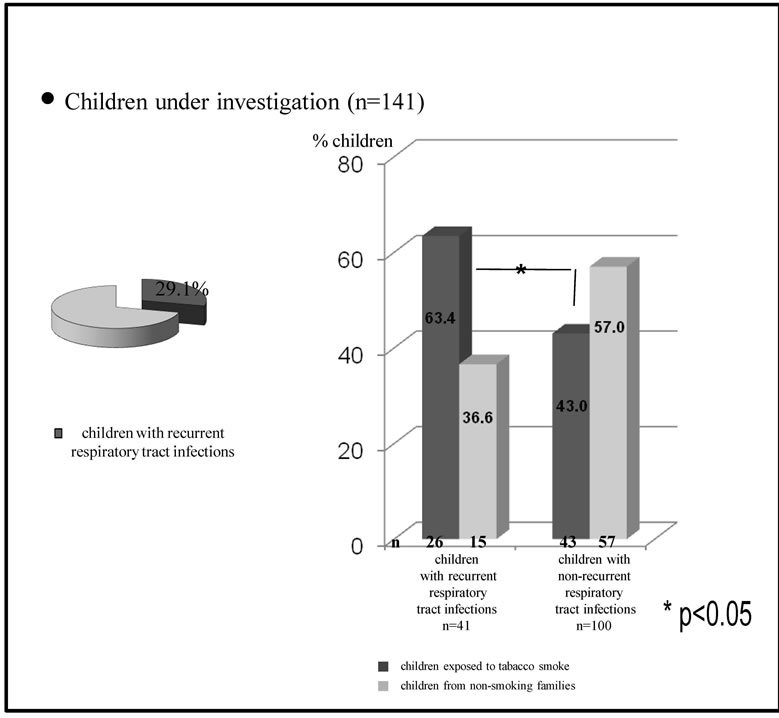
Figure 3. Frequency of occurrence of passive smokers among children with allergies in relation to recurrent and non-recurrent infections.
whereas 30.8% had higher education and only 11.0% completed primary education and 16.6% had vocational education. The detailed analysis of father’s and mother’s education among the two groups was shown in Figure 5. It was observed that significantly more parents with primary and vocational education were in the group of smoking mothers and fathers and those with higher education were mostly in the non-smoking group.
Next, fetal life expectancy and birth weight in children from the examined groups were analyzed, and those results are shown in Table 1. It was verified that among children exposed to tobacco smoke, essentially more
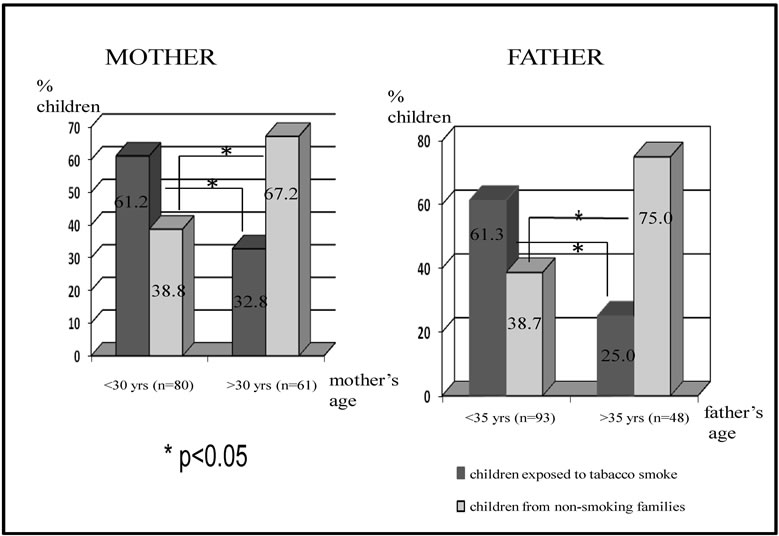
Figure 4. Analysis of age of parents of children under investigation.
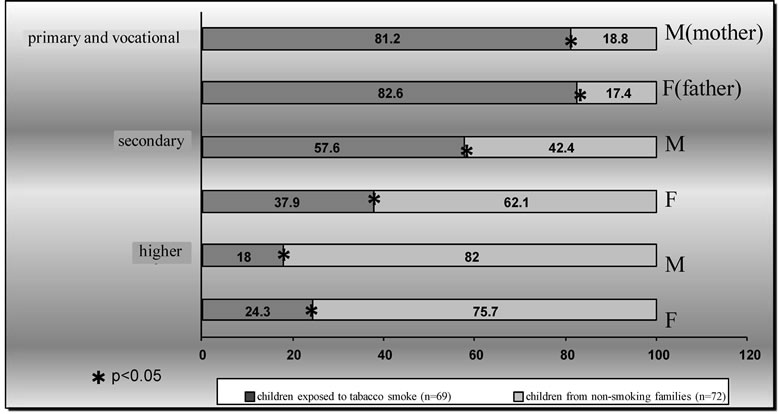
Figure 5. Analysis of parents’ education taking into account an exposure to tobacco smoke.
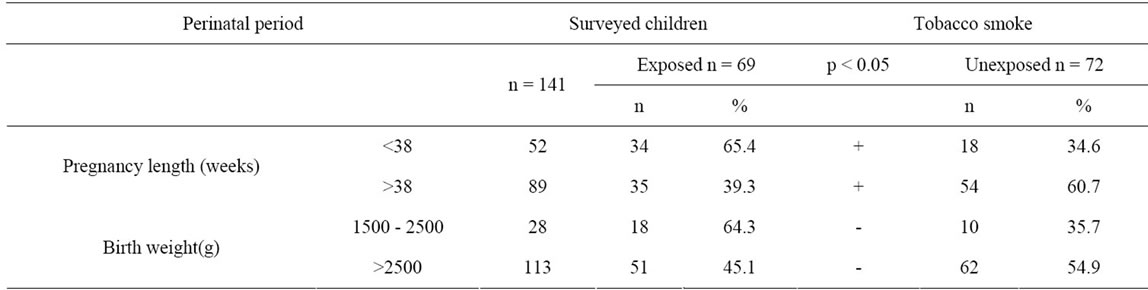
Table 1. Analysis of fetal length of life and birth weight in children from two groups.
individuals under study were born before 38 weeks of pregnancy. However, no differences in the birth weight were observed.
Among 69 children from smoking families the cotinine/creatinine index was quantified in urine using the HPLC method. 26 children suffered from recurrent infections whereas 43 children made up the group of nonrecurrent infections. Shapiro-Wilk’s tests of normality were carried out for 2 groups of passive smokers: children with recurrent infections and children with nonrecurrent infections. The obtained test probabilities (p < 0.05) are the basis for rejection of the null hypothesis about normal distribution. Mann-Whitney’s non-parametric test was carried out. Accounted test probabilities indicate the insignificance of the null hypothesis (below 0.05), and therefore it can be rejected and an alternative hypothesis can be accepted. Due to the diversified character of variables in groups, median should be applied to describe the statistics. Table 2 shows that the average cotinine/creatinine index expressed as median is significantly higher (over 2-fold) in passive smokers with recurrent respiratory infections than among passive smokers with non-current respiratory infections.
The group of 69 individuals exposed to tobacco smoke with determined cotinine/creatinine indexes in urine was divided into 2 subgroups depending on the number of cigarrettes smoked in households: 1) 41 patients whose parents smoke together fewer than 20 cigarrettes per day (average 13.34 ± 5.89); and 2) 28 patients whose parents smoke more than 20 cigarrettes (average 36.83 ± 12.58). Because the distribution of the variable cotinine/creatinine was not normal, for statistical description median was used. Median for the first group was 24.08 μmol/ mmol, and for the second group 19.37 μmol/mmol.
Among the 69 patients housing conditions were as follows: an average density index (person/room) according to surveys was: 2.19 ±1.03 for children with recurrent infections and 1.86 ± 1.08 for children with nonrecurrent infections. An average declared number of cigarettes smoked per day in households was: 23.33 ± 13.64 for parents of patients with recurrent infections and 22.93 ± 15.57 for parents of patients with non-recurrent infections.
4. Discussion
The current results showed that 48.9% of the children under investigation in the first six years of their life were exposed to cigarette smoke. Such a high percentage is in compliance with the results of other authors i.e. those of Kardas-Sobantka [25] for pre-school and Sabanty and Brózik [24] and Majkowska-Wojciechowska [49] for school age children.
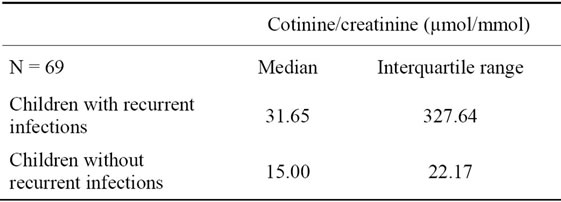
Table 2. Median values and interquartile ranges among two subgroups of patients exposed to tobacco smoke in the home environment according to surveys: children with recurrent infections and children without recurrent infections.
Tobacco smoke toxically affects different organs, which is emphasized by many authors [3,4,11,23,27,50]. Thus, it is a very substantial health threat inversely proportional to the age. It should also be emphasized that nicotine passes into breast milk. The investigations of Mennell et al. [51] proved the presence of cotinine in the samples of breast milk, taken just before suckling. It was stated that nicotine is a psycho stimulator which affects the development of infants’ brains, which in the future can cause long-term problems in learning and behavioral disorders. Moreover, troubles with sleeping, including a shortening of duration in 24 hours, were observed. The influence of nicotine on the respiratory system is especially disadvantageous because apart from the general effects like bronchial hyperreactivity, an intensification of immunoglobulin E production and an increased risk of bronchial asthma, nicotine topically affect the ciliary epithelium of respiratory tracts leading to its damage [2,6].
In the literature, authors accentuate that children exposed to passive smoking suffer more often from recurrent respiratory tract infections [13,23,45]. This is favored by the fact that passive smoking of young children influences their still immature immune system and may impair lymphocytes T cell function [10]. In turn, Marseglia et al. [52] indicated that passive smoking causes the increase in Interferon-gamma (IFN-γ).
Also, our results showed that among children with recurrent respiratory tract infection the number of children exposed to tobacco smoke was statistically significantly higher.
A lot of authors indicate the usefulness of cotinine determination for the evaluation of exposure to tobacco in children from smoking families [18,30,31,33] especially taking into consideration much longer, as compared with nicotine, half-life exposure (up to 87 hours in the case of young children) [53]. In the case of our children, urine was collected during the first two days of their stay in the Department.
Many authors emphasize that young parents as well as low educated persons smoke more [18,21,24,54]. Also, in our studies the frequency of exposure to tobacco smoke was higher among children of younger mothers (<30 years) and fathers (<35 years) and lower of educated parents. Also, living conditions are of importance because higher housing density may increase the exposure to tobacco smoke [21,54]. Our results, although statisticcally insignificant, also show such a tendency. However, the results of other authors i.e. Sabanty and Brózik [24] did not show the dependence between exposure to tobacco smoke and housing conditions.
Summarizing, the results of our studies showed that the exposure to tobacco smoke in home environment can lead to the increase in frequency of respiratory tract infections. The average cotinine/creatinine index expressed as median is significantly higher (over 2-fold) in children with recurrent respiratory tract infections. It was observed that the parents’ age and education and housing conditions influence the frequency of exposure to cigarette smoke. The studies confirmed that there is a need for extensive education on the harmful effects of passive smoking on recurrent infections.
REFERENCES
- H. Heydarnejad, H. R. Jamaati, S. F. Tafti, G. R. Heydari, S. Shariat-Panahi and M. Hosseini, “Comparison of Clinic Visits among Children of Smoking and Non-Smoking Families,” Tanaffos, Vol. 5, No. 4, 2006, pp. 47-52.
- P. Keskinoglu, D. Cimrin and G. Aksakoglu, “Relationships between Cotinine, Lower Respiratory Tract Infection, and Eosinophil Cationic Protein in Children,” European Journal of Pediatrics, Vol. 166, No. 5, 2007, pp. 455-459. doi:10.1007/s00431-006-0263-4
- I. Pirogowicz, M. Bujnowska-Fedak, M. Piorek and A. Steciwko, “Effect of Passive Cigarette Smoking on the Frequency of Respiratory Tract Infections, Allergy and Bronchial Asthma in Children,” Przegląd Lekarski, Vol. 61, No. 10, 2004, pp. 1061-1064.
- K. Tziomalos and F. Charsoulis, “Endocrine Effects of Tobacco Smoking,” Clinical Endocrinology, Vol. 61, No. 6, 2004, pp. 664-674. doi:10.1111/j.1365-2265.2004.02161.x
- S. Farchi, F. Forastiere, G. Cesaroni and C. A. Perucci, “Environmental Exposures and Hospitalisation for Respiratory Conditions in Children: A Five Year Follow up Study in Rome, Italy,” Occupational and Environmental Medicine, Vol. 63, No. 8, 2006, pp. 573-576. doi:10.1136/oem.2005.023556
- O. C. Ilicali, N. Keles, K. Dèger, O. F. Sàgun and Y. Guldiken, “Evaluation of the Effect of Passive Smoking on Otitis Media in Children by an Objective Method: Urinary Cotinine Analysis,” Laryngoskope, Vol. 111, No. 1, 2001, pp. 163-167. doi:10.1097/00005537-200101000-00028
- B. Kamer, R. Pasowska, K. Pyziak and A. Kamer-Bartosińska, “Evaluation of Exposure to Passive Smoking in Infants and Small Children with Food Allergy,” Środowiskowe Źródła Zagrożeń Zdrowotnych, Lublin, 2007.
- J. Kołodziejczyk, “The Effect of Passive Smoking on the Health of Nonsmokers,” Kosmos Seria A, Biologia, Vol. 51, No. 1, 2002, pp. 47-55.
- M. H. J. Sikorska-Jaroszyńska, M. Mielnik-Błaszczak, D. Krawczyk, A. Nasiłowska-Barud and J. Błaszczak, “Passive Smoking as an Environmental Health Risk Factor,” Annals of Agricultural and Environmental Medicine, Vol. 19, No. 3, 2012, pp. 547-550.
- C. I. Vardavas, M. N. Tzatzarkis, M. Plada, A. M. Tzatzarkis, A. Papadki, W. H. Saris, L. A. Moreno and A. G. Kafatos, “Biomarker Evaluation of Greek Adolescents’ Exposure to Secondhand Smoke,” Human & Experimental Toxicology, Vol. 29, No. 6, 2010, pp. 459-466. doi:10.1177/0960327109354941
- K. Yolton, K. Dietrich, P. Auinger, B. P. Lanphear and R. Hornung, “Exposure to Environmental Tobacco Smoke and Cognitive Abilities among U.S. Children and Adolescents,” Environmental Health Perspectives, Vol. 113, No. 1, 2005, pp. 98-103. doi:10.1289/ehp.7210
- J. M. Samet, E. M. Lewit and K. E. Warner, “Involuntary Smoking and Children’s Health,” Critical Health Issues for Children and Youth, Vol. 4, No. 3, 1994, pp. 94-114.
- K. Polańska and W. Hanke, “Postnatal Environmental Tobacco Smoke Exposure and Children’s Health”, Przegląd Pediatryczny, Vol. 35, No. 4, 2005, pp. 225-228.
- E. Florek, M. K. Kornacka, A. Hubert, J. Wilimowska and A. Basior, “The Level of Cotinine in Urine and Placenta of Women Smoking or Exposure to Tobacco Smoke during Pregnancy,” Przegląd Lekarski, Vol. 61, No. 10, 2004, pp. 1090-1093.
- S. Gidding, W. Morgan, C. Perry, J. Isabel-Jones and T. Bricker, “Active and Passive Tobacco Exposure: A Serious Pediatric Health Problem,” AHA Medical/Scientific Statement Special Report, Vol. 90, No. 5, 1994, pp. 2581- 2590.
- G. S. Metsios, A. D. Flouris, M. Angioi and Y. Koutedakis, “Passive Smoking and the Development of Cardiovascular Disease in Children: A Systematic Review,” Cardiology Research and Practice, Vol. 2011, 2011. doi:10.4061/2011/587650
- P. Tutka, M. Wielosz and W. Zatoński, “Exposure to Environmental Tobacco Smoke and Children Health,” International Journal of Occupational Medicine and Environmental Health, Vol. 15, No. 4, 2002, pp. 325-335.
- T. Jędrzejczyk, M. Zarzeczna-Baran and Ł. Balwicki, “Smoking among Pregnant Women in Gdansk—Social Background of the Phenomenon,” Problemy Higieny i Epidemiologii, Vol. 87, No. 4, 2006, pp. 265-269.
- Y. Lu, S. Tong and B. Oldenburg, “Determinants of Smoking and Cessation during and after Pregnancy,” Health Promotion International, Vol. 16, No. 4, 2001, pp. 355- 356. doi:10.1093/heapro/16.4.355
- K. Polańska, W. Hanke, W. Sobala and J. Jurewicz, “Exposure to Tobacco Smoke of Pregnant Women—Result of Prospective Study in Lodz Region,” Przegląd Lekarski, Vol. 64, No. 10, 2007, pp. 824-826.
- M. Baumert, “Maternal Smoking and Fetal and Neonatal Development,” Przegląd Pediatryczny, Vol. 34, No. 2, 2004, pp. 79-83.
- A. Castles, E. K. Adams, C. L. Melvin, C. Kelsh and M. L. Boulton, “Effects of Smoking during Pregnancy. Five Meta-Analyses,” American Journal of Preventive Medicine, Vol. 16, No. 3, 1999, pp. 208-215. doi:10.1016/S0749-3797(98)00089-0
- J. R. DiFranza, C. A. Aligne and M. Weitzman, “Prenatal and Postnatal Environmental Tobacco Smoke Exposure and Children’s Health,” Pediatrics, Vol. 113, 2004, pp. 1007-1015.
- W. Sabanty and H. Brózik, “Selected Parameters of Health Condition and the Concentration of Cotinine in Urine in Children from Primary Schools in Lodz Exposed to Tobacco Smoke in their Home Environment,” Przegląd Pediatryczny, Vol. 34, No. 2, 2004, pp. 52-59.
- D. Kardas-Sobantka, D. Chlebna-Sokół and A. Stańczyk, “Nałóg Palenia Tytoniu `w Środowisku Domowym Dzieci Przedszkolnych,” IV Francusko-Polskie Spotkanie Pediatryczne, Warszawa, 1995, p. 197.
- C. E. Bartecchini, T. MacKenzie and R. Schrier, “Zdrowotne Skutki Palenia Papierosów,” Świat Nauki, Vol. 47, No. 7, 1995, pp. 26-34.
- W. Zatoński, “Democracy and Health: Tobacco Control in Poland,” In: J. de Beyer and W. Brigden, Eds., Tobacco Control Policy, Strategies, Successes, and Setbacks, World Bank and TITC, Washington, 2003, pp. 97-120.
- H.-S. Shin, J.-G. Kim, Y.-J. Shin and S. H. Jee, “Sensitive and Simple Method for the Determination of Nicotine and Cotinine in Human Urine, Plasma and Saliva by Gas Chromatography-Mass Spectrometry,” Journal of Chromatography B, Vol. 769, No. 1, 2002, pp. 177-183. doi:10.1016/S1570-0232(02)00007-7
- J. W. Leong, N. D. Dore, K. Shelley, E. J. Holt, I. A. Laing, L. J. Palmer and P. N. LeSouef, “The Elimination Half-Life of Urinary Cotinine in Children of TobaccoSmoking Mothers,” Pulmonary Pharmacology & Therapeutics, Vol. 11, No. 4, 1998, pp. 287-290. doi:10.1006/pupt.1998.0153
- C. Barbier, N. Houdret, C. Vittrant, A. Deschildre and D. Turk, “Study of Passive Smoking Measured by Urinary Cotinine in Maternal and Child Protective Health Centers in North-Pas-de-Calais,” Archives de Pédiatrie, Vol. 7, No. 7, 2000, pp. 719-724. doi:10.1016/S0929-693X(00)80151-7
- R. Bono, M. Vincenti, T. Schiliro, D. Traversi, C. Pignata, E. Scursatone, G. Dotti and G. Gilli, “Cotinine and N-(2- hydroxyethyl)valine as Markers of Passive Exposure to Tobacco Smoke in Children,” Journal of Exposure Analysis and Environmental Epidemiology, Vol. 15, No. 1, 2005, pp. 66-73. doi:10.1038/sj.jea.7500344
- P. B. Doctor, V. N. Gokani, P. K. Kulkarni, J. R. Parikh and H. N. Saiyed, “Determination of Nicotine and Cotinine in Tobacco Harvesters’ Urine by Solid-Phase Extraction and Liquid Chromatography,” Journal of Chromatography B, Vol. 802, No. 2, 2004, pp. 323-328. doi:10.1016/j.jchromb.2003.12.013
- H. W. Kuo, J. S. Yang and M. C. Chiu, “Determination of Urinary and Salivary Cotinine Using Gas and Liquid Chromatography and Enzyme-Linked Immunosorbent Assay,” Journal of Chromatography B, Vol. 768, No. 2, 2002, pp. 297-303. doi:10.1016/S1570-0232(01)00613-4
- B. Koszowski, J. Czołga, M. Ł. Goniewicz, A. Sobczak, E. Kolasińska, L. Kośmider and T. Kuma, “Use of Hair Nicotine as a Tool to Assess Tobacco Smoke Exposure,” Przeglad Lekarski, Vol. 65, No. 10, 2008, pp. 696-699.
- N. L. Benowitz, “Cotinine as a Biomarker of Environmental Tobacco Smoke Exposure,” Epidemiologic Reviews, Vol. 18, No. 2, 1996, pp. 188-204. doi:10.1093/oxfordjournals.epirev.a017925
- R. Kronstrand, I. Nystrom, J. Strandberg and H. Druid, “Screening for Drugs of Abuse in Hair with Ion Spray LC-MS-MS,” Forensic Science International, Vol. 145, No. 2-3, 2004, pp. 183-190. doi:10.1016/j.forsciint.2004.04.034
- C. Oddoze, A. M. Pauli and J. Pastor, “Rapid and Sensitive High Performance Liquid Chromatographic Determination of Nicotine and Cotinine in Nonsmoker Human and Rat Urines,” Journal of Chromatography B, Vol. 708, No. 1-2, 1998, pp. 95-101. doi:10.1016/S0378-4347(97)00632-4
- E. Peterson, C. Johnson and D. Ownby, “Use of Urinary Cotinine and Questionnaires in the Evaluation of Infant Exposure to Tobacco Smoke in Epidemiologic Studies,” Journal of Clinical Epidemiology, Vol. 50, No. 8, 1997, pp. 917-923. doi:10.1016/S0895-4356(97)00095-4
- G. Scherer, I. Meger-Kossien, K. Riedel, T. Renner and M. Meger, “Assessment of the Exposure of Children to Environmental Tobacco Smoke (ETS) by Different Methods,” Human & Experimental Toxicology, Vol. 18, No. 4, 1999, pp. 297-301. doi:10.1191/096032799678840075
- M. Krzywiecka, A. Obuchowicz, C. Bukowska, T. Wielkoszynski, A. Świętochowska-Chechlińska and J. KulaGradzik “Evaluation of Infants and Younger Children Passive Smoking at Home and Prevalence of their Lower Respiratory Tract Infections,” Przegląd Lekarski, Vol. 63, No. 10, 2006, pp. 827-830.
- C. Bell, “Nicotine in Psychiatry: Psychopathology and Emerging Therapeutics,” JAMA, Vol. 284, No. 6, 2000, pp. 764-765. doi:10.1001/jama.284.6.764
- Y.-C. Chen, C.-H. Tsai and Y. L. Lee, “Early-Life Indoor Environmental Exposures Increase the Risk of Childhood Asthma,” International Journal of Hygiene and Environmental Health, Vol. 215, No. 1, 2011, pp. 19-25. doi:10.1016/j.ijheh.2011.07.004
- V. Ernster, R. Kaufman, M. Nichter, J. Samet and S. Y. Yoon, “Women and Tobacco: Movingfrom Policy to Action,” Bulletin of the World Health Organization, Vol. 78, No. 7, 2000, pp. 891-901.
- J. J. Lee, D. Liu, J. S. Lee, J. M. Kurie, F. R. Khuri, H. Ibarguen, R. C. Morice, G. Walsh, J. Y. Ro, A. Broxson, W. K. Hong and W. N. Hittelman, “Long-Term Impact of Smoking on Lung Epithelial Paroliferation in Current and Former Smokers,” Journal of the National Cancer Institute, Vol. 93, No. 14, 2001, pp. 1081-1088. doi:10.1093/jnci/93.14.1081
- M. Wróblewska and P. Kuna, “Tobacco Smoke and Diseases of the Respiratory System,” Terapia, Vol. 15, No. 4, 2007, pp. 90-96.
- H. Kimata, A. Yoshida, C. Ishioka, M. Fujimoto, I. Lindley and K. Furusho, “RANTES and Macrophage Inflammatory Protein 1 Selectively Enhance Immunoglobulin E (IgE) and IgG4 Production by Human B Cells,” The Journal of Experimental Medicine, Vol. 183, No. 5, 1996, pp. 2397-2402. doi:10.1084/jem.183.5.2397
- S. Popović-Grle, “Allergic Diseases and Smoking,” Acta Medica Croatica, Vol. 65, No. 2, 2011, pp. 141-146.
- K. Kuśmierek, R. Głowacki and E. Bald, “Analysis of Urine for Cysteine, Cysteinylglycine, and Homocysteine by High-Performance Liquid Chromatography,” Analytical and Bioanalytical Chemistry, Vol. 385, No. 5, 2006, pp. 855-860. doi:10.1007/s00216-006-0454-x
- B. Majkowska-Wojciechowska, B. Laskowska, Z. Wojciechowski and M. Kowalski, “Prevalence of Allergy in School Children in Lodz. Relation to Home and School Environment,” Annals of Allergy, Asthma & Immunology, Vol. 5, No. 2, 2000, pp. 115-122.
- M. D. Lebowitz, D. Sherill and C. J. Holberg, “Effects of Passive Smoking on Lung Growth in Children,” Pediatric Pulmonology, Vol. 12, No. 1, 1992, pp. 27-42. doi:10.1002/ppul.1950120109
- J. A. Mennella, L. M. Yourshaw and L. K. Morgan, “Breastfeeding and Smoking: Short-Term Effects on Infant Feeding and Sleep,” Pediatrics, Vol. 120, No. 3, 2007, pp. 497-502. doi:10.1542/peds.2007-0488
- G. L. Marseglia, M. A. Avanzini, S. Caimmi, A. Marseglia, C. Valsecchi, D. Poddighe, G. Ciprandi, F. Pagella, C. Klersy and A. M. Castellazzi, “Passive Exposure to Smoke Results in Defective Interferon-Gamma Production by Adenoids in Children with Recurrent Respiratory Infections,” Journal of Interferon & Cytokine Research, Vol. 29, No. 8, 2009, pp. 427-432. doi:10.1089/jir.2008.0108
- J. M. Knight, C. Eliopoulos, J. Klein, M. Greenwald and G. Koren, “Passive Smoking in Children. Racial Differerences in Systemic Exposure to Cotinine by Hair and Urine Analysis,” Chest, Vol. 109, No. 2, 1996, pp. 446- 450. doi:10.1378/chest.109.2.446
- D. M. Mannino, R. Caraballo, N. Benowitz, J. Repace, “Predictors of Cotinine Levels in US Children: Data from the Third National Health and Nutrition Examination Survey,” Chest, Vol. 120, No. 3, 2001, pp. 718-724. doi:10.1378/chest.120.3.718

