International Journal of Clinical Medicine
Vol.5 No.8(2014), Article ID:45200,7 pages DOI:10.4236/ijcm.2014.58066
A Real World Experience of Guidewire-Induced Perforations during Percutaneous Intervention and Their Successful Management
Sridhar Kasturi1, Vinothkumar Vilvanathan1, Srinivas Polasa1, Shiva Kumar Bandimida1, Kripa Patel2, Ashok Thakkar3
1Sunshine Heart Institute, Hyderabad, India
2Department of Pharmacology, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology Campus, Changa, India
3Department of Clinical Trials, Sahajanand Medical Tech. Pvt. Ltd., Surat, India
Email: sridharkasturi@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 18 March 2014; revised 3 April 2014; accepted 20 April 2014
Abstract
Complication rates following percutaneous interventions have decreased over the past decade due to advancement in both interventional equipment and procedure technique. Despite these advances, the risk of iatrogenic perforations still exists with associated high morbidity and mortality. We are presenting three cases of guidewire-induced perforations including coronary artery and renal artery perforation with their complications and successful management in these case-series.
Keywords
Percutaneous Intervention, Guidewire, Coronary Artery Perforation, Renal Artery Perforation

1. Introduction
In this age of advancing technology and expertise, catheter-based diagnostic and interventional procedures are now being performed with increasing frequency. This has inadvertently led to an increase in the incidence of catheter-related complications. One such potentially critical complication is guidewire-induced perforations. There are several factors which influence the selection of treatment strategies. The patient can be managed conservatively, deploying covered stents or through surgical intervention.
Although these are rare events following percutaneous intervention, the fact that these can lead to near-fatal iatrogenic complications makes their timely diagnosis and subsequent successful management vital. We report three cases of guidewire-induced perforations, including two coronary artery perforations and one renal artery perforation and discuss the management of these cases. All these cases are unusual in their presentation (clinically apparent after the interventional procedure). The follow-up events and their management would provide an important guidance for any such unforeseen complications.
2. Case-Series
Case-Report 1
A 67 year old female underwent percutaneous coronary intervention (PCI) as the coronary angiogram (CAG) done in the past revealed single vessel disease (Figure 1(A)) with ostio-proximal tight lesion in the first diagonal branch (D1). However, left anterior descending artery (LAD) left main coronary artery (LMCA) and left circumflexes (LCX) as well as dominant right coronary artery (RCA) were normal. She was a known case of non-insulin dependent diabetes mellitus (NIDDM) presented with non-ST-segment elevation myocardial infarction (NSTEMI).
During the PCI procedure LMCA was engaged with 5-Fr JL 3.5 Cordis™ guiding catheter (Cordis Corp., Johnson & Johnson, New Jersey) through the right femoral approach and the lesion was crossed with 0.014" × 190 cm Shinobi Plus guidewire (Cordis Corp., Johnson & Johnson, New Jersey). It was pre-dilated with 1.5 mm × 10 mm balloon at 14 atmospheres for 15 sec and stented with 2.25 mm × 24 mm BioMatrix drug eluting coronary stent (Biosensors Interventional Technologies Pvt Ltd., Singapore) at 10 atmospheres for 15 sec. Intracoronary nitroglycerine 100 µg and 2 mg nicorandil were given. A final check angiogram showed good result with TIMI-III flow without any dissection/perforation (Figure 1(B)). The patient was shifted to intensive cardiac care unit (ICCU) for post-surgical observation with a blood pressure (BP) of 110/80 mm Hg; heart rate (HR) 85 beats/minute (bpm) without any chest pain or shortness of breath and was administered intravenous (IV) infusion of glycoprotein IIb/IIIa inhibitor (Eptifibatide). After approximately 6 hours, the patient developed sudden hypotension and tachycardia. On examination, there was no evidence of any retroperitoneal bleed or hematoma at the puncture site. Electrocardiogram (ECG) did not show any fresh ST-T changes. Patient was given IV fluids and inotropic support but systolic BP remained between 70 to 80 mm of Hg. To rule out other causes of the persistent hypotension, an echocardiography was done. The echocardiography revealed pericardial effusion with right atrium (RA), right ventricle (RV) collapse with inferior vena cava (IVC) plethora suggesting pericardial tamponade. The patient was immediately managed conservatively with an emergency pericardiocentesis followed by aspiration of 250 ml of hemorrhagic pericardial fluid using 6-Fr pigtail which resulted in markedly improved hemodynamic status with HR 80 bpm, BP 140/90 mm Hg. Pigtail drainage was continued for twelve hours. A repeat echocardiography revealed no re-collection of pericardial fluid. A check angiogram revealed a patent stent with no evidence of coronary perforation/dissection with TIMI-III flow (Figure 1(C)) and pigtail was subsequently removed from the pericardial space. Patient was discharged after her condition stabilized and was prescribed atorvastatin 40 mg, antiplatelet agents (aspirin + clopidogrel), ramipril 2.5 mg and oral antidiabeic agents.
Case-Report 2
A 51-year-old male, a known case of diabetes and hypertension, was admitted for classical effort angina and breathlessness on minimal exertion for the last 2 months and recent episodes of rest angina. He was an ex-smoker. ECG showed ST depression in antero-septal leads during angina. 2-D Echocardiography revealed no regional wall motion abnormalities and good left ventricular function. CAG showed normal LMCA, LAD—mid segment 80% - 90% two lesions, LCX—proximal 95%, obtuse marginal (OM) branch—90% lesion, RCA— proximal mild disease with mid total occlusion and retrograde filling of distal RCA (Figure 2(A) and Figure 2(B)) through left coronary collaterals.
Percutaneous transluminal coronary angioplasty (PTCA) of LAD and LCX was done through right radial route due to unwillingness of patient for coronary artery bypass grafting (CABG). LMCA was engaged with 6-Fr 3.0 X JL guiding catheter (Cordis Corporation, Miami, Florida) and intra-coronary eptifibatide was given. LAD lesion was crossed with 0.014" BMW guidewire (Abbott Vascular, USA), predilated with 2.0 mm × 10 mm Maverick balloon TM (Boston Scientific Corp., USA). The 2.75 mm × 32 mm PROMUS ElementTM stent
 (A)
(A)  (B)
(B)  (C)
(C)
Figure 1. (A) CAG-D1 long segment tight lesion proximally; (B) Check angiogram showed good result with TIMI-III flow any dissection/perforation; (C) Well patent diagonal stent with pig tail catheter in the pericardial sac.
 (A)
(A)  (B)
(B)  (C)
(C)  (D)
(D)
Figure 2. (A) RCA mid total occlusion; (B) Retrograde filling of RCA; (C) Mild staining & extravasation of distal RCA; (D) Perforated RCA with pig tail in the pericardial sac; (E) Total occlusion of RCA due to dissection with total sealing of perforation.
(Boston Scientific Corp., USA) was deployed at 10 atmospheres for 16 sec. Post stenting LAD angiogram showed TIMI-III flow. LCX and OM lesions were crossed with 0.014" BMW guidewire, pre-dilated with 1.5 mm × 15 mm Minitrek (Abbott Vascular, USA) and 2.0 mm × 10 mm Maverick balloons (Abbott Vascular, USA). The stenting of LCX to OM branch was done with 2.5 mm × 32 mm PROMUS ElementTM stents (Boston Scientific Corporation, USA) at 12 atmospheres and 14 atmospheres respectively. Post-stent dilatation was carried out with 2.5 mm × 15 mm Sapphire non-compliant balloon (Asia Cardiovascular Pvt. Ltd., Hong Kong) with TIMI-III antegrade-flow.
In view of diabetes with multi-vessel stenting and total occlusion of RCA, PCI of RCA was planned 2 days later to avoid contrast nephropathy. During PCI of RCA, the vessel was engaged through right femoral route using 6-Fr sheath and 6-Fr JR side hole guiding catheter. We used 0.014" Shinobi guidewire to cross mid RCA lesion. After crossing mid RCA lesion, the lesion was dilated with 1.2 mm × 15 mm Minitrek balloon which resulted in antegrade flow across mid to distal RCA. Check angiogram (Figure 2(C)) showed mild staining and extravasation of distal RCA due to guidewire-induced perforation, treated with prolonged balloon inflations to occlude mid RCA to reduce extravasation of distal RCA. Procedure was completed and the patient was shifted to ICCU with stable haemodynamic status. The patient developed hypotension and tachycardia along with profuse sweating and cold and clammy extremities after 1 hour of PCI. Echocardiography showed pericardial effusion and dilated IVC. Emergency pericardiocentesis was done using pigtail catheter through the sub costal approach and about 300 ml of haemorrhagic fluid was aspirated. Patient was shifted back to ICCU with markedly improved hemodynamic status, pigtail drainage was continued. Approximately after 4 hours, patient suddenly deteriorated with cardio respiratory arrest. Pigtail was replaced due to suspicion of pigtail blockage and 200 ml of haemorrhagic fluid was aspirated which resulted in improved haemodynamic status and recovery of consciousness. Check angiogram of RCA showed persistent extravasation (Figure 2(D)) and so coil closure of RCA was planned by using two 3 mm NESTERTM embolization coils (COOK, Bjaeverskov Denmark). RCA was engaged with right guiding catheter and 0.014" BMW guidewire was placed in RCA over which 2.6 Frmicrocatheter was introduced to mid RCA level over the exchange length stabilizer guidewire. Prior to coiling, check angiogram showed dissection of proximal to distal RCA which resulted in total occlusion of mid RCA after removal of micro catheter, hence coiling was avoided. Final check angiogram of RCA showed total occlusion of mid RCA with no extravasation of distal RCA (Figure 2(E)). The patient was shifted to ICCU in a stable status with markedly improved hemodynamic status and he was discharged 3 days later with total relief of cardiovascular symptoms.
Case-Report 3
A 18-year-old female, a known case of renovascular hypertension secondary to Takayasu arteritis, presented with signs and symptoms of acute renal failure with severe acidosis. She was in aneuric phase in spite of daily haemodialysis for the last 4 days. She also had asymmetric pulses of both upper limbs (right upper limb BP 210/110 mm Hg, left upper limb BP 170/100 mm Hg and no right radio-femoral delay). Abdominal ultrasound & color Doppler evaluation showed total occlusion of left subclavian, celiac axis, superior mesenteric artery as well as occlusion of both renal arteries. These findings were confirmed with computerized tomography (CT) angiogram (performed along with dialysis support) (Figure 3(A)-Figure 3(D)). Right kidney was normal in size (10.5 cm × 4.3 cm) whereas left kidney (5.5 cm × 2.6 cm) was non-functioning which was confirmed with Diethylene Triamine Penta-acaetic Acid (DTPA) scan.
Right renal artery stenting was planned through left femoral route due to hemodialysis catheters in the right groin. Due to total occlusion of the right renal artery just at the ostium with cranially looking origin of right renal artery resulted in lack of guide catheter support from the left femoral approach, could not cross the occluded right renal artery in spite of using various guidewires. Finally, the right renal artery was engaged through 6-Fr Judkins guiding catheter (Precision Extrusion, Inc., New Jersey) through the right brachial approach. Various approaches were attempted to cross the lesion with 0.014" BMW and pilot guidewires but the approaches failed and ultimately the lesion was crossed with 0.014" Shinobi guidewire, and serial dilations were carried out with 11.5 mm × 10 mm Europa CTO Balloon (Rantis, Switzerland), 2.0 mm × 15 mm Mini Treck Balloon (Abbott Vascular, US), 3.5 mm × 15 mm NC Treck Balloon (Abbott Vascular, US). While crossing with balloon support, the guidewire tip inadvertently migrated distally (due to the tight lesion) and it resulted in perforation of one of the peripheral branches of the right renal artery (Figure 4).
After balloon angioplasty, check angiogram showed well dilated proximal portion of right renal artery with good antegrade flow, irregular margins of the proximal right renal artery and extravasation of one of the peripheral branches of right renal artery resulting in loin pain. Stenting of right proximal portion of the right renal artery was done with 5.0 mm × 15 mm Express® balloon mounted stent (Boston scientific). In view of the persistent extravasation with loin pain, coil embolization of peripheral branches of the right renal artery was done with 3 straight coils 0.014" × 1 cm (Cock Company) using 0.21 microcatheters and 0.18 guidewire (Figure 4). Check angiogram showed well implanted stent with good antegrade flow without any extravasation.
The patient developed mild hematomas at brachial & left femoral catheter sites and abdominal ultrasound showed hypoechoic collection in the right renal subcapsular space measuring 10.6 cm × 3.6 cm with thick internal echos. A hypoechoic collection was seen in the posterior para-renal space, extending along the psoas muscle.
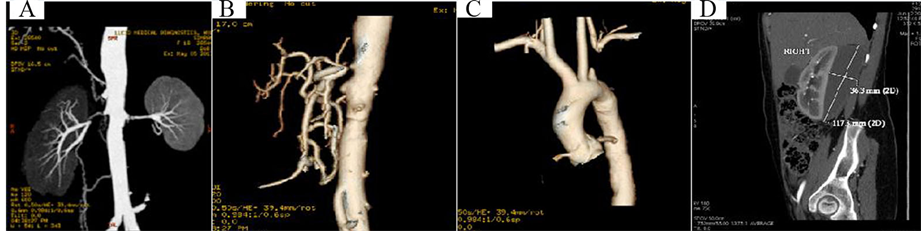
Figure 3. (A) Bilateral renal artery total occlusion; (B) Celiac/mesentric artery; (C) Subclavian artery; (D) Perinephrotic hematoma.
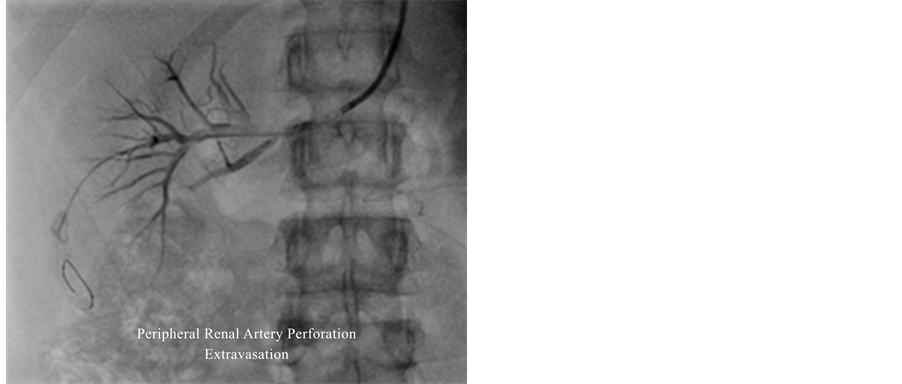
Figure 4. Guidewire induced perforation of peripheral branch of renal artery showing extravasation.
Hemoglobin level dropped from initial 9.1 gm% to 5.7 gm% and 2 units of blood transfusion was given. After 36 hours of the procedure, the patient had improved urine output with fall of serum creatinine from initial 7.7 gm% to 1.1 gm% without any dialysis support from second post-stent day onwards. Patient also had relief of abdominal pain, recovery of ileus, resumed oral fluids and was ambulated from the 4th day. The medications administered were immunosuppressant (azathioprine), steroid (methylprednisolone) and antihypertensive medications (metoprolol and amlodipine). Patient was discharged in a stable condition with total relief of symptoms after 6 days of the procedure.
3. Discussion
Guidewire-induced perforation is a known but rare and fatal aftermath of percutaneous interventional procedures. The reported incidence of angiographic evident coronary artery perforation is found to be 0.1% which may likely to increase from 0.5% to 3.0% while treating more complex lesion (chronic total occlusion, angulated calcified type B2 and type C lesion, eccentric lesion, small vessel size, long > 10 mm lesion) and with more aggressive interventional techniques [1] -[4] . Risk factors for perforation include diseases i.e. hypertension, diabetes and chronic renal failure as they tend to increase calcification. The association between the use of glycoprotein IIb/IIIa and perforation is controversial. In this case-series, we have reported two cases of iatrogenic perforations of coronary artery and one case of renal artery along with their successful management.
As per Ellis et al. [5] classification, angiographically documented perforations are classified into 3 categories: Type-1 refers to an extraluminal crater without contrast extravasation and rarely developed tamponade or resulted in ischemia and they may be very difficult rather impossible to distinguish angiographically from localized dissections. Type 2 perforation indicates presence of pericardial or myocardial blush without contrast jetting, associated with delayed tamponade (up to 24 hours at least) and if there was a contrast jetting with frank perforation, it is defined as type 3 perforation. Type 3 perforations are associated with abrupt tamponade, may need for urgent bypass surgery with high mortality.
Perforation at the site is usually immediately apparent in most of the patients. However in many reported cases this complication had become clinically apparent even after 24 hour of the intervention [6] [7] . After the perforation has been diagnosed, the treatment strategy will vary depending on the clinical situation including the size of the perforation, the hemodynamic status of the patients as well as the extent of contrast extravasation, if any. The conservative management strategy of the perforation include rapid reversal of anticoagulation or antiplatelet therapy, balloon tamponade-prolonged inflation of an angioplasty balloon proximal to or at the level of the perforation to seal the site of perforation and if leakage persists the occlusion of the distal vessel by delivering thrombin, collagen, polyvinyl alcohol, gel foam, subcutaneous fat or even autologous blood using microcatheter at the target vessel site [5] [8] -[10] . An emergency pericardiocentesis performed if patient develop cardiac tamponade. Echocardiography guided pericardiocentesis has been shown to be safe and effective for the management of catheter related perforation and tamponade [11] . Further management depends upon control of bleeding and ranges from percutaneous covered stents/grafts to surgery.
In our first reported case, the patient became hemodynamically unstable due to pericardial effusion possibly because of perforation after six hour of the stent implantation in D1 branch. However, we did not see any extravasation either during the procedure or during follow-up check angiography. We suspected type-1 perforation in this patient. Cardiac tamponade can occur in about 11% - 46% patients experiencing coronary artery perforation post-PCI [12] . We aggressively treated the patient with an emergency pericardiocentesis. The patient became hemodynamically stable within twelve hours of drainage of hemorrhagic fluid and discharged in stable condition.
In second case also, the patient repeatedly developed pericardial effusion and finally cardiac arrest after 4 hours of the intervention even though the perforation (which was of type-2) in the RCA during procedure was successfully treated with prolonged balloon inflation. The patient was successfully managed by removing haemorrhagic fluid from pericardial space using pigtail but it should be noteworthy that pigtail drainage block can compromise the clinical status of the patient. Due to the persistent extravasation, coil closure of RCA was planned. However, prior to coiling check angiogram showed dissection of proximal to distal RCA which resulted in total occlusion of mid RCA and hence coiling was avoided. The patient discharged from the hospital without any complication.
Similarly, in third case, iatrogenic type-2 perforation occurred in the peripheral branches of the right renal artery while treating the patient for total occlusion just at the ostium of the artery. As patient experienced loin pain due to extravasation, coil embolization of peripheral branches of the right renal artery was done. However, the extravasation resulted in to hemorrhage in the right renal subcapsular space. Renal subcapsular hemorrhage may be a life-threating problem as seen in this case. It requires early detection and immediate intervention.
4. Conclusion
Despite the availability of innovative equipment and novel techniques, the risk of arterial perforation still exists when performing percutaneous interventions. These three cases demonstrate the possible complications including cardiac tamponade, pigtail drainage block, and persistent exravasation in spite of the management of the guidewire-induced perforations. Close observation of the patient is crucial for the management of these secondary potentially catastrophic complications.
References
- Mulvihill, N.T., Boccalatte, M., Sousa, P., Farah, B., Laborde, J.C., Fajadet, J. and Marco, J. (2003) Rapid Sealing of Coronary Perforations Using Polytetrafluoroethylene-Covered Stents. American Journal of Cardiology, 91, 343-346. http://dx.doi.org/10.1016/S0002-9149(02)03166-1
- Roguin, A. and Beyar, R. (2005) Coronary Perforation 2006—Watch for the Wire. Journal of Invasive Cardiology, 17, 606-608.
- Ajluni, S.C., Glazier, S., Blankenship, L., O’Neill, W.W. and Safian, R.D. (1994) Perforations after Percutaneous Coronary Interventions: Clinical, Angiographic, and Therapeutic Observations. Catheterization and Cardiovascular Diagnosis, 32, 206-212. http://dx.doi.org/10.1002/ccd.1810320303
- Holmes Jr., D.R., Reeder, G.S., Ghazzal, Z.M., Bresnahan, J.F., King 3rd, S.B., Leon, M.B. and Litvack, F. (1994) Coronary Perforation after Excimer Laser Coronary Angioplasty: The Excimer Laser Coronary Angioplasty Registry Experience. Journal of the American College of Cardiology, 23, 330-335. http://dx.doi.org/10.1016/0735-1097(94)90415-4
- Ellis, S.G., Ajluni, S., Arnold, A.Z., Popma, J.J., Bittl, J.A., Eigler, N.L., Cowley, M.J., Raymond, R.E., Safian, R.D. and Whitlow, P.L. (1994) Increased Coronary Perforation in the New Device Era. Incidence, Classification, Management, and Outcome. Circulation, 90, 2725-2730. http://dx.doi.org/10.1161/01.CIR.90.6.2725
- Vakili, H., Sadeghi, R., Biranvand, M.R., Namazi, M.H., Saadat, H., Safi, M. and Motamedi, M.R. (2009) Coronary Artery Perforation after Guidewire Removal. The Journal of Tehran University Heart Center, 4.
- Senguttuvan, N.B., Ramakrishnan, S., Gulati, G.S., Seth, S. and Bhargava, B. (2012) How Should I Treat Guidewire-Induced Distal Coronary Perforation? EuroIntervention, 8, 155-163. http://dx.doi.org/10.4244/EIJV8I1A23
- Al-Lamee, R., Ielasi, A., Latib, A., Godino, C., Ferraro, M., Mussardo, M., Arioli, F., Carlino, M., Montorfano, M., Chieffo, A. and Colombo, A. (2011) Incidence, Predictors, Management, Immediate and Long-Term Outcomes Following Grade III Coronary Perforation. JACC: Cardiovascular Interventions, 4, 87-95. http://dx.doi.org/10.1016/j.jcin.2010.08.026
- Yoo, B.S., Yoon, J., Lee, S.H., Kim, J.Y., Lee, H.H., Ko, J.Y., Lee, B.K., Hwang, S.O. and Choe, K.H. (2001) Guidewire-Induced Coronary Artery Perforation Treated with Transcatheter Injection of Polyvinyl Alcohol Form. Catheterization and Cardiovascular Interventions, 52, 231-234. http://dx.doi.org/10.1002/1522-726X(200102)52:2<231::AID-CCD1055>3.0.CO;2-Q
- Tanaka, S., Nishigaki, K., Ojio, S., Yasuda, S., Okubo, M., Yamaki, T., Kubota, T., Takasugi, N., Ishihara, Y., Kawasaki, M. and Minatoguchi, S. (2008) Transcatheter Embolization by Autologous Blood Clot Is Useful Management for Small Side Branch Perforation Due to Percutaneous Coronary Intervention Guide Wire. Journal of Cardiology, 52, 285-289. http://dx.doi.org/10.1016/j.jjcc.2008.04.003
- Almdahl, S.M., Veel, T., Halvorsen, P. and Rynning, S.E. (2013) Immediate Rescue Operations after Failed Diagnostic or Therapeutic Cardiac Catheterization Procedures. Interactive CardioVascular and Thoracic Surgery, 17, 314-317. http://dx.doi.org/10.1093/icvts/ivt214
- Gunning, M.G., Williams, I.L., Jewitt, D.E., Shah, A.M., Wainwright, R.J. and Thomas, M.R. (2002) Coronary Artery Perforation during Percutaneous Intervention: Incidence and Outcome. Heart, 88, 495-498. http://dx.doi.org/10.1136/heart.88.5.495

