Open Journal of Thoracic Surgery
Vol.2 No.3(2012), Article ID:23046,3 pages DOI:10.4236/ojts.2012.23017
Simplified Technique for Excision of Metastasizing Leiomyoma of Suprahepatic Inferior Vena Cava with Intracardiac Extension
![]()
1Department of Cardiovascular Surgery; 2Pathology Hospital Universitario de La Princesa, Universidad Autónoma de Madrid, Madrid, Spain.
Email: *anas_sarraj@hotmail.com
Received April 23rd, 2012; revised July 14th, 2012; accepted July 27th, 2012
Keywords: Cardiac-General; Extracorporeal Circulation; Minimal Invasive Surgery
ABSTRACT
Uterine leiomyoma may metastasize via intravascular to the right heart cavities several years after hysterectomy. The symptoms are usually produced by compression of the tissues beside. We describe a simplified technique for excision of a benign metastasizing leiomyoma of suprahepatic segment of inferior vena cava with intracardiac extension.
1. Introduction
Metastatic leiomyoma is a rare benign tumor affects women with history of uterine leiomyoma. The primary tumor generally extends or spreads via intravascular to the right heart cavities several years after hysterectomy [1]. The symptoms are usually produced by a mass effect and compression of the tissues where the metastasis settles [2,3].
2. Case Report
A 52-year-old woman with history of smoking and arterial hypertension underwent hysterectomy 15 years ago because of uterine leiomyoma. She was admitted in our hospital because of transient cerebral ischemic accident. The routine laboratory results were within normal limits. Electrocardiogram, Holter 24 hours, and Echo-Doppler of great vessels of aortic arch were normal. Cerebral computer tomographic (CT) scan showed infarction in the territory of the left postero-inferior cerebellar artery. Transthoracic echocardiogram showed a mobile mass of 53X10 mm arisen from the inferior vena cava (IVC) and extended to the right atrium (RA). Cardiac magnetic resonance image (MRI) revealed a pedunculated mass of 56 × 15 mm arisen from the suprahepatic segment of IVC 2 - 3 cm away from the RA. Thoracoabdominal CT scan did not reveal any another tumor. The early differential diagnoses were organized thrombus, metastatic leiomyoma, or fibroma.
After full neurological recovery, the patient was admitted again for surgery. A consensus was reached initially to excise the tumor without caval replacement. She was placed on normothermic cardiopulmonary bypass using a double venous cannulation of superior vena cava and right femoral vein. The RA was opened and a Foley catheter (18 French) was introduced into the IVC, inflated and connected to the pump suction to avoid venous return to the RA (Figure 1). The tumor was excised and sent for histopathogical study. The base of implantation was carefully scraped. The postoperative course was unremarkable and the patient was discharged one week after surgery.
The histopathogical study showed predominant stroma, spindle cells interlaced with vascular structures, and focal calcifications without evidence of mitosis, necrosis or signs of malignancy. Desmin, actin and Caldesmon were positive for metastasizing uterine leiomyoma (Figure 2). One year follow-up at out-patient clinic, she was asymptomatic in functional class I (NYHA). Thoraco-abdominal CT scan and transthoracic echocardiogram showed no evidence of recurrent metastatic disease.
3. Discussion
Metastatic leiomyoma is a rare benign tumor spreads from uterine leiomyomatosis via intravascular and usually affects lungs, brain, bone, lymph, heart, and skin. The tumor is incidentally discovered by echocardiography or thoraco-abdominal CT scan [2]. The symptoms are produced by a mass effect and compression of the
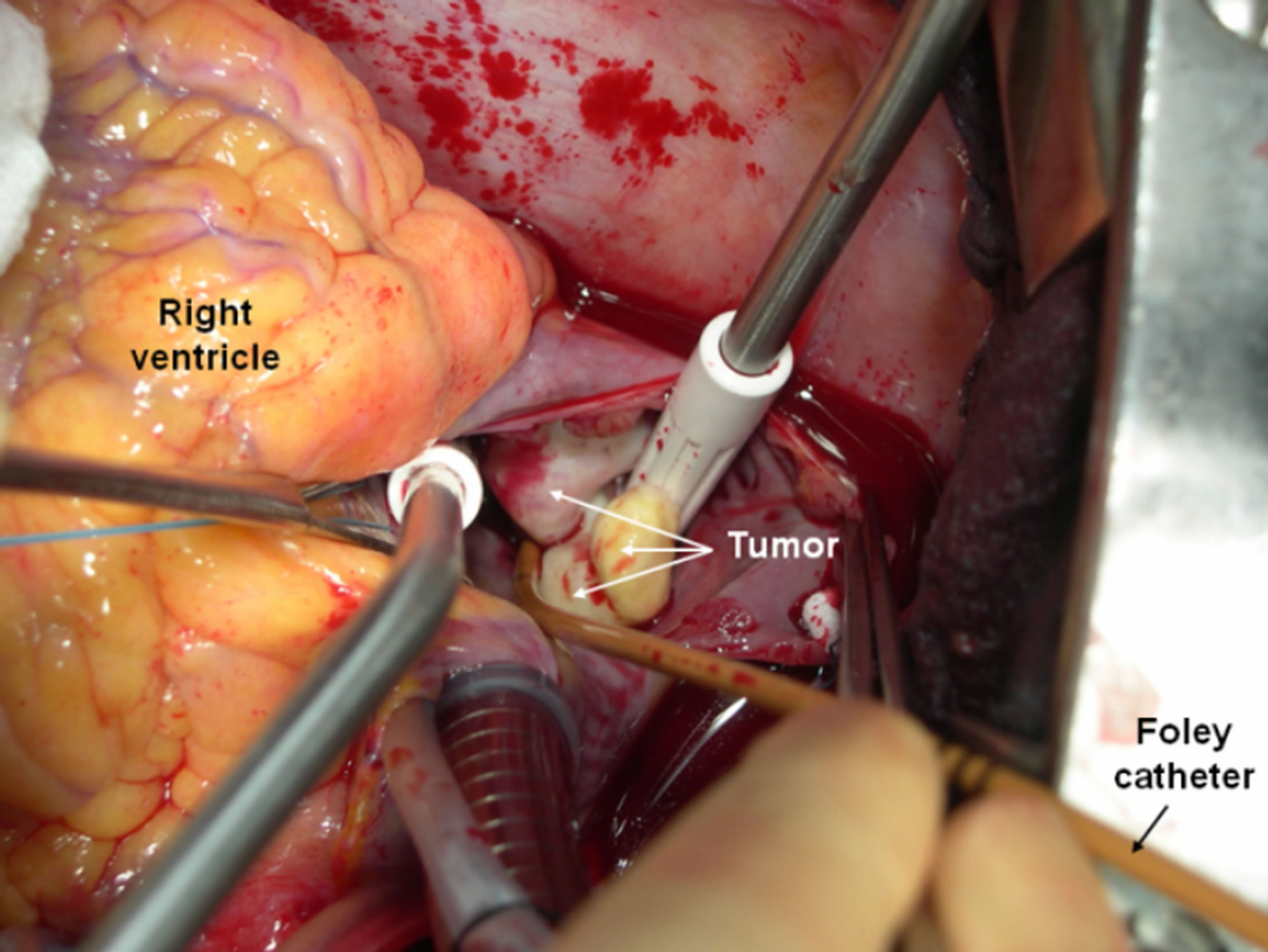
Figure 1. An operating view showed the suprahepatic IVC metastatic leiomyoma tumor. Foley catheter is introduced into IVC and connected to the pump suction.
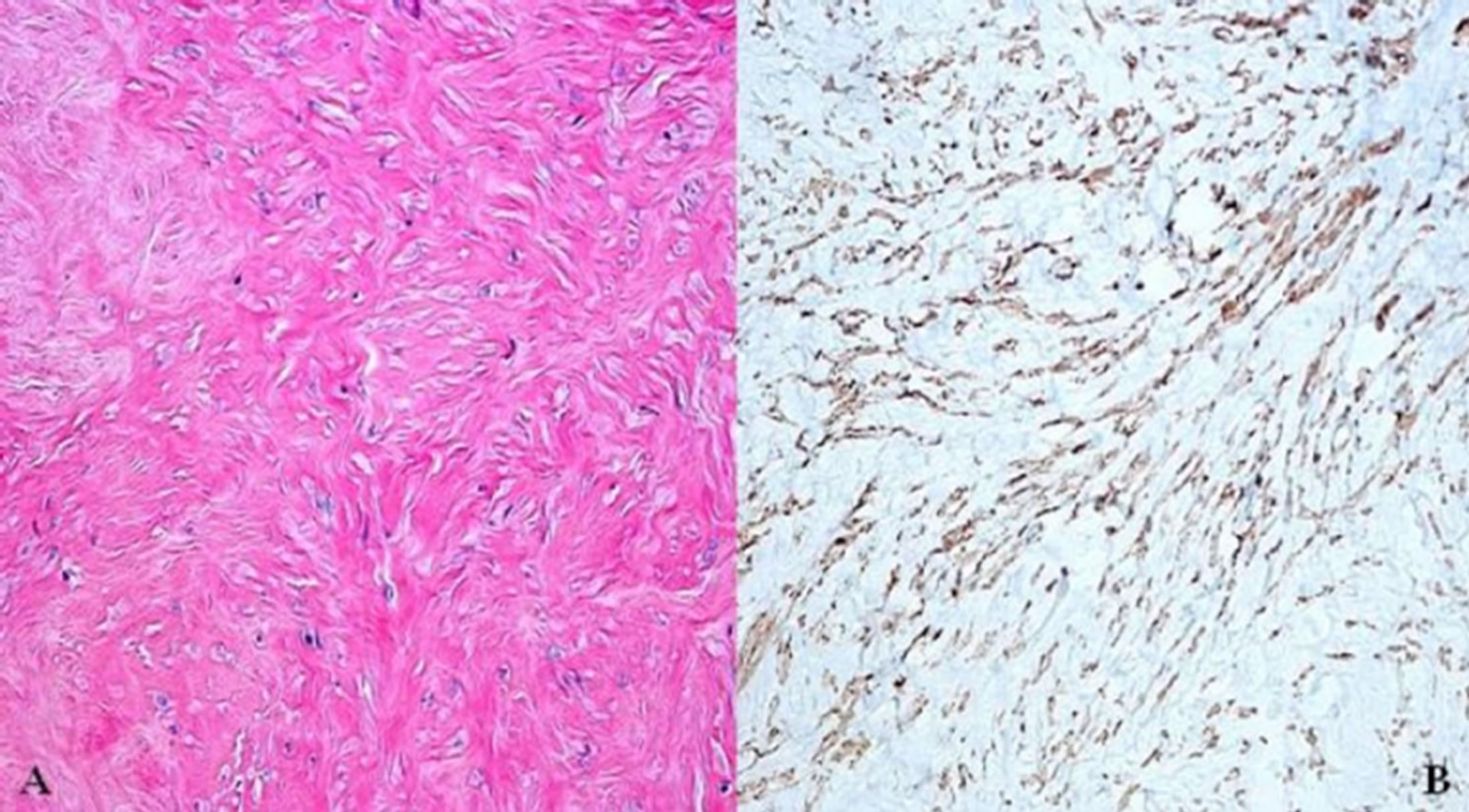
Figure 2. Immunohistopathogical study of metastasizing uterine leiomyoma of inferior venacava. (A): Stained with hematoxylin-eosin; (B): Stained with caldesmon.
tissues where the metastasis settles. The treatment varies according to the affected organ. Surgical excision of the tumor is recommended in cardiac involvement to avoidserious complications such as pulmonary thromboembolism, obstruction of right ventricular out-tract or IVC drainage [4,5].
The tumoral involvement of IVC is classified into 3 groups: segment I, infrarenal; segment II, interand suprarenal up to but not including the main suprahepatic veins; and segment III, suprahepatic with possible intracardiac extension. Fabre et al., use venovenous shunting with selective hypothermia hepatic perfusion in surgical treatment of tumors involving the suprarenal segment of the IVC [6]. Kieffer et al., recommend circulatory arrest at 18˚C for surgical excision of tumors involving supraheptic segment of IVC with intracardiac extension so as to perform in a blood-free operating field. They use cardiopulmonary bypass with double venous cannulation; one through the appendage of RA and the other in femoral vein or infrarenal IVC [7]. Based on experience of Hardwigsen et al., the material of choice for caval replacement is reinforced PTFE. They also strongly recommend the creation of an arteriovenous fistula to ensure patency and eliminate the need for long-time anticoagulation therapy [8].
Herein, we describe a simplified and efficacious technique for excision of a metastatic leiomyoma as a benign tumor involving the suprahepatic segment of IVC with intracardiac extension. Normothermic cardiopulmonary bypass using a double venous cannulation; superior vena cava and right femoral vein, and introduction of a Foley catheter into IVC do not only eliminate the side effects of circulatory arrest but also provide an excellent exposure with blood-free operating field and prevent the falling of tumor down into IVC.
REFERENCES
- J. M. Rosenberg, M. A. Marvasti, A. Obeid, L. W. Johnson and M. Bonaventura, “Intravenous Leiomyomatosis: A Rare Cause of Right Sided Cardiac Obstruction,” European Journal of Cardiothoracic Surgery, Vol. 2, No. 1, 1988, pp. 58-60. doi:10.1016/1010-7940(88)90099-1
- D. M. Koh, P. R. Burn and D. M. King, “Benign Metastasizing Leiomyoma with Intracaval Leiomyomatosis,” British Journal of Radiology, Vol. 73, No. 868, 2000, pp. 435-437.
- G. P. Sutton, R. E. Rogers and W. W. Hurd, “Benign Tumors of the Uterus,” In: S. I. Swartz, G. T. Shires, F. C. Spencer, J. E. Fisher, J. M. Daly and A. C. Galloway, Eds., Principios de Cirugia, 7th Edition, McGraw-Hill, Mexico, 2000, pp. 1960-1994.
- S. D. Galvin, B. Wademan, J. Chu and R. W. Bunton, “Benign Metastasizing Leiomyoma: A Rare Metastatic Lesion in the Right Ventricle,” Annals of Thoracic Surgery, Vol. 89, No. 1, 2010, pp. 279-281.
- R. F. Padera Jr. and F. J. Schoen, “Pathology of Cardiac Surgery,” In: L. H. Cohn and L. H. Edmunds Jr., Eds., Cardiac Surgery in the Adult, 3rd Edition, McGraw-Hill, New York, 2007, pp. 168-172.
- J. M. Fabre, J. Domergue, H. Fagot, F. Guillon, B. Souche, M. Joswik and H. Baumel, “Leiomyosarcoma of the Inferior Vena Cava Presenting as Budd-Chiari Syndrome: Vena Cava Replacement under Veno-Venous Bypass and Liver Hypothermic Perfusion,” Eurpean Journal of Surgical Oncology, Vol. 21, No. 1, 1995, pp. 86-87.
- E. Kieffer, M. Alaoui, J.-C. Piette, P. Cacoub and L. Chiche, “Leiomyosarcoma of the Inferior Vana Cava: Experience in 22 Cases,” Annals of Surgery, Vol. 244, No. 2, 2006, pp. 289-295.
- J. Hardwigsen, P. Baque, B. Crespy, V. Moutardier, J. R. Delpero and Y. P. Le Treut, “Resection of the Inferior Vena Cava for Neoplasms with or without Prosthetic Replacement: A 14-Patient Series,” Annals of Surgery, Vol. 233, No. 2, 2001, pp. 242-249.
NOTES
*Corresponding author.

