Open Journal of Anesthesiology
Vol.3 No.3(2013), Article ID:31257,5 pages DOI:10.4236/ojanes.2013.33036
Thoracic Epidural Morphine for Postoperative Analgesia after Hemiclamshell Incision in Castleman Disease
![]()
1Department of Anaesthesiology, Critical Care and Pain Medicine, Donostia University Hospital, San Sebastián, Spain; 2Department of Thoracic Surgery, Donostia University Hospital, San Sebastián, Spain; 3Department of Maxillo-Facial Surgery, Donostia University Hospital, San Sebastián, Spain.
Email: mugabure@yahoo.es
Copyright © 2013 Borja Mugabure et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 22nd, 2013; revised March 29th, 2013; accepted April 15th, 2013
Keywords: Hemiclamshell; Epidural Morphine; Postoperative Pain; Multimodal Analgesia
ABSTRACT
The hemiclamshell incision is a valid but infrequent surgical alternative for the resection of tumors from mediastinum. This point makes this approach impossible to be studied under a randomized controlled trial. The triple association of thoracotomy, sternotomy and cervicothomy makes the hemiclamshell approach a high intensity postoperative pain surgery. However, there is no published data on this topic, and the reviewed articles only mention the analgesic regimens as a secondary point. Indeed, no author defines the best epidural drugs mixture for the patients. Multimodal analgesic regimen based on regional anesthesia should be used. Based on the recent advances on spinal opioids, morphine could be a good choice as epidural coadjutant to local anesthetics for hemiclamshell incision.
1. Introduction
Classical incisions, such as postero-lateral thoracotomy or median sternotomy, do not provide optimal exposure of the intraoperative field for surgical treating of tumors arising from the cervico-thoracic junction or in the anterior mediastinum with extension to the chest cavity [1]. The hemiclamshell approach (HCS, trap door incision or book thoracotomy) has been proposed as a better alternative for these cases [2-7].
There are few articles on this topic, less than 15 PubMed citations until December-2012 using “hemiclamshell” as keyword (5 of all as a surgical case report) [8- 12]. Most of them, focusing on the thoracic surgeon choice of the most appropriate surgical approach to obtain complete tumor resection and repair of damaged structures but not on the management of postoperative pain. This one should be treated with a multimodal analgesic regimen based on continuous thoracic epidural analgesia but the best choice for epidural drugs mixture has not been yet well defined [13]. We present the case of a young man, which was treated with thoracic epidural bupivacaine plus morphine during 72 hours for the treatment of postoperative pain after hemiclamshell incision for the resection of a mediastinal Castleman disease. Moreover, this approach was successfully accepted either by the patient or the multidisciplinary medical and nursing team.
2. Case Description
2.1. Anesthetic and Analgesic Procedures
43 years old, ASA II, 95 kg Caucasian man, was programmed for an elective thoracic surgery for the resection of a mediastinum tumor. The mass size was 9.01 × 5.15 cm (Figure 1) and due to the infiltration of the right Brachiocephalic plexus, a hemiclamshell approach plus cervicothomy was decided by the multidisciplinary surgical team. Indeed, a loss of the right radial artery pulse was observed during surgery in relation to tumor traction. A combined general/epidural anesthesia was made. General anesthesia was based on a balanced combination of intravenous (IV) dexamethasone (8 mg), midazolam (2 mg), propofol 200 mg, rocuronium 50 mg plus an infusion of 0.3 mg/kg/h, and fentanyl 2 μg/kg/h. Unipulmonar ventilation was used with a mixture of 50% O2 + Air and sevoflurane as inhaled agents to maintain a Bispectral
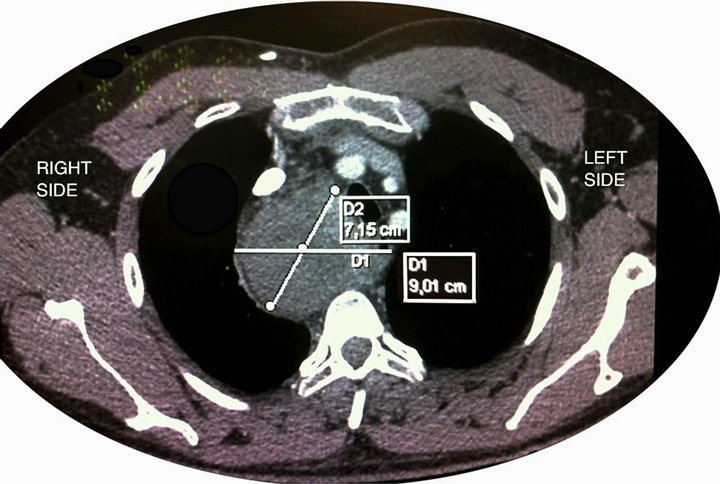
Figure 1. CT Scan imagen showing the localization and size of the mediastinum tumor.
index (BIS) between 40 - 60, during 10 hours that lasted the whole surgical procedure. A resistive-heating system was used to prevent intraoperative hypothermia.
The perioperative analgesic regimen was based on a multimodal approach with IV Nonsteroidal anti-inflammatory agents NSAIDs (acetaminophen 1 gr/8 h + Desketoprofen Trometamol 50 mg/12 h, both started into the operating room), epidural analgesia and 2 mg IV morphine bolus as needed. An epidural thoracic catheter was placed before surgery between the 7th and 8th thoracic levels and it was tested for intravascular or subarachnoid placement with 3 ml of 1.5% lidocaine plus epinephrine 5 μg/ml. Afterwards, 5 mg of morphine diluted into 5 ml of saline solution was administrated at the beginning of the surgery and the same dose was repeated at the end, 10 hours later. After 2 hours in the post-anesthesiacare unit (PACU) the patient was extubated, and an epidural infusion with bupivacaine 0.125% plus morphine 40 μg/ml, prepared under sterile conditions by the Pharmacy service, was started at a flow rate of 6 - 8 ml/h and maintained for 72 h. The total amount of epidural morphine was 28 mg and the rescue IV morphine 8 mg, only needed during the first 24 h after surgery. He remained in the PACU for adequate monitorization to prevent from delayed respiratory depression and the unique adverse effect observed was a low intensity pruritus. Ondasetrón 4 mg each 8 hours was also administrated to prevent postoperative nausea and vomiting. The visual analogic scale (VAS) was ≤3 along the three days in the PACU, and the global patient satisfaction was excellent. The hospital length stay was 7 days, and the tumor was diagnosed as a rare non-clonal lymphproliferative disorder called Castleman disease (Figure 2).
2.2. Patient’s Surgical Approach and Position
Unilateral stern thoracotomy, or HCS, consists of antero-lateral thoracotomy into the 4th or 5th intercostal space, extending median longitudinal partial sternotomy with
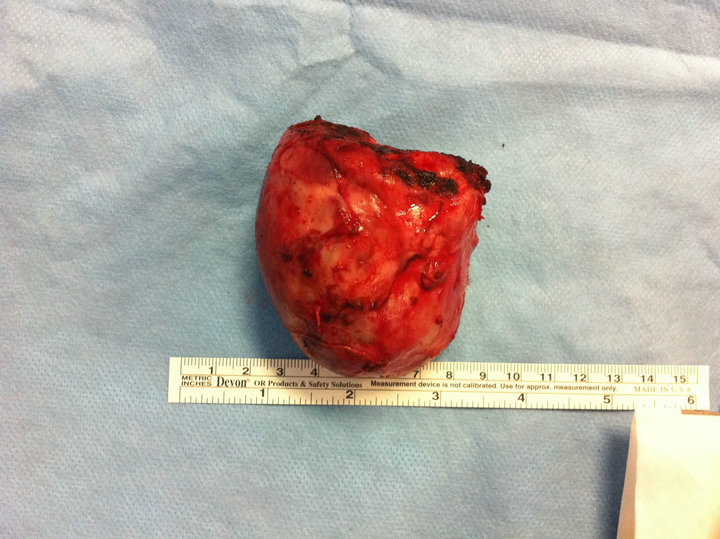
Figure 2. Direct view of the thoracic Castleman disease.
possible extension of the incision along the sterno-cleidomastoidian [2], (Figure 3). This approach requires ligature and sacrificing of the internal thoracic pedicle but provides a large view to the thoracic cavity, pulmonary apex and cervicothoracic junction [4]. Thus, HCS allows lengthy access to the thoracic inlet, enabling the excision of large masses as well as parietal resections without any size limitation. These can all be achieved with greater safety as the pulmonary and intra-pericardial vessels can be easily controlled [4,6]. HCS also has the advantage of the patient being in dorsal decubitus position, thereby limiting the risk of compression of the cardiac cavity and problems with venous filling that are encountered in lateral decubitus in patients with a large intra-thoracic mass. In addition, this route, which preserves the sternoclavicular articulation, decreases post-operative functional disability, which results from most other antero-lateral routes and preserves the morbility and appearance of the shoulder and brachial plexus, as well as offering excellent surgical exposure [6] (Figure 4).
3. Analgesic Epidural Drugs Literature Review
In the review of Lardinois D et al. [4], 25 patients under HCS incision were studied. The indications for this approach were chest trauma with massive hemorrhage (40%), lesions involving one or both chest cavity and the mediastinum (32%) and tumors of the anterior cervicothoracic junction with suspicion of vascular involvement (28%). The 30-day mortality was only 8% and the complication rate was 24%. They concluded that HCS improved exposure of the operative field in selected patients without causing more postoperative morbidity, chest wall complaints, shoulder girdle dysfunction or impairment of pulmonary function than standard approaches. In the abstract, they affirm that analgesia requirements for postoperative pain relief were not increased compared to
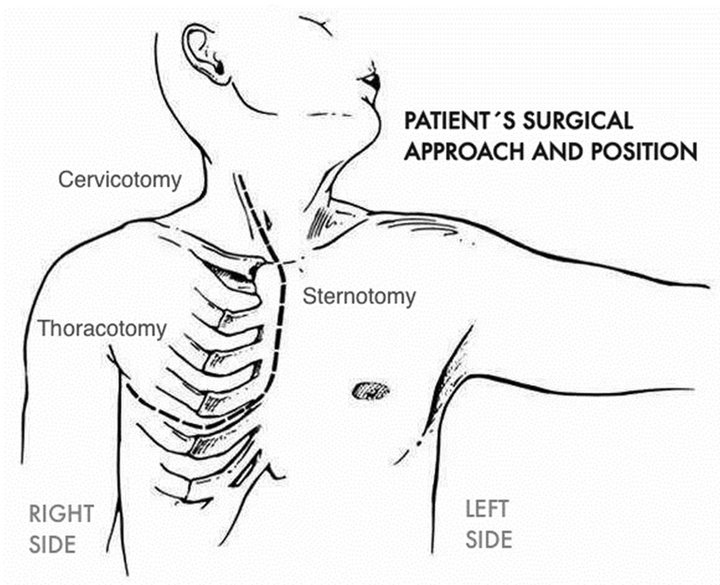
Figure 3. Incision for right hemiclamshell thoracotomy with right neck extension (Adapted from R. J. Korst, et al. [2]).
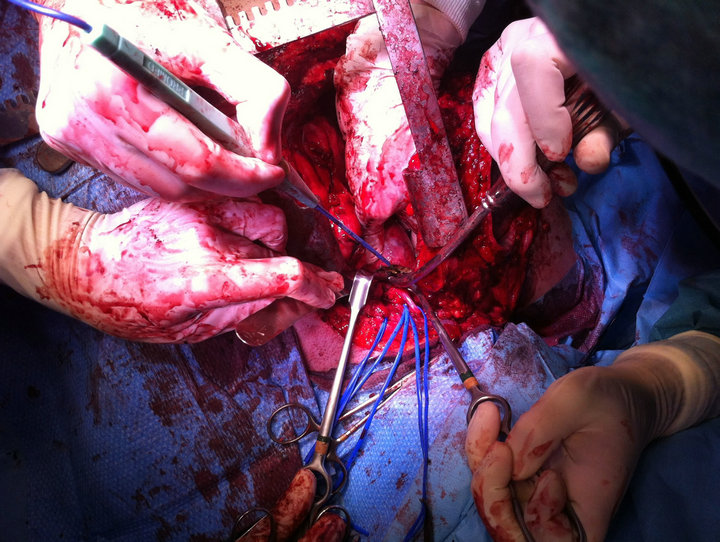
Figure 4. View of the surgical HCS plus cervicothomy approach.
those following a standard thoracotomy, but however no more data were available along the article exposure.
In a most recent retrospective review, Lebreton G., et al. [6] studied the HCS approach in 50 patients undergoing mass resection in thoracic surgery (46% mediastinal mass or tumors of the cervicothoracic junction, 38% pulmonary apex tumor, 10% primary thoracic wall tumor and 6% compressive bulky thoracic tumor), analyzing the indications, morbidity and outcome (pain, neurological or shoulder defects and mortality) one month and one year. Postoperative complications occurred in eight patients, atelectasis and pneumopathy in five, chylotorax treated medically in two and phrenic paralysis in one. Mortality was 6% at one month and 8% at a year (two sepsis shocks and one acute respiratory distress syndrome). Concerning postoperative pain, the authors comment also in the abstract, that the analgesic requirements were similar to those after other thoracic surgery approaches and that 12% of patients suffered pain for one month and 6% for one year, but no more data were added during the text.
In the only case report that explains the postoperative analgesia [11], epidural tramadol infusion was administered only in the first postoperative day. The second day epidural catheter was removed and parenteral tramadol combined with NSAIDs were given. Tramadol was stopped in 8th postoperative day and analgesia was continued with NSAIDs. The patient was internalized with pneumonia after 12 days.
4. Discussion
Postoperative analgesia for thoracic surgery is actually well defined and implies a multimodal approach based on regional analgesia before surgery (metameric epidural or paravertebral blockade) associated with the administration of IV NSAIDs and IV opioids as a rescue regimen [13]. However, the PROSPECT GROUP, specialized in providing evidence-based procedure-specific recommendations and clinical decision support for the management of postoperative pain, just recommends a strong opioid added to LA when an epidural analgesia is used but they do not define the best option for epidural mixture [13].
In a review by Scarci et al. [14], paravertebral blockade (PVB) was found to be of equal efficacy to epidural anesthesia, but with a favorable side effect profile, and lower complication rate. The reduced morbidity rate was most marked for pulmonary complications and was accompanied by quicker return to normal pulmonary function. Epidural block was associated with frequent side effects [urinary retention (42%), nausea (22%), itching (22%) and hypotension (3%) and, rarely, respiratory depression (0.07%)]. Additionally, it prolonged operative time and was associated with technical failure or displacement (8%). Epidurals were also related to a higher complication rate (atelectasis/pneumonia) compared to the PVB.
The same results favorable to PVB were found in the study of Powell et al. [15], about analgesia following thoracotomy in the UK. Data on the type of analgesic techniques were available for 98.1% of cases (n = 306). The most common type of analgesia was epidural catheter (n = 187, 61.1%) followed by paravertebral blockade (n = 95, 31%). In the epidural analgesia group (n = 187), a combination of epidural local anesthetic and opioids was used in 62.6% (n = 117). For 33.2% (n = 62) of epidural analgesia recipients, the drug mixture used was not specified. For epidural analgesia, the most common local anesthetic used was bupivacaine (concentration range 0.08% - 0.15%) and the most common opioid used was fentanyl (concentration range 2 - 10 μg/ml). So we can conclude that epidural morphine is not a widespread practice for thoracic surgery.
The reviews about HCS cited above [4,6], affirm that analgesia regimens were similar to the current use in their institutions for thoracic surgery and we suppose it’s based on epidural analgesia because PVB only offers unilateral thoracic analgesia and is not suitable for HCS surgery. Further, classic thoracic surgery uses a metameric incision which can be easily covered in most cases by an epidural continuous infusion, but the sternotomy and as in our patient, the cervicothomy incision, make this point more difficult because the upper thoracic and even cervical metameric levels are involved. In these cases, we think that the combination of a local anesthetic with a strong opioid (morphine due to its rostral spinal diffusion) by the epidural thoracic route is an appropriate choice.
The spinal administration of an opioid drug does not guarantee selective action neither segmental analgesia in the spine [16]. Evidence from experimental studies in animals indicates that bioavailability in the spinal cord biophase is negatively correlated with liposolubility. It’s higher for hydrophilic opioids, such as morphine, than lipophilic opioids, such as fentanyl, sufentanil and alfentanil [16]. All opioids administered produce part of their analgesic effect via spinal selectivity, although lipophilic opioids also rapidly reach higher centers of the brain due to their good vascular uptake and redistribution [16,17]. Clinical trials have demonstrated that the administration of lipophilic opioids by continuous epidural infusion does not produce analgesia due to a spinal mechanism, although strengthening local anesthesia enables total doses to be reduced [17]. This contrasts with single epidural injections of fentanyl as a bolus, which with sufficiently high quantities of the drug can reach specific areas at the spinal level [18]. The continuous alone epidural administration of fentanyl and sufentanil offers very few advantages compared to its IV administration, which is why it is associated to LA to reduce its minimum effective analgesic concentration and also related adverse effects. The consequence is an analgesic effect mainly by systemic reuptake to blood and secondary to brain opioids receptors [16,17].
Morphine is probably the opioid with the greatest medullary selective action after its epidural or intradural administration. Morphine has been the most used epidural opioid, and it could be considered the “gold standard” of spinal drugs, which does not imply the ideal one. Due to its high medullar selectivity, the epidural dose used is much lower than the parenteral dose (1/5 - 1/10), with a recommended daily maximum dose of 10 mg [16-19]. It can be administered both either in the form of boluses (30 - 100 µg/kg) or continuous infusion (0.2 - 0.4 mg/h), as the latter appears to induce a greater analgesic quality. Also as a single drug or together with LA. Because these two drugs potentiate the global analgesic effect by means of a synergistic action, resulting in a postoperative analgesia of great quality and duration, but at the expense of a greater incidence of adverse effects. Risk factors for the development of respiratory depression include large doses, concomitant use of additional opioids and/or sedatives, administration in opioid-naïve patients, age > 65 years, sleep apnoea and obesity. Protocols for monitoring vary, but typically recommended duration is 18 to 24 hours after spinal morphine and during the whole time of the epidural infusion [19,20].
5. Conclusion
Recent reviews have demonstrated that pararavertebral blockade is superior to epidural analgesia due to minor incidence of major morbility and better outcome with similar analgesic effect for patients undergoing thoracic surgery. However, for HCS approach, only continuous thoracic epidural analgesia will provide an appropriate pain control, but the most suitable epidural drug mixture is not yet well defined. In our patient, the association of bupivacaine 0.125% plus morphine 40 μg/ml by the thoracic epidural route produced a very good control for the postoperative pain during 72 hours, without major adverse effects. This epidural opioid concentration allows the physician easily calculate the daily morphine amount because it’s very similar to the epidural rate infusion, (mg/day 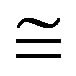 ml/h). The complete surgical medical and nursery team’s opinion is that the multimodal management of postoperative pain after HCS incision should be based on thoracic epidural analgesia composed of a long action LA plus morphine, combined with physiotherapy and a rehabilitation programme in order to minimise the period of postoperative recovery, hospital stay and convalescence.
ml/h). The complete surgical medical and nursery team’s opinion is that the multimodal management of postoperative pain after HCS incision should be based on thoracic epidural analgesia composed of a long action LA plus morphine, combined with physiotherapy and a rehabilitation programme in order to minimise the period of postoperative recovery, hospital stay and convalescence.
REFERENCES
- M. S. Bains, R. J. Ginsberg, W. G. Jones, P. M. McCormack, V. W. Rusch, M. E. Burt and N. Martini, “The Clamshell Incision: An Improved Approach to Bilateral Pulmonary and Mediastinal Tumor,” Annals of Thoracic Surgery, Vol. 58, No. 1, 1994, pp. 30-32. doi:10.1016/0003-4975(94)91067-7
- R. J. Korst and M. E. Burt, “Cervicothoracic Tumors: Results of Resection by the Hemi-Clamshell Approach,” Journal of Thoracic and Cardiovascular Surgery, Vol. 115, No. 2, 1998, pp. 286-295. doi:10.1016/S0022-5223(98)70271-5
- M. Rusca, P. Carbognani and P. Bobbio, “Cervicothoracic Tumors: Results of Resection by the ‘Hemi-Clamshell’ Approach,” Journal of Thoracic and Cardiovascular Surgery, Vol. 117, No. 5, 1999, p. 1040. doi:10.1016/S0022-5223(99)70400-9
- D. Lardinois, M. Sippel, M. Gugger, M. Dusmet and H. R. Ris, “Morbidity and Validity of the Hemiclamshell Approach for Thoracic Surgery,” European Journal of Cardiothoracic Surgery, Vol. 16, No. 2, 1999, pp. 194-199. doi:10.1016/S1010-7940(99)00156-6
- P. D. Rio, L. Bezer, M. F. Arcuri and M. Sianesi, “Hemiclamshell Incision in the Treatment of Mediastinal Goiter,” Langenbecks Archives of Surgery, Vol. 394, No. 3, 2009, pp. 457-459. doi:10.1007/s00423-008-0342-2
- G. Lebreton, J. M. Baste, M. Thumerel, F. Delcambre, J. F. Velly and J. Jougon, “The Hemiclamshell Approach in Thoracic Surgery: Indications and Associated Morbidity in 50 Patients,” Interactive Cardiovascular Thoracic Surgery, Vol. 9, No. 6, 2009, pp. 965-969. doi:10.1510/icvts.2009.211623
- I. S. Sarkaria, M. S. Bains, S. Sood, C. S. Sima, V. E. Reuter, R. M. Flores, R. J. Motzer, G. J. Bosl and V. W. Rusch, “Resection of Primary Mediastinal Non-Seminomatous Germ Cell Tumors: A 28-Year Experience at Memorial Sloan-Kettering Cancer Center,” Journal of Thoracic Oncology, Vol. 6, No. 7, 2011, pp. 1236-1241. doi:10.1097/JTO.0b013e31821d513e
- M. Menth, M. C. Balmer and H. B. Ris, “Blunt Thoracic Trauma with Hemorrhage into a Mediastinal lymphangioma: Case Report,” Swiss Surgery, Vol. 4, No. 2, 1998, pp. 58-60.
- A. Karajiannis, T. Krueger, E. Stauffer and H. Ris, “Large thoracic Duct Cyst: A Case Report and Review of the Literature,” European Journal of Cardiothoracic Surgery, Vol. 17, No. 6, 2000, pp. 754-756. doi:10.1016/S1010-7940(00)00447-4
- M. Okuno, M. Kawashima, K. Miura, E. Kadota, S. Goto and M. Kato, “Resection of Giant Mediastinalliposarcoma Using the Hemiclamshell Incision,” General Thoracic and Cardiovascular Surgery, Vol. 58, No. 12, 2010, pp. 654- 656. doi:10.1007/s11748-010-0584-0
- N. KanlioğluKuman, S. Sen, S. Cokpinar, E. Ceylan, C. Tataroğlu and M. Boğa, “A Hemiclamshell Incision for a Giant Solitary Fibrous Tumor of the Right Hemithorax,” Case Reports in Surgery, Vol. 2012, 2012, Article ID: 826454. doi:10.1155/2012/826454
- K. Kurisu and M. Hisahara, “Repair of a Ductal Aneurysm Using a Hemi-Clamshell Incision in an Elderly Patient,” Annals of Thoracic and Cardiovascular Surgery, Vol. 18, No. 3, 2012, pp. 268-270. doi:10.5761/atcs.cr.11.01724
- H. Kehlet, R. C. Wilkinson, H. B. Fisher and F. Camu, “Prospect Working Group. Prospect: Evidence-Based, Procedure-Specific Postoperative Pain Management,” Best Practice & Research Clinical Anaesthesiology, Vol. 21, No. 1, 2007, pp. 149-159. doi:10.1016/j.bpa.2006.12.001
- M. Scarci, A. Joshi and R. Attia, “In Patients Undergoing Thoracic Surgery, Is Paravertebral as Effective as Epidural Analgesia for Pain Management?” Interactive Cardiovascular of Thoracic Surgery, Vol. 10, No. 1, 2010, pp. 92-96. doi:10.1510/icvts.2009.221127
- E. S. Powell, D. Cook, A. C. Pearce, P. Davies, G. M. Bowler, B. Naidu, F. Gao and UKPOS Investigators, “A Prospective, Multicentre, Observational Cohort Study of Analgesia and Outcome after Pneumonectomy,” British Journal of Anaesthesiology, Vol. 106, No. 3, 2011, pp. 364-370. doi:10.1093/bja/aeq379
- C. M. Bernards, “Recent Insights into the Pharmacokinetics of Spinal Opioids and the Relevance to Opioid Selection,” Current Opinion in Anaesthesiology, Vol. 17, No. 5, 2004, pp. 441-447. doi:10.1097/00001503-200410000-00015
- B. M. Bujedo, S. G. Santos and A. U. Azpiazu, “A Review of Epidural and Intrathecal Opioids Used in the Management of Postoperative Pain,” Journal of Opioid Management, Vol. 8, No. 3, 2012, pp. 177-192. doi:10.5055/jom.2012.0114
- Y. Ginosar, E. T. Riley and M. S. Angst, “The Site of Action of Epidural Fentanyl in Humans: The Difference between Infusion and Bolus Administration,” Anesthesia & Analgesia, Vol. 97, No. 5, 2003, pp. 1428-1438. doi:10.1213/01.ANE.0000081793.60059.10
- B. M. Bujedo, “A Clinical Approach to Neuraxial Morphine for the Treatment of Postoperative Pain,” Pain Research and Treatment, Vol. 2012, 2012, Article ID: 612145. doi:10.1155/2012/612145
- O. A. de Leon-Casasola and M. J. Lema, “Postoperative Epidural Opioid Analgesia: What Are the Choices?” Anesthesia & Analgesia, Vol. 83, No. 4, 1996, pp. 867-875. content/83/4/867.long
NOTES
*Acute postoperative pain management leader team.

