Open Journal of Gastroenterology
Vol.2 No.3(2012), Article ID:22042,12 pages DOI:10.4236/ojgas.2012.23027
Suggested guidelines for the diagnosis and management of chronic HCV infection in children
![]()
Ysbyty Gwynedd Hospital, Salisbury, UK
Email: drfazal2000@yahoo.com
Received 25 June 2012; revised 22 July 2012; accepted 31 July 2012
Keywords: Chronic Hepatitis C; Children; Guidelines; Antiviral Therapy
ABSTRACT
HCV infection in children is different from the adult infection in many ways like natural course of the disease; duration, therapeutic response and side effects profile of the drug therapy; and prognosis. Special considerations include what is the appropriate time to investigate a suspected child, when to institute and choice of drug therapy and how to prevent vertical transmission. In this article, based on the current evidence suggested guidelines for the diagnosis and management of chronic HCV infection in children is given. Feedback to help improve/modify these recommendations by those experienced in dealing with the children will be welcome.
1. INTRODUCTION
Antiviral therapy for chronic hepatitis C has traditionally been considered contraindicated in children. A review of the recent literature however suggests that this view is no more true. Many recent studies have suggested that antiviral therapy can be safely given in children with excellent results, though some important diagnostic and therapeutic considerations need to be addressed. In the light of the current evidence, this article presents a practical and succinct diagnostic and therapeutic approach to HCV-infected children.
2. DISCUSSION
Approximately, 0.2% and 0.4% of children under the age of 12 and between 12 - 19 years respectively are infected with hepatitis C in Pakistan [1] (roughly, 75% - 90% of them have HCV genotype 3). Based upon studies in adults, it is estimated that 75% - 80% of these children are HCV RNA positive as well (i.e. have active disease and potentially require treatment). In USA, an estimated 240,000 children (28,000 new each year) [2] are infected with HCV with viremia present in 50% - 80% of them [3]. The prevalence of HCV infection among women of childbearing age is estimated to be 1.2% and the risk of HCV transmission at the time of delivery is 2% - 5% (the higher the HCV viral load in mother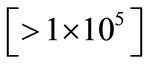 , higher the risk of transmission and vice versa) [4]. In mothers suffering from HCV/HIV coinfection, the risk further increases by at least four folds [5]. Whereas, in the developed world maternal drug use and vertical (perinatal) transmission appears to be the major mode of HCV transmission in children [6], in Pakistan we cannot overlook transmission by blood/blood products transfusion. Thus all children who have ever been transfused unscreened blood/blood products in Pakistan for any reason, and children born to infected mothers (all pregnant ladies should be screened for HBV & HCV) should be considered as potentially infected and investigated appropriately. Other potential modes of exposure in pediatric age-group patients include hemodialysis, extracorporeal membrane oxygenation, surgery for congenital heart disease and high-risk behaviours such as intravenous or intranasal drug use and use of shared tattoo equipment, Seroprevalence rates as high as 10% - 20% have been reported in such cases [7,8]. In quite an appreciable percentage (63% in one study) [9] of HCV-positive pediatric age-group patients, no obvious cause of viral exposure is found.
, higher the risk of transmission and vice versa) [4]. In mothers suffering from HCV/HIV coinfection, the risk further increases by at least four folds [5]. Whereas, in the developed world maternal drug use and vertical (perinatal) transmission appears to be the major mode of HCV transmission in children [6], in Pakistan we cannot overlook transmission by blood/blood products transfusion. Thus all children who have ever been transfused unscreened blood/blood products in Pakistan for any reason, and children born to infected mothers (all pregnant ladies should be screened for HBV & HCV) should be considered as potentially infected and investigated appropriately. Other potential modes of exposure in pediatric age-group patients include hemodialysis, extracorporeal membrane oxygenation, surgery for congenital heart disease and high-risk behaviours such as intravenous or intranasal drug use and use of shared tattoo equipment, Seroprevalence rates as high as 10% - 20% have been reported in such cases [7,8]. In quite an appreciable percentage (63% in one study) [9] of HCV-positive pediatric age-group patients, no obvious cause of viral exposure is found.
2.1. Natural Course of HCV Infection in Children
The natural course of the disease is different in children as compared to the adults. As compared to verticallytransmitted HCV infection in whom most patients are asymptomatic [10] with normal or near-normal ALT levels, transfusion-acquired pediatric HCV infection is more commonly associated with symptomatology (jaundice in 1/3rd of the cases) and raised ALT levels (in almost all cases). In cases of vertically-transmitted HCV infection, although ALT levels are often normal at birth, many studies have reported that the levels may rise either transiently or persistently at 4 - 6 months of age, and may continue to remain high till 2 years of age with substantial fall thereafter [11,12]. Nonetheless, it is pertinent to mention here that ALT concentration is not a criterion to suspect or rule out the presence of significant liver damage. Many studies have repeatedly shown that ALT levels may be persistently normal in patients who otherwise are HCV infected with high viral loads and evidence of severe hepatocellular damage or even significant fibrosis on liver biopsy [13-15]. Therefore, normal ALT levels should never be interpreted as normal liver. Unlike adults, higher rates of spontaneous resolution (ranging from 20% - 45% especially in genotype 3 cases) have been demonstrated in children [16,17]. It appears that children with transfusion-acquired infection are more likely to undergo spontaneous resolution than those who acquired the disease vertically. Also, unlike adults, in children the disease progression per se is slower and there is less probability of development of end-stage-liver disease or hepatocellular carcinoma [18]. Data however shows that the frequency of development of fibrosis is no different in children than adults, although progression to cirrhosis is slower (most children develop advanced liver disease >30 years after infection) [19-21]. Interestingly, periportal fibrosis appears to be commoner in children; it may worsen with age [20,21]. It appears that the probability of persistent viraemia and development of end-stage liver disease is relatively higher in children who are infected with genotype 1a, are polymerase chain reaction (PCR)- positive after the first year of life [22], are obese, have HCV/HIV coinfection or those whose mothers abuse drugs [9]. This probably represents the groups of patients who should be offered the benefits of antiviral therapy early in the course of the disease. Conversely, spontaneous viral clearance is more likely with genotype 3 cases as depicted in one study by Cox regression analysis (hazard ratio 6.44; 95% confidence interval: 2.7 - 15.5) [9]. In this group of patients, it is reasonable to give some time for spontaneous resolution to take place. Children who present with symptomatic acute HCV infection are probably less likely to develop chronic infection and thus should be given time for spontaneous resolution to take place [23].
2.2. Investigative Work-Up
Only those children whose mothers are both anti-HCV antibody & HCV-RNA positive should be investigated for possible vertical transmission of HCV infection. Mothers who are anti-HCV antibody positive but HCVRNA negative virtually never transmit infection to their infants [24-30]. Diagnostic work-up of children suspected of having chronic HCV should proceed similar to that of adults. A sensitive serological test (3rd generation enzyme linked immunoassay—EIA) for the detection of anti-HCV antibodies (not done during the first year of life due to the presence of maternal IgG antibodies) followed by a sensitive HCV RNA assay for definite confirmation is the usual diagnostic protocol [31,32]. Presence of HCV-RNA in both the mother and the infant is the most reliable indicator of vertical transmission of HCV infection. In anti-HCV positive persons, a single positive HCV RNA assay confirms the diagnosis. Having said this please remember that false positive result is also a possibility from contamination due to errors in sampling, storage, or extraction of RNA. Since, HCV RNA is normally present only intermittently in the circulation, a single negative assay doesn’t rule out the diagnosis of HCV infection. Thus, all negative test results should be reconfirmed with a repeat test done approximately 1 - 2 months later so as to reliably rule out the presence of ongoing HCV infection. If HCV RNA is lost due to errors in sampling, storage, or extraction of RNA, or insensitivity of the assay (caused by improperly selected probes or primers), false negative results can come. The special precautions that need to be observed in all such cases include urgent shipping of the samples on dry ice; rapid separation of the serum within 2 to 4 hours of collection; and storage at –20˚C.
Unlike hepatitis B virus (HBV) cases, no test is currently available to determine infectivity in a HCV-infected case. Therefore, all patients who are anti-HCV positive should be taken as potentially infectious.
Because of the probability of spontaneous clearance of HCV RNA from circulation during the first few years of life, infants born to HCV-infected mothers (particularly genotype 2 & 3 cases, which represent most of the Pakistani patients) should best be given time for spontaneous resolution to take place. Investigative work up can thus be deferred till 18 months of age or later. There is 80% probability that children who are PCR-positive at the age of 18 months won’t develop spontaneous resolution and will end up having chronic infection albeit asymptomatic [22]. Since antiviral treatment is contraindicated in children under the age of 3 years, there is no need to induce undue anxiety in the family just to have an earlier diagnosis. However, if parents insist on an earlier diagnosis, a qualitative PCR for HCV RNA may be performed at or after the infant’s first well-child visit at 4 months of age [33]. Anti-HCV antibodies passively acquired by infants from the infected mothers may persist in the circulation for up to 12 months. Therefore, serological testing (antiHCV detection by enzyme immunoassay—EIA) should better be avoided in infants <1 year of age [34]. Because of the possibility of contamination with the maternal blood, cord blood should never be used for any HCV-related testing [35].
Adolescents/young adults who need to be screened for HBV/HCV include those who were transfused blood before 1992 or who had had admission in a neonatal intensive care unit (NICU) prior to 1992 for any reason. This represents a large risk group (approximately 500,000 in the United States). Although there is a documented [36] seroprevalence of about 3% in this group, whether systematic screening of all NICU graduates for HCV to identify previously unrecognized cases is practicable &/or cost-effective is not yet clear [37].
2.3. Role & Utility of Liver Biopsy in Hepatitis C
The role and utility of liver biopsy in hepatitis C cases is still debatable and no definite consensus exists in any guideline. Subgroup of patients in which liver biopsy may be useful include:
1) >5 years olds who have acquired HCV infection vertically in which aminotransferase levels remain persistently normal and parents are unsure about whether or not to proceed to treatment [15]. As is well known that ALT levels do not correspond accurately to the degree of hepatic fibrosis, [38] in patients with persistently normal aminotransferase levels, the only way to reliably determine the extent and severity of liver disease and thus to make timely decisions regarding therapeutic interventions is to go for liver biopsy.
2) >5 years olds who have acquired HCV infection vertically and are suffering from HCV genotype 1 (less likely to respond to treatment) and parents are unsure about whether or not to proceed to treatment; [liver biopsy is not routinely recommended for genotype 2 or 3 cases because these patients respond very favorably to antiviral therapy regardless of the stage of liver disease and degree of fibrosis].
If liver biopsy in such patients shows only a minimal fibrosis limited to the portal tract (Metavir [39] score < 2 or Ishak [40] score < 3), initiation of interferon therapy may be delayed/individualized. Repeat biopsies done at 4 - 5 years intervals can be used to monitor the progression of fibrosis in such cases. If repeat biopsies show worsening of fibrosis, especially more-than-portal fibrosis (i.e. Metavir score ≥ 2 or Ishak score ≥ 3), patients should be offered antiviral therapy lest fibrosis worsens and cirrhosis develops when the treatment success rate and prognosis will be relatively poorer [41].
In children who have recently acquired HCV infection horizontally (via IV drug abuse etc), liver biopsy may not be necessary. This is because such patients are unlikely to develop advanced liver disease within a few years of transmission. If a decision to treat such a case is being made, determining the degree of fibrosis on histology is irrelevant.
2.4. Treatment
One rule of thumb that must be kept in mind while making a treatment decision in pediatric age-group HCVinfected cases is that although less common than adults, cirrhosis, end-stage liver disease and HCC do develop in some cases during childhood/adolescence. Therefore, deferring antiviral treatment on the assumption that these complications may not develop till 3rd/4th decade of life is not a very safe and prudent approach. Nonetheless, treatment plan may be deferred in patients suffering with genotype 1 who are approaching their 18th birthday in the near future because at least one of the protease inhibitors (telaprevir) is likely to be available to adults soon.
Monitoring for HCC is only recommended in patients who have already developed advanced cirrhosis (a rarity) and those coinfected with HBV (which can cause HCC without first causing cirrhosis). Liver ultrasonography and serum alpha fetoprotein levels both done annually generally suffice to monitor for HCC. Importantly the monitoring business should continue even after institution and successful completion of antiviral treatment. This is because successful treatment of HCV infection in cirrhotic patients probably does not eliminate the risk of development of HCC.
It is suggested that all ≥3 years old patients with positive serology & HCV RNA be offered antiviral therapy. Children with persistently normal ALT levels repeatedly 6 monthly, however, can be monitored with serial liver biopsies as mentioned above.
Controversy exists regarding who to treat and who not to treat amongst infected children. Since disease progresssion is less likely in children, it appears reasonable not to expose the children to the adverse events associated with antiviral therapy. On the other hand, when we look at the life expectancy of an average infected child, it doesn’t seem rational and ethical to let a patient live for 40 or more years with ongoing infection and suffer its potentially avoidable complications.
Antiviral therapy should not be given in children <3 years of age [31,42]. The reason is potential neurotoxicity of interferon and thus its deleterious effects on the developing brain [31]. Because of the high rate of spontaneous resolution, the need for interferon therapy, if any, is only minimal in children <3 years of age. Children aged 3 - 17 who are infected with hepatitis C and are considered appropriate candidates for treatment may be offered antiviral therapy [31,42,43]. Encouragingly, compared to adults, antiviral therapy in children yields relatively higher SVR rates [genotype 2/3 (84%); genotype 1 (36%)] [44,45] and fewer adverse events. This is probably due to earlier stage of the disease, higher relative IFN dosage, & relative lack of comorbid conditions in paediatric patients [46].
Earlier studies recommended treating with non-pegylated interferon alfa-2b (3 MU/m2 three times a week) and ribavirin (15 mg/kg) for 24 and 48 weeks in genotypes 2 & 3 and 1 cases respectively [31,32,42,44]. A recent comparative analysis of efficacies of different therapeutic options available for children however has revealed that PEG-IFN-alpha-ribavirin combination therapy yields better results (in terms of % ETR & SVR achieved) as compared to non-pegylated interferon monotherapy or its combination with ribavirin [47].
US Food and Drug administration (FDA) approved PEG-IFN-alfa-2b-ribavirin combination therapy for use in children >3 years old with compensated liver disease in 2008. Although not yet approved, Peg-IFN alfa 2a can be used in doses of 180 micrograms/1.73 m2 subcutaneously once weekly in combination with ribavirin in children ≥5 years of age. The approval of Peg-IFN use in children came following the publication of the results of a large multicenter trial [48] which evaluated the efficacy and safety of PEG-IFN-alpha-ribavirin combination therapy in 107 children with chronic HCV infection. The dosage regimens used in this trial were pegylated interferon alfa-2b 60 micrograms/m2 subcutaneously once weekly & ribavirin 15 mg/kg/day in two divided doses orally. Children with genotype 2 or 3 were treated for 24 or 48 weeks depending upon the viral load—less than or more than 600,000 IU/mL respectively. Those with genotype 1 or 4 were treated for 48 weeks regardless of the viral load. As expectedly much higher SVR rates of 93% were attained in patients with genotypes 2 or 3 compared to 53% in those with genotypes 1 or 4. Although anxiety and depression were reported in 28% of the cases, in great majority of them the side effects were not serious enough to warrant starting antidepressant treatment. Hematological side effects i.e. anemia & neutropenia serious enough to require dose reductions were reported in 25% of the subjects.
In another study aimed at ascertaining the efficacy and safety of PEG-IFN-alpha-ribavirin combination therapy in children, [49] 30 children between 3 - 6 years of age were selected for antiviral therapy based on positive HCV RNA for ≥3 years and elevated ALT levels. They received PEG-IFN-alpha2b 1.0 µg/kg/wk plus ribavirin 15 mg/kg/d for 24 weeks (genotype 2/3) or 48 weeks (genotype 1/4). Primary endpoint i.e. attainment of SVR (defined as undetectable HCV RNA (<50 IU/mL) at week 24 of follow-up) was achieved in 50% of the patients - (3/3 genotype 3; 12/27 genotype 1/4). No patient required ribavirin dose reduction; because of neutropenia, PEG-IFNalpha-2b dose was reduced in 23% of the patients and stopped in 3 subjects.
In a multicenter trial (called PEDS-C trial), [50] comparative efficacies of pegylated interferon-ribavirin combination and pegylated interferon-placebo combination were compared in 114 children with chronic HCV infection. Former combination yielded higher SVR rates (53%) compared to the latter (21%).
It is not clear at this point whether to use early virologic response (EVR) as a criterion, similar to adults, to stop therapy at week 12 or not. Refer to Table 1 for definitions of treatment responses, and Tables 2 and 3 for a schematic lay out of the management plan in children with genotypes 2 & 3 (the most prevalent genotypes in Pakistan) and genotype 1 (the most prevalent genotype in West) respectively [51,52].
2.5. Nonresponders & Relapsers
Nonresponders are those cases in whom the quantitative HCV RNA assay done at 12 weeks into the therapy shows either no decline in the HCV RNA titer (compared with the pre-treatment assay) or a decline of <2 log [31]. Relapsers, on the other hand, are those cases in whom the qualitative HCV RNA assay done at the end of the treatment course comes out to be negative (<50 IU/mL) i.e. end-of-treatment response (ETR) achieved, but 24 weeks later, qualitative HCV RNA assay done to ascertain sustained virologic response (SVR) comes out to be positive [31]. In Pakistani patients, since genotype 3 accounts for almost 75% - 90% of all cases, we (rightly) only do qualitative PCR at week 12 to see achievement of early
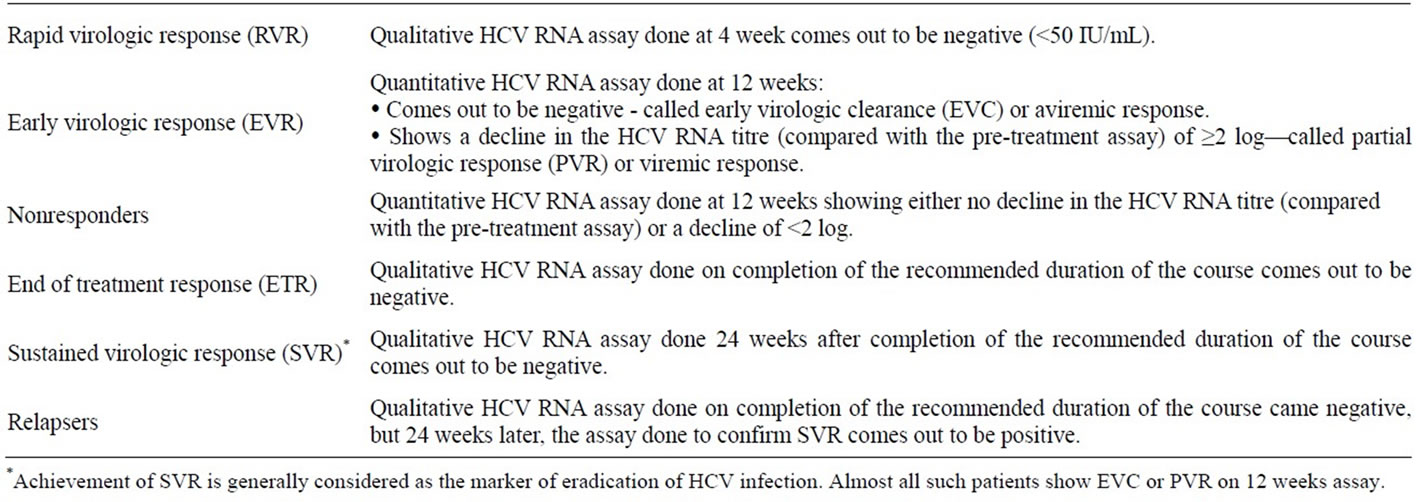
Table 1. Definitions of treatment responses.

Table 2. Suggested management plan in children with genotypes 2 & 3.


Table 3. Suggested management plan in children with genotypes 1.
virologic response (EVR) [52]. Quantitative PCR is only reserved for genotype 1 cases and the above-mentioned definition of non-responders is primarily true for this group of patients.
How do we approach nonresponders and relapsers basically depends upon the previous drug regimen administered in the patients (peginterferon-ribavirin combination; nonpegylated interferon-ribavirin combination; peginterferon monotherapy; nonpegylated interferon monotherapy) and the presence of negative predictors to drug therapy. In patients who were prescribed any regimen other than peginterferon-ribavirin combination therapy can be prescribed this regimen regardless of the genotype, [31] and sustained virologic response rates of 25% - 40% for nonpegylated interferon monotherapy cases, and 10% for nonpegylated interferon-ribavirin combination therapy cases can be expected [45].
2.6. Acute HCV Infection in Children
There is limited data and experience available to treat acute HCV infection in children. This is because except in rare outbreaks, [23] acute symptomatic HCV infection is rarely encountered in pediatric clinical practice. Based on empirical evidence a reasonable recommendation would be to allow 6 - 8 weeks observation time for spontaneous resolution to take place and treat only those who demonstrate persistent viremia in the form of positive PCR for HCV RNA after this period.
2.7. Monitoring of Antiviral Therapy
Although unlike adults, children surprisingly appear to tolerate interferon therapy much better, [53,54] antiviral therapy needs to be monitored to look for the development of potentially serious side effects and also to determine the response to therapy (see Table 4) [51]. Monitoring of antiviral therapy includes checking complete blood counts (CBC) at weeks 1, 2, 4, 6, 8 and then monthly; every 3 months, all baseline investigations should be repeated including LFT’s, creatinine, glucose and TSH.
Since retinopathy & uveitis have been reported in 2% - 3% of the patients treated with IFN, a baseline ophthalmologic examination prior to the commencement of treatment, and repeat examination thereafter if symptoms develop is recommended in all patients.
Interference with both linear growth and weight gain have been reported in children during the months of treatment [55]. Almost 80% of the children catch-up the growth following completion of the antiviral course. In 20% of the cases however growth velocity remains inhibited (<3rd percentile) six months after cessation of the treatment. Data regarding recovery of growth after longer time periods are awaiting publication.
Obesity is known to be inversely related to the success of antiviral therapy in both adults and children. In one study [56], each standard deviation (1 z-score unit) increase in BMI was associated with a 12% reduction in the probability of SVR attained. Based on this we recommend that a period of weight loss for several months or a year be given to obese children before commencing antiviral treatment. Since the disease progression is appreciably slow in children, this delay in the commencement of antiviral treatment is unlikely to affect the SVR except positively.
As with ribavirin therapy, the incidence of hemolytic anemia appears to be less than that in adults [57]; also it appears that the incidence doesn’t rise when higher doses (15 mg/kg) are used as compared to lower doses (8 - 12 mg/kg) [43]. Anemia, however, is a particular problem in those having renal insufficiency, cirrhosis, thalassemia, or HIV co-infection. There are reports of ribavirin-induced worsening of anemia in such patients with consequent rise in transfusion requirements (especially, in thalassemia patients) [4]. Anemia usually develops within the first 4 weeks of starting antiviral therapy and persists till the end of the course [58]. Almost 40% patients suffer a drop in Hb concentration of ≥3 gm/dl [58]. Most published studies recommend RBV dose-reduction if Hb level falls to or below 10g/dl, and discontinue it if it falls to <8 g/dL [42,59]. The current recommendation is to reduce IFN dose if neutrophil count falls to <0.5 × 109/L, and discontinue it if it falls to <0.3 × 109/L [31,59]. Regarding platelet count, it is recommended to reduce IFN dose if platelet count falls to <30 × 109/L, and discontinue if it falls to <20 × 109/L [42,59]. Because of the risk of development of life-threatening infection (very low!),
Table 4. Monitoring of anti-viral therapy.
patients who already have neutropenia or thrombocytopenia below the permissible limits (neutrophil count > 1500/mm3 & thrombocyte count > 75,000/mm3) should not be started with antiviral therapy. Although haematopoietic growth factors (erythropoietin and filgrastim) have been used in adults to help avoid antiviral dose reductions and attain optimum adherence (defined as the administration of interferon-ribavirin combination therapy in an optimum dose for more than 80% of the prescribed duration), [60] our experience with these drugs in children is almost non-existent. Therefore, despite of promising results in adults, the use of these agents as adjuncts to antiviral therapy is not recommended at this moment.
2.8. Antiviral Therapy in Decompensated Cirrhotic Patients
Traditionally, despite of the known theoretical benefits of antiviral therapy (improvement in liver histology, partial reversal of established cirrhosis, and prevention of life-threatening complications), many cirrhotic patients have not been offered antiviral therapy. Current literature review, however, shows that because of the unstandardized dosage schedules being administered over variable periods of time in the past studies, we have under- & overestimated the potential benefits & risks of antiviral therapy respectively, in decompensated cirrhotic patients. Based on the current literature review it is suggested that cirrhotic patients with a CTP score ≤9 and a decompensated event that abated with common management may be considered for antiviral therapy [61,62] although more data in pediatric age group is needed to recommend routine usage of this therapy. Because of the high risk of septic complications and low probability of attainment of an SVR, patients with Child-Pugh class C, CTP score ≥10 or MELD score 18 disease are not considered appropriate candidates to institute antiviral therapy [63]. The ideal candidate for antiviral therapy remains a patient with Child-Pugh class A disease in whom the risk of druginduced side effects is almost identical to that of the controls [51]. Whether or not to institute antiviral therapy in Child-Pugh class B patients should be individualized on case-to-case basis giving due consideration to factors like genotype & pre-treatment viral loads with antiviral therapy discontinued after 4 or 12 weeks if there is no virological response [63]. Standard schedules of treatment may be considered in all patients with genotype 2 and 3 HCV infection; in genotype 1 cases, however, the riskbenefit ratio still needs to be defined. All cirrhotic patients on antiviral therapy need adjustment of the dosage schedule in accordance with the tolerability of the patient, especially in response to the development of haematologic side effects [64]. Additionally, norfloxacin prophylaxis has been shown to substantially reduce the risk of superadded infections. One thing that has increasing become clear from the existing trials date is that cirrhotic patients who achieve SVR are less likely to develop liver-related complications as compared to the non-responders [63]. Despite of the many encouraging studies in the recent past, however, data on the long-term disease progression, avoidance of transplantation, and most importantly, improvement of life expectancy is still sparse. Although liver functions have clearly been shown to improve with antiviral therapy (as indicated by significant reductions in CTP and MELD scores), the same are more likely to deteriorate within a few years in patients with advanced cirrhosis thus explaining the need to accumulate data on the survival benefit conferred by antiviral therapy in cirrhotic patients.
2.9. Vertical Transmission of HCV Infection
Vertical transmission primarily occurs in women with demonstrable HCV viremia during pregnancy or delivery (<10% risk) [65]. Transmission in non-viremic mothers is unusual. Similarly, the lower the level of viremia, the lesser is the risk of vertical transmission and vice versa. For example, many studies have demonstrated that a viral titer of <1 × 105 copies per mL is associated with a much lower risk of vertical transmission [66-68]. Although HIV coinfection appears to be the single most important cofactor associated with almost fourfold increased risk of vertical transmission, [5] the increased risk at least in part may be explained by higher levels of viremia in coinfected cases [26]. In fact the increased risk of vertical transmission in HIV coinfected cases may possibly be abrogated after adjusting for viral titres. Also, antiretroviral therapy against HIV infection possibly reduces the risk of vertical transmission [30]. Although more validating studies are needed, at least one study [27] demonstrated increased risk (~40% - 50%) of vertical transmission in genotype 3 cases compared to genotype 1—a finding that may have important implications in the development of Pakistani guidelines (screen women of child bearing age and offer them antiviral therapy if found HCV infected before they marry/attempt to conceive!). Certain other studies, however, have failed to find an association between a given genotype and an increased risk of vertical transmission [24,26]. Doctors working in high HCV seroprevalence countries (like Pakistan) are recommended to conduct some statistically significant studies on this issue.
All pregnant women in Pakistan should undergo routine testing for HCV (this recommendation, however, is not valid for most of the western countries!). Because of the risk of teratogenicity both interferon alpha and ribavirin are absolutely contraindicated in pregnancy [47]. Since, vertical transmission usually occurs at the time of birth, in order to reduce its risk, most pediatricians recommend going for delivery within 6 hours of membrane rupture in HCV infected mothers and avoidance of the use of invasive procedures on the fetus such as fetal blood sampling or internal fetal (fetal scalp) monitoring [69,70]. No measure, however, fully prevents vertical transmission [71,72]. Current evidence has not proved cesarean section to be a useful way of reducing the transmission risk. Thus most authorities in this field do not advise to have cesarean sections, other than for the usual obstetric indications [73]. Probably, the only valid exception to this recommendation are the HIV/HCV coinfected cases in whom the risk of vertical transmission appears to reduce if elective cesarean sections are done before membrane rupture [65,74]. Infant’s exposure to infected blood is more likely during vaginal delivery compared to cesarean section; [75] thus the need to go for as bloodless a cesarean section as possible in coinfected cases. In HCV mono-infection cases, however, cesarean section has not been proven to be protective [29].
Although HCV-RNA is detectable in maternal colostrum and breast milk, [76] the risk of HCV transmission via breast feeding has not been proven [77,78]. Probably, this is because of very low levels of HCV-RNA in breast milk, and its inactivation by gastric HCl. Thus both the American Academy of Pediatrics [79] and American College of Obstetricians and Gynecologists [74] support breastfeeding by HCV-infected mothers. The only exceptions are the HCV/HIV coinfected cases in which breast feeding should be avoided. If nipples are cracked or bleeding, breast feeding should be withheld temporarily [79].
Following observations have been made in different papers studying the effects of HCV infection on the mother/fetus:
1) Effects on mother: High ALT levels at the beginning of pregnancy may actually normalize in a significant proportion of pregnant ladies by 3rd trimester (probably due to the effects of pregnancy on the immune system) and rise again within a few months after delivery. Paradoxically, HCV RNA titre may actually rise during the 3rd trimester. Although Histologic Activity Index may show transient histological deterioration during pregnancy, [80] studies have shown pregnancy to be associated with improvement in long-term progression of fibrosis [81]. Increased risk of gestational diabetes (OR 2.5; 95% CI 1.0 - 6.0) especially if mother is overweight has also been suggested.
2) Effects on fetus (other than vertical transmission of HCV infection): Increased risk of low birth weight (OR 2.2; 95% CI 1.2 - 3.8), small for gestational age (OR 1.5; 95% CI 1.0 - 2.1), need for assisted ventilation (OR 2.4; 95% CI 1.4 - 3.9) or neonatal intensive care (OR 2.9, 95% CI 1.9 - 4.6).
More studies are needed to understand the significance of the above-mentioned observations.
2.10. Horizontal Transmission of HCV Infection
Unless blood is transmitted somehow, horizontal transmission from child to child within households, school, or daycare settings does occur and thus HCV infected children can intermingle with other children as otherwise [79,82]. In order to avoid the possibility of blood transmission, sharing of razors, toothbrushes, nail clippers, or other objects that may be contaminated with blood is highly discouraged. Since the risk of transmission through saliva appears to be minimal, avoiding sharing eating utensils, drinking glasses, towels or other potential sources of saliva transmission is not required [83]. Adolescents should be educated, encouraged and monitored to avoid high-risk behaviors including sex with multiple partners, body piercing, tattooing, IV drug abuse, intranasal cocaine etc. In adolescents who have already acquired HCV infection, measures should be taken to minimize disease progresssion. These include treating with antiviral therapy, avoiding alcohol consumption, checking hepatitis A and hepatitis B status and immunizing accordingly if required. NSAID’s should better be avoided; if required Paracetamol can be used as an analgesic agent although it’s recommended should never be exceeded.
3. CONCLUSION
A large-scale, population-based seroprevalence survey is the need of the hour in order to reliably estimate the true disease burden of hepatitis C in Pakistan. The study design should take into account the risk factors particularly strong in our population (e.g. unscreened blood transfusion). A well streamlined surveillance, data collection and reporting process should also be developed at national level in order to determine the true incidence of new cases. Diagnostic work-up (serology by 3rd generation EIA/ELIZA [not if <1 year of age] followed by qualitative HCV RNA assay) of children suspected of having chronic HCV should proceed similar to that of adults. Liver biopsy may be considered in HCV-positive children with persistently normal aminotransferase levels. Because of the potential interferon-induced neurotoxicity, antiviral therapy is contraindicated in children <3 years of age. Infected children aged 3 - 17 who are selected for treatment may receive therapy with pegylated interferon alfa-2b 60 micrograms/m2 subcutaneously once weekly & ribavirin 15 mg/kg/day in two divided doses orally. Peginterferon-alfa-2a in a dose of 180 micrograms/1.73 m2 subcutaneously once weekly can also be used in combination with ribavirin in children ≥5 years old. Nonpegylated interferon yields inferior therapeutic response in terms of ETR & SVR rates achieved, nonetheless, if chosen for affordability reasons the recommended dose regimen is interferon alfa-2b 3 MU/m2 subcutaneously three times a week & ribavirin 15 mg/kg/day in two divided doses orally for 24 and 48 weeks in genotypes 2&3 and 1, respectively. In non-responding patients/relapsers, peginterferon-ribavirin combination therapy may be prescribed regardless of the genotype provided the same was not given beforehand. Cirrhotic patients with a CTP score ≤ 9 and a decompensated event that abated with common management may be considered for antiviral therapy, although more data in pediatric age group is needed to recommend routine usage of this therapy. Despite of promising results in adults, the use of haematopoietic growth factors (erythropoietin & filgrastim) as adjuncts in the management of HVC infection in children is not recommended at this moment.
REFERENCES
- Hamid, S., Umar, M., Alam, A., Siddiqui, A., Qureshi, H., Butt, J., et al. (2004) PSG consensus statement on the management of hepatitis C virus infection-2003. Journal of the Pakistan Medical Association, 54, 146-150.
- Alter, M.J. (1997) Epidemiology of hepatitis C. Hepatology, 26, 62S. doi:10.1002/hep.510260711
- Jonas, M.M. (2002) Children with hepatitis C. Hepatology, 36, S173-S178. doi:10.1002/hep.1840360722
- Davison, S.M. and Kelly, D.A. (2008) Management strategies for hepatitis C virus infection in children. Pediatric Drugs, 10, 357-365. doi:10.2165/0148581-200810060-00003
- Yeung, L.T., King, S.M. and Roberts, E.A. (2001) Mother-to-infant transmission of hepatitis C virus. Hepatology, 34, 223. doi:10.1053/jhep.2001.25885
- Bortolotti, F., Resti, M., Giacchino, R., Crivellaro, C., Zancan, L., Azzari, C., et al. (1998) Changing epidemicologic pattern of chronic hepatitis C virus infection in Italian children. Journal of Pediatrics, 133, 378-381. doi:10.1016/S0022-3476(98)70273-2
- Nelson, S.P. and Jonas, M.M. (1996) Hepatitis C infection in children who received extracorporeal membrane oxygenation. Journal of Pediatric Surgery, 31, 644. doi:10.1016/S0022-3468(96)90665-X
- Ni, Y.H., Chang, M.H., Lue, H.C., et al. (1994) Posttransfusion hepatitis C virus infection in children. Journal of Pediatrics, 124, 709. doi:10.1016/S0022-3476(05)81359-9
- Bortolotti, F., Verucchi, G., Cammà, C., Cabibbo, G., Zancan, L., Indolfi, G., et al. (2008) Long-term course of chronic hepatitis C in children: From viral clearance to end-stage liver disease. Gastroenterology, 13, 1900-1907. doi:10.1053/j.gastro.2008.02.082
- Hsu, S.C., Chang, M.H., Chen, D.S., Hsu, H.C. and Lee, C.Y. (1991) Non-A, non-B hepatitis in children: A clinical, histologic, and serologic study. Journal of Medical Virology, 35, 1-6. doi:10.1002/jmv.1890350102
- Bortolotti, F., Vajro, P., Cadrobbi, P., Lepore, L., Zancan, L., Barbera, C., et al. (1992) Cryptogenic chronic liver disease and hepatitis C virus infection in children. Journal of Hepatology, 15, 73-76. doi:10.1016/0168-8278(92)90014-G
- Tovo, P.A., Pembrey, L.J. and Newell, M.L. (2000) Persistence rate and progression of vertically acquired heaptitis C infection. European paediatric hepatitis C virus infection. Journal of Infectious Diseases, 181, 419.
- Gholson, C.F., Morgan, K., Catinis, G., Favrot, D., Taylor, B., Gonzalez, E. and Balart, L. (1997) Chronic heaptitis C with normal aminotransferase levels: A clinical histologic study. The American Journal of Gastroenterology, 92, 1788-1792.
- Martinot-Peignoux, M., Boyer, N., Cazals-Hatem, D., Pham, B.N., Gervais, A., Le Breton, V., Levy, S., et al. (2001) Prospective study on anti-hepatitis C virus positive patients with persistently normal serum alanine transaminase with or without detectable serum hepatitis C virus RNA. Hepatology, 34, 1000-1005. doi:10.1053/jhep.2001.28458
- Pradat, P., Alberti, A., Poynard, T., Esteban, J.I., Weiland, O., Marcellin, P., Badalamenti, S., et al. (2002) Predictive value of ALT levels for histologic findings in chronic hepatitis C: A European collaborative study. Hepatology, 36, 973-977.
- Bortolotti, F., Jara, P., Diaz, C., Vajro, P., Hierro, L., Giacchino, R., et al. (1994) Post transfusion and community-acquired hepatitis C in childhood. Journal of Pediatric Gastroenterology and Nutrition, 18, 279-283. doi:10.1097/00005176-199404000-00005
- Casiraghi, M.A., De Paschale, M., Romanò, L., et al. (2004) Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology, 39, 90. doi:10.1002/hep.20030
- Bortolotti, F., Resti, M., Giacchino, R., Azzari, C., Gussetti, N., Crivellaro, C., et al. (1997) Hepatitis C virus infection and related liver disease in children of mothers with antibodies to the virus. Journal of Pediatrics, 130, 990-993. doi:10.1016/S0022-3476(97)70289-0
- Kage, M., Fujisawa, T., Shiraki, K., Tanaka, T., Kimura, A., Shimamatsu, K., et al. (1997) Pathology of chronic hepatitis C in children. Child Liver Study Group of Japan. Hepatology, 26, 771-775. doi:10.1002/hep.510260333
- Badizadegan, K., Jonas, M.M., Ott, M.J., Nelson, S.P. and Perez-Atayde, A.R. (1998) Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology, 28, 1416-1423. doi:10.1002/hep.510280534
- Guido, M., Rugge, M., Jara, P., Hierro, L., Giacchino, R., Larrauri, J., et al. (1998) Chronic hepatitis C in children: the pathological and clinical spectrum. Gastroenterology, 115, 1525-1529. doi:10.1016/S0016-5085(98)70032-0
- European Paediatric Hepatitis C Virus Network (2005) Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clinical Infectious Diseases, 41, 45. doi:10.1086/430601
- Jonas, M.M., Baron, M.J., Bresee, J.S. and Schneider, L.C. (1996) Clinical and virologic features of hepatitis C virus infection associated with intravenous immunoglobulin. Pediatrics, 98, 211.
- Kuroki, T., Nishiguchi, S., Fukuda, K., et al. (1993) Vertical transmission of hepatitis C virus (HCV) detected by HCV-RNA analysis. Gut, 34, S52. doi:10.1136/gut.34.2_Suppl.S52
- Matsubara, T., Sumazaki, R. and Takita, H. (1995) Mother-to-infant transmission of hepatitis C virus: A prospective study. European Journal of Pediatrics, 154, 973. doi:10.1007/BF01958640
- Sabatino, G., Ramenghi, L.A., Di Marzio, M. and Pizzigallo, E. (1996) Vertical transmission of hepatitis C virus: An epidemiological study on 2,980 pregnant women in Italy. European Journal of Epidemiology, 12, 443. doi:10.1007/BF00143994
- Mazza, C., Ravaggi, A., Rodella, A., et al. (1998) Prospective study of mother-to-infant transmission of hepatitis C virus (HCV) infection. Study Group for Vertical Transmission. Journal of Medical Virology, 54, 12. doi:10.1002/(SICI)1096-9071(199801)54:1<12::AID-JMV3>3.0.CO;2-S
- Giacchino, R., Tasso, L., Timitilli, A., et al. (1998) Vertical transmission of hepatitis C virus infection: Usefulness of viremia detection in HIV-seronegative hepatitis C virus-seropositive mothers. Journal of Pediatrics, 132, 167. doi:10.1016/S0022-3476(98)70507-4
- Manzini, P., Saracco, G., Cerchier, A., et al., Human immunodeficiency virus infection as risk factor for motherto-child hepatitis C virus transmission (1995) persistence of anti-hepatitis C virus in children is associated with the mother’s anti-hepatitis C virus immunoblotting pattern. Hepatology, 21, 328. doi:10.1016/0270-9139(95)90088-8
- Paccagnini, S., Principi, N., Massironi, E., et al. (1995) Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. The Pediatric Infectious Disease Journal, 14, 195. doi:10.1097/00006454-199503000-00005
- Sherman, M., Shafran, S., Burak, K., Doucette, K., Wong, W., Girgrah, N., et al. (2007) Management of chronic hepatitis C: Consensus guidelines. Canadian Journal of Gastroenterology, 21, 25C-34C.
- Fazal, A.D. (2008) Current Standards in the Pharmacotherapy of chronic hepatitis C and local practices. Infectious Diseases Journal, 17, 93-97.
- Conte, D., Fraquelli, M., Prati, D., et al. (2000) Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology, 31, 751. doi:10.1002/hep.510310328
- England, K., Pembrey, L., Tovo, P.A., Newell, M.L., European Paediatric HCV Network (2005) Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatrica, 94, 444. doi:10.1111/j.1651-2227.2005.tb01916.x
- American Academy of Pediatrics, Committee on Infectious Diseases (1997) 1997 Red book: Report of the committee on infectious diseases. 24th Edition, American Academy of Pediatrics, Elk Grove Village, 263.
- Cagle, H.H., Jacob, J., Homan, C.E., et al. (2007) Results of a general hepatitis C lookback program for persons who received blood transfusions in a neonatal intensive care unit between January 1975 and July 1992. Archives of Pediatrics & Adolescent Medicine, 161, 125. doi:10.1001/archpedi.161.2.125
- Jonas, M.M. (2007) Finding adolescents and young adults with transfusion-associated hepatitis C: Looking forward to looking back. Archives of Pediatrics & Adolescent Medicine, 161, 202. doi:10.1001/archpedi.161.2.202
- Goodman, Z.D, Makhlouf, H.R., Liu, L., et al. (2008) Pathology of chronic hepatitis C in children: Liver biopsy findings in the Peds-C Trial. Hepatology, 47, 836. doi:10.1002/hep.22094
- Bedossa, P. and Poynard T. (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology, 24, 289-293. doi:10.1002/hep.510240201
- Ishak, K., Baptista, A., Bianchi, L., Callea, F., De Groote, J., Gudat, F., et al. (1995) Histological grading and staging of chronic hepatitis. Journal of Hepatology, 22, 696- 699. doi:10.1016/0168-8278(95)80226-6
- Marcellin, P., Asselah, T. and Boyer, N. (2002) Fibrosis and disease progression in hepatitis C. Hepatology, 36, S47-S56. doi:10.1002/hep.1840360707
- Strader, D.B., Wright, T., Thomas, D.L. and Seeff, L.B. (2004) Diagnosis, management, and treatment of hepatitis C. Hepatology, 39, 1147-1171. doi:10.1002/hep.20119
- Gonzalez-Peralta, R., Haber, B.A., Jonas, M., Martin, S., Lang, T., Geffner, M., et al. (2002) Interferon-alfa 2b in combination with ribavirin for the treatment of chronic hepatitis C in children [Abstract]. Hepatology, 36, 311A.
- González-Peralta, R.P., Kelly, D.A., Haber, B., Molleston, J., Murray, K.F., Jonas, M.M., et al. (2005) International Pediatric Hepatitis C Therapy Group. Interferon alpha-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: Efficacy, safety, and pharmacokinetics. Hepatology, 42, 1010-1018.
- Jacobsen, I.M., Ahmed, F., Russo, M.W., Brown, R.S., Lebovics, E., Min, A., et al. (2003) Pegylated interferon alfa-2b plus ribavirin in patients with chronic hepatitis C: A trial in non-responders to interferon monotherapy or combination therapy and in combination therapy relapsers: Final results. Gastroenterology, 124, A540.
- Palumbo, E. (2009) Treatment for chronic hepatitis C in children: A review. American Journal of Therapeutics, 16, 446-450. doi:10.1097/MJT.0b013e31818a88c5
- Ghany, M.G., Strader, D.B., Thomas, D.L. and Seeff, L.B., American Association for the Study of LIver Diseases (2009) Diagnosis, management, and treatment of hepatitis C: An update. Hepatology, 49, 1335. doi:10.1002/hep.22759
- Wirth, S., Ribes-Koninckx, C., Calzado, M.A., et al. (2010) High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. Journal of Hepatology, 52, 501. doi:10.1016/j.jhep.2010.01.016
- Jara, P., Hierro, L., de la Vega, A., Díaz, C., Camarena, C., Frauca, E., et al. (2008) Efficacy and safety of peginterferon-alpha2b and ribavirin combination therapy in children with chronic hepatitis C infection. The Pediatric Infectious Disease Journal, 27, 142-148.
- Schwarz, K.B., Gonzalez-Peralta, R.P., Murray, K.F., et al. (2008) Peginterferon with or without ribavirin for chronic hepatitis C in children and adolescents: Final results of the PEDS-C trial. Hepatology, 48, 418A.
- Fazal, A.D., Salman, S.K., Fazal, R.S., Ahmed, E.R. and Saeeda, Y. (2009) Diagnosis and treatment of HCV infection in children: Suggested recommendations for the development of local guidelines. Journal of Pakistan Paediatric Association, 33, 203-210
- Danish, F.A., Koul, S.S., Subhani, F.R., Rabbani, A.E. and Yasmin, S. (2010) Managing HCV infection in paediatric age group: Suggested recommendations. Saudi Journal of Gastroenterology, 16, 230-235. doi:10.4103/1319-3767.65182
- Comanor, L., Minor, J., Conjeevaram, H.S., Roberts, E.A., Alvarez, F., Bern, E.M., et al. (2001) Impact of chronic hepatitis B and interferon-alpha therapy on growth of children. Journal of Viral Hepatitis, 8, 139-147. doi:10.1046/j.1365-2893.2001.00262.x
- Gottrand, F., Michaud, L., Guimber, D., Ategbo, S., Dubar, G., Turck, D., et al. (1996) Influence of recombinant interferon alpha on nutritional 1168 STRADER et al. Hepatology, April 2004 status and growth pattern in children with chronic viral hepatitis. European Journal of Pediatrics, 155, 1031-1034. doi:10.1007/BF02532525
- (2009) Prescribing information for pegintron® package insert.
- Delgado-Borrego, A., Healey, D., Negre, B., et al. (2010) Influence of body mass index on outcome of paediatric chronic hepatitis C virus infection. Journal of Pediatric Gastroenterology and Nutrition, 51, 191. doi:10.1097/MPG.0b013e3181d32756
- Bunn, S., Kelly, D., Murray, K.F., Shelton, M., Olson, A., Mieli-Vergani, G., et al. (2000) Safety, efficacy and pharmacokinetics of interferonalfa-2b and ribavirin in children with chronic hepatitis C [Abstract]. Hepatology, 32, 350A.
- Maddrey, W.C. (1999) Safety of combination interferon alfa-2b/RBV therapy in chronic hepatitis C-relapsed and treatment-naive patients. Seminars in Liver Disease, 19, 67-75.
- Mirza, R., Memon, I., Cheema, H.U., Ali, S., Thobani, S. and Shah, U. (2007) Recommendations for the treatment of chronic Hepatitis C infection in children. Journal Pakistan Medical Association, 57, 419-420
- [61] Danish, F.A., Koul, S.S., Subhani, F.R., Rabbani, A.E. and Yasmin, S. (2008) Role of haematopoietic growth factors as adjuncts in the treatment of chronic hepatitis C patients. Saudi Journal of Gastroenterology, 14, 151-157. doi:10.4103/1319-3767.41739
- [62] Iacobellis, A., Siciliano, M., Perri, F., Annicchiarico, B.E., Leandro, G., Caruso, N., et al. (2007) Peginterferon alfa- 2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: A controlled study. Journal of Hepatology, 46, 206-212. doi:10.1016/j.jhep.2006.08.020
- [63] Tekin, F., Gunsar, F., Karasu, Z., Akarca, U. and Ersoz, G. (2008) Safety, tolerability, and efficacy of pegylatedinterferon alfa-2a plus ribavirin in HCV-related decompensated cirrhotics. Alimentary Pharmacology & Therapeutics, 27, 1081-1085. doi:10.1111/j.1365-2036.2008.03680.x
- [64] Danish, F.A., Koul, S.S., Subhani, F.R., Rabbani, A.E. and Yasmin, S. (2010) Antiviral therapy in HCV-infected decompensated cirrhotics. Saudi Journal of Gastroenterology, 16, 310-314. doi:10.4103/1319-3767.70632
- [65] Danish, F.A., Koul, S.S., Subhani, F.R., Rabbani, A.E. and Yasmin, S. (2010) Considerations in the management of hepatitis C virus-related thrombocytopenia with eltrombopag. Saudi Journal of Gastroenterology, 16, 51-56. doi:10.4103/1319-3767.58772
- [66] Thomas, S.L., Newell, M.L., Peckham, C.S., et al. (1998) A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. International Journal of Epidemiology, 27, 108. doi:10.1093/ije/27.1.108
- [67] Zanetti, A.R., Tanzi, E., Romanò, L., et al. (1998) A prospective study on mother-to-infant transmission of heaptitis C virus. Intervirology, 41, 208. doi:10.1159/000024938
- [68] Terrault, N. (1998) Epidemiological evidence for perinatal transmission of hepatitis C virus. Viral Hepatitis Reviews, 4, 245.
- [69] Moriya, T., Sasaki, F., Mizui, M., et al. (1995) Transmission of hepatitis C virus from mothers to infants: Its frequency and risk factors revisited. Biomedicine & Pharmacotherapy, 49, 59. doi:10.1016/0753-3322(96)82587-X
- [70] Azzari, C., Resti, M., Moriondo, M., et al. (2000) Vertical transmission of HCV is related to maternal peripheral blood mononuclear cell infection. Blood, 96, 2045.
- [71] Mast, E.E., Hwang, L.Y., Seto, D.S., et al. (2005) Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. Journal of Infectious Diseases, 192, 1880. doi:10.1086/497701
- [72] Centers for Disease Control and Prevention (1998) Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Morbidity and Mortality Weekly Report, 47, 1-39.
- [73] Mast, E.E. and Alter, M.J. (1997) Hepatitis C. Seminars in Pediatric Infectious Diseases, 8, 17-22. doi:10.1016/S1045-1870(97)80005-2
- [74] McIntyre, P.G., Tosh, K. and McGuire, W. (2006) Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database of Systematic Reviews, 18, Article ID: CD005546.
- [75] ACOG Committee Opinion (1999) Breastfeeding and the risk of hepatitis C virus transmission. Number 220, August 1999. Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. International Journal of Gynecology & Obstetrics, 66, 307.
- [76] Zanetti, A.R., Tanzi, E., Paccagnini, S., et al. (1995) Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet, 345, 289. doi:10.1016/S0140-6736(95)90277-5
- [77] Lin, H.H., Kao, J.H., Hsu, H.Y., et al. (1995) Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. Journal of Pediatrics, 126, 589. doi:10.1016/S0022-3476(95)70356-X
- [78] Polywka, S., Schröter, M., Feucht, H.H., et al. (1999) Low risk of vertical transmission of hepatitis C virus by breast milk. Clinical Infectious Diseases, 29, 1327. doi:10.1086/313473
- [79] Kumar, R.M. and Shahul, S. (1998) Role of breast-feeding in transmission of hepatitis C virus to infants of HCVinfected mothers. Journal of Hepatology, 29, 191. doi:10.1016/S0168-8278(98)80003-2
- [80] American Academy of Pediatrics, Committee on Infectious Diseases (2009) Hepatitis C. In: Pickering, L.K., Ed., Red Book: 2009 Report of the Committee on Infectious Diseases, 28th Edition, American Academy of Pediatrics, Elk Grove Village, 357.
- [81] Fontaine, H., Nalpas, B., Carnot, F., et al. (2000) Effect of pregnancy on chronic hepatitis C: A case-control study. Lancet, 356, 1328. doi:10.1016/S0140-6736(00)02823-3
- [82] Di Martino, V., Lebray, P., Myers, R.P., et al. (2004) Progression of liver fibrosis in women infected with hepatitis C: Long-term benefit of estrogen exposure. Hepatology, 40, 1426. doi:10.1002/hep.20463
- [83] Vegnente, A., Iorio, R., Saviano, A., et al. (1994) Lack of intrafamilial transmission of hepatitis C virus in family members of children with chronic hepatitis c infection. The Pediatric Infectious Disease Journal, 13, 886-889.
- [84] Suzuki, T., Omata, K., Satoh, T., et al. (2005) Quantitative detection of hepatitis C virus (HCV) RNA in saliva and gingival crevicular fluid of HCV-infected patients. Journal of Clinical Microbiology, 43, 4413-4417.
- [85] Buti, M., Valdes, A., Sanchez-Avila, F., Esteban, R. and Lurie, Y. (2003) Extending combination therapy with peginterferon-alpha-2b plus ribavirin for genotype 1 chronic hepatitis C late responders: A report of 9 cases. Hepatology, 37, 1226-1227. doi:10.1053/jhep.2003.50107
- [86] Berg, T., von Wegner, M., Nasser, S., et al. (2006) Extended treatment duration for hepatitis C virus type 1: Comparing 48 versus 72 of peginterferon-alpha-2a plus ribavirin. Gastroenterology, 130, 1086-1097. doi:10.1053/j.gastro.2006.02.015
- [87] Ferencie, P., Lafen, H., Scherzer, T.M., et al. (2006) Customizing treatment with peginterferon-alpha-2A (40KD) (Pegasys[R]) plus ribavirin in patients with HCV genotype 1 or 4 infection. Hepatology, 44, 336A.

