Open Journal of Forestry
Vol.05 No.07(2015), Article ID:60128,11 pages
10.4236/ojf.2015.57063
Responses of Native Tree Species to Soil Water Stress in a Tropical Forest on Limestone, Vietnam
Le Van Binh1,2*, Nguyen Van Thinh1,2, Reinhard Köpp1, Vo Dai Hai2, Ralph Mitlöhner1
1Department of Tropical Silviculture and Forest Ecology, Georg-August-Universität Göttingen, Göttingen, Germany
2Vietnamese Academy of Forest Sciences, Ha Noi, Vietnam
Email: *binhfsiv@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 9 July 2015; accepted 27 September 2015; published 30 September 2015
ABSTRACT
Forests over limestone in the tropics have received little attention and limestone forests in Vietnam have been overlooked to an even greater extent in terms of tree physiology. In Ba Be National Park, Vietnam, soil water availability in limestone forests seems to be the most limiting factor in the dry season. Therefore, in order to enhance the preliminary knowledge of choosing native tree species for enrichment planting in the restoration zone, characteristics of the 20 native tree species to soil water stress were investigated in a limestone forest. One-ha plot each consisting of twenty-five 20 m × 20 m plots was established in undisturbed forests. All trees ≥ 10 cm DBH were measured in 20 m × 20 m plots, while twenty-five 5 m × 5 m subplots were established in order to sample the regeneration of tree species with a DBH < 10 cm. The Scholander apparatus and freezing point osmometry were used in order to measure the leaf water potential (Ψw) and leaf osmotic potential (Ψπ) of the 20 native tree species, respectively in this study. 61 species belonging to 34 families of all trees with a DBH ≥ 10 cm were recorded in one ha, while 31 species representing 18 families of trees < 10 cm DBH were identified in 625 m2. The 20 species’ leaf water and osmotic potential values revealed significant differences among species. The maximum leaf water potential was not affected by any anticipated sources of variation, while the minimum water potential, however, showed significant variation to soil water stress. The results in the study area emphasized the importance of water factors in influencing tree species distribution; it could be concluded that native species with wide water potential ranges would be better able to withstand water changes and might be thus good candidates for reforestation (enrichment planting) in limestone areas.
Keywords:
Tropical Limestone Forests, Floristic Composition, Native Tree Species, Leaf Water Potential, Leaf Osmotic Potential

1. Introduction
Tropical limestone forests and karsts often contain high plant diversity combined with a large degree of endemism (Vermeulen & Whitten, 1999, Clements et al., 2006) . Vietnam’s total natural area is approximately 330,000 km2, and three-quarters of Vietnam is composed of hills and mountains originally covered by tropical forests (Trung, 1998) . Vegetation on limestone is one of the most diverse ecological systems classified in Vietnam; in this case, limestone mountains make up about 18% Vietnam’s area (Qin et al., 2012) . The studied forest grows on a limestone substrate estimated to be about 1,150,000 ha (FIPI, 1995) where botanical scientists have discovered many animal species and rare plants (Dung, 2001) . Limestone karst ecosystems in Vietnam are important reservoirs for biodiversity but are currently experiencing forest degradation due to land use changes and increasing pressure from the extractive and tourism industries (Furey et al., 2010) . Therefore, Vietnam’s forests have severely declined in both in quantity and quality (Dang et al., 2001) ; many species in Vietnam are therefore at risk for extinction (Nghia, 2005) , and 150 are listed as either vulnerable or critically endangered (IUCN, 2006) . Vietnam’s conservationists feel that these remaining forest blocks, though hard to reach, are threatened by the local population’s high demand for fuelwood (Dzung et al., 2004) , particularly in the mountainous areas. In contrast with other natural systems, karst ecosystems cannot be reestablished once damaged (Tuyet, 2001) and recent studies have indicated that their biodiversity goes largely unknown in the face of ongoing forest degradation.
The terrestrial vegetation of Ba Be National Park is dominated by a tropical forest on limestone (Whitmore, 1984) that is under severe pressure (Dzung et al., 2004) . A total of 638 vascular plants belonging to 450 genera and 140 families were recorded in the park. The flora includes a large number of broad-leaved species, while coniferous species are rare and only found in high mountain areas; many of the fore species are rare and have high economic value (e.g., Burretiodendron tonkinense, Chukrasia tabularis, Garcinia fagraeoides, Fokienia hodginsii, and Nageia fleuryi) (Chan, 2006) . 69 of the total tree species identified in the park were endangered and listed in the Red Data Book of Vietnam (Anon, 1996) ; of these, 24 were found in the red list of the IUCN in 1997 (Anonymous, 2012) . In the park, soil water availability in limestone forests seems to be the most limiting factor in the dry season but up until now, studies and the understanding concerning these forest’s responses to environmental stressors such as water and light have not been carried out and information about choosing site condition for planting native tree species in the restoration zone has not been available yet. Thus, in the present study the basic information on the floristic composition of an undisturbed tropical forest over limestone was provided firstly. Our second objective was to examine responses of 20 native tree species to water factors in the dry season and we predicted that differences in species’ responses to water availability would be reflected in the distribution of the species relative to the soil water availability. This study will provide data and preliminary knowledge for predicting the responses of tree species in forests on limestone and will have further silviculture implications on their distribution in case of choosing site conditions for enrichment planting in restoration zones of the park.
2. Materials and Methods
2.1. Study Site
The study was carried out in Ba Be National Park (from 22˚21' to 22˚29'N and from 105˚34' to 105˚42'E), located in Northern Vietnam. The park was established as a protected area in 1977 with a total area of approximately 10,000 ha. At its center is Ba Be Lake, which, with an area of 450 ha, is the largest natural freshwater mountain lake in all of Vietnam. The local geology and terrain are predominantly limestone under tall mountains with numerous rugged peaks and deep, steep-sided river valleys (Anonymous, 2012) . Ba Be National Park is characterized by a tropical monsoon climate; its average annual rainfall is 1364 mm, while the average temperature is 22.5˚C. Two main seasons are recorded in the study site; the rainy season lasts from April to September, and nearly 75% of the annual rainfall is observed during this period; the dry season is from October to March. The park’s soil is developed on sedimentary rock of silt and clay and is mainly covered by yellow-red ferralit, the layers of which range from thin to thick. Three main soil types can be found here: 1) humicacrisols, 2) ferralit alisols, and 3) yellow-red ferralsols with medium to thick soil horizons formed over the shale rock (MARD, 2004; Anonymous, 2012) (Figure 1).
2.2. Sampling Procedure, Data Collection and Laboratory Experiments
25 sample plots 400 m2 (20 m × 20 m) in size were set systematically with a random starting point in an undisturbed forest (Figure 2). Sub-plot of 5 m × 5 m (Veríssimo et al., 1992) was set up at the center of each 20 × 20 m sample plot to investigate all trees with a DBH < 10 cm (Figure 3). All trees with a DBH ≥ 10 cm were measured, and their scientific name and DBH recorded in twenty-five 400 m2. Tree species were identified in the field with specialists’ help. In the case of unidentified specimens, leaves and fruits were taken and identified by a botanist at the Vietnamese Academy of Forest Sciences. All trees (regeneration) with a DBH < 10 cm were sampled in twenty-five compartment B.
Field measurements of the leaf water and osmotic potentials were carried out during the middle of the dry season (in December 2013). The 20 most dominant native tree species based on the importance value index (IVI) were collected in order to measure water potential. Leaf samples were collected from six randomly selected trees per species. Fully developed leaves from the branches on each tree’s southward exposure were used to measure leaf water potential at midday (12-14:30 p.m.) and predawn (3:30-6 a.m.) (Mitlöhner, 1997a; Mitlöhner et al., 1997b) . A pressure chamber (Scholander Apparatus) was used, as it has become one of the most widely used field methods for determining total plant water potential.
In these measurements, a fully expanded leaf exposed to direct sunlight was first chosen for measurement. Leaf samples measured for leaf water potentials from the same six individuals per species were collected in or-
Figure 1. Map showing the study site in Ba Be National Park, Vietnam.
Figure 2. Layout of the sampling design in which the size of each sample plot was 400 m2 (20 × 20 m). The interval sample plot was 50 m.
Figure 3. Compartment B (5 × 5 m) was set up at the center of each 400 m2 sample plot to collect all trees with a DBH < 10 cm.
der to calculate the leaf osmotic potential. For midday leaf osmotic potential measurements, 5 - 10 g of fresh leaves were weighed using a balance under field conditions. After balancing the weights, the leaf samples were dried in a stainless steel pan over a gas stove, a vitally important process necessary in order to avoid leaf enzymatic changes. 3 - 5 small branches per species were collected to measure the saturated osmotic potential. The branches absorbed water and re-saturated overnight; the saturated leaf samples were then cut from the branches, balanced, dried under field conditions, labelled, and stored for further analysis. Further processing began with kiln-drying leaf samples in an oven (Memmert, Schwabach, Germany) at 103˚C until mass constancy (~12 h) in the lab at the Georg-August University of Göttingen. Laboratory experiments were performed to measure the plant osmotic potentials cryoscopically using a semi-micro osmometer (Knauer, Germany). For the osmotic potential measurement, each 1 g (≈1 g) of leaf powder was diluted with demineralized water (dilution × 7), stored in a water bath at 55˚C for 24 hours, and centrifuged at 4000 rpm for 15 - 20 minutes to separate the solute and residue. A 0.15 ml solution for each sample was cryoscoped. Results from the freezing points were recalculated using the following equations developed by Kreeb (1990) :
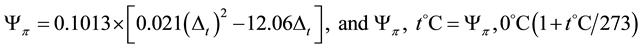
where: Δt = the depression at freezing point; Yp, 0˚C = the osmotic potential at 0˚C, and Yp, t˚C = the osmotic potential at t˚C.
2.3. Data Analysis
Tree abundance per ha was calculated with the count of all trees with a DBH ≥ 10 cm from 25 sample plots of 400 m2; the basal area of the trees was calculated by using the following equation: BA = πr2 = 3.142 × (DBH/200)2, where BA = tree basal area (m2) and r = radius (cm). The total basal area of one ha was calculated by the sum of the BA of all trees ≥ 10 cm DBH in the 25 plots. The species richness was easily taken by counting the number of species occurring in all plots (Rana & Gairola, 2009) . The Shannon-Wiener index as mentioned by Shannon (1948) was used to assess species diversity. The evenness index was determined in accordance with Pielou (1966) . The midday and predawn values of the selected tree species were compared separately in graphs for a particular sampling site. A 45˚ bisecting line approach as detailed by Slatyer (1957) was created on the graph to mark the threshold value of tree species’ water stress. The Kruskal-Wallis test was used to compare the mean values of midday and predawn leaf water potentials among 20 native tree species. The relationships between the leaf water potential at midday and leaf osmotic potential at saturated of these 20 species were analyzed using an individual treatment and via regression analysis using linear. All statistical analyses were performed with Statistica, Version 12.
3. Results and Discussion
3.1. Main Characteristics of Forest Type
One-ha tropical forests over limestone yielded a total of 630 stems with 61 tree species belonging to 34 families all adult trees, while 31 species representing 18 families of regeneration were identified in 625 m2. The Evenness index of the former was 81%, while it was 77% for the latter. The Shannon-Wiener index of trees with a DBH ≥ 10 cm and < 10 cm was 3.33 and 2.65, respectively (Table 1). Tree abundance of this study was in keeping with other studies carried out in limestone forests all over the world such as 644 individuals ha−1 (DBH 10 ≥ cm) were counted in such forests in Gunung Mulu National Park, Malaysia (Proctor et al., 1983) . Our findings were lower than results from limestone forests in the Ayawasi area in Indonesia, where the tree abundance ranged from 960 to 1390 stems・ha−1 (DBH ≥ 10 cm) (Polak, 2000) . However, the tree abundance of the three forest types in this study was higher than in either a tropical forest over limestone (210 - 390 stems ha−1) with a DBH ≥ 10 cm in Visayas, the Philippines (Peque & Hölscher, 2014) or a lowland limestone forest on Seram Island, Indonesia (438 stems ≥ 10 cm DBH) (Ranlund, 2011) . Tree species richness in this study showed similar trends as in the undisturbed stands of a tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas, India (Bhuyan et al., 2003) . Concerning regeneration, each family was represented by 1 - 3 species. Moraceae was had the highest number of abundance; it was followed by Annonaceae and Rutaceae (data not shown in Table 1). Our findings demonstrated similar patterns of population structure as were observed for the regeneration of a dominant family in the limestone forest of three sites in Xuan Son, Cuc Phuong, and Pu Mat national parks in North Vietnam (Toai, 2012) .
3.2. Plant Water Relations of the Native Tree Species
The water potential at predawn/midday values for the 20 native tree species are shown in Table 2 and Figure 4.
Table 1. Main characteristics of undisturbed limestone forest in Ba Be National Park, Vietnam.
Figure 4. Midday and predawn leaf water potentials Yw (MPa) of the 20 native tree species collected in the undisturbed limestone forest in Ba Be National Park, Vietnam.
Table 2. The leaf water potentials of the 20 native tree species collected in the undisturbed limestone forest in Ba Be National Park, Vietnam.
A significant response pattern was observed among these 20 native species at midday and predawn (p < 0.05). Among all species, the lowest water potentials were found at midday but even here significant differences were found among the 20 species, the exceptions being Chukrasia tabularis, Canthium dicoccum, Goniothalamus macrocalyx, Syzygium wightianum, Hydnocarpus ilicifolia, and Garcinia fagraeoides. Diospyros susarticulata had both the lowest midday (−3.1 ± 0.1 MPa) and predawn (−1.7 ± 0.09 MPa) values, followed by Streblus ilicifolius, and Alphonsea philastreana. Streblus macrophyllus, Litsea monopetala, Turpinia Montana, Aglaia gigantean, Sterculia nobilis, and Deutzianthus tonkinensis demonstrated maximum leaf water potentials varying from −0.7 to −1.0 MPa. The paired t-test between the midday and predawn water potentials showed significant differences in night recovery from the midday water stress for all species.
3.3. Plant Osmotic Potentials for the Native Tree Species
Results in relation to the leaf osmotic potential for the 20 native tree species are given in Table 3 and the relationship between the leaf water potential at midday and leaf osmotic potential at saturated is indicated in Figure 5. All species responded to soil water stress and exhibited their lowest osmotic potential values (from −1.4 to −3.3 MPa among species) at midday. Of these 20 species, Syzygium wightianum had the lowest midday (−3.3 MPa) and saturated (−3.1 MPa) osmotic potential values, while Canthium dicoccum, Turpinia Montana, and Deutzianthus tonkinensis had the highest midday (−1.4 MPa) and saturated (−1.3 MPa) osmotic potentials. Litsea monopetala, Sterculia nobilis, Garcinia fagraeoides, and Vitex trifolia responded with relatively high midday and saturated osmotic potential values. Significant differences in the osmotic potentials were found for all species measured at midday (p < 0.05); however, Chukrasia tabularis, Goniothalamus macrocalyx, Diospyros susarticulata, Aglaia gigantean, Burretiodendron hsienmu, and Hydnocarpus ilicifolia showed no significant differences in their midday osmotic values.
Table 3. The leaf osmotic potential of the 20 native tree species collected in the undisturbed limestone forest in Ba Be National Park, Vietnam.
Figure 5. The relationship between the leaf water potential at midday and leaf osmotic potential at saturated of 20 native trees species collected in the undisturbed limestone forest during the dry season, 2013 in Ba Be National Park, Vietnam.
The lowest water and osmotic potential values of all studied species suggest that these species have an advantage in drought -and salinity-affected areas. This finding was in agreement with similar results such as in a riparian forest, Northeast Vietnam (Anh, 2006) ; in the evergreen moist forest of Diepwalle South Africa (Mitlöhner, 1998) ; at a savanna-scrub ecotone, Ethiopia (Gebrekirstos et al., 2014) , and in Central Myanmar (Khaing, 2013) . However, Abrams’ (1988) observations indicated that the leaf osmotic potentials at full turgor of several North American tree species ranged from −1.5 to −3.2 MPa under dry conditions. Scholander et al. (1965) found that the initial osmotic potentials of Avicennia nitida and Laguncularia racemosa in a mangrove forest were approximately −4.5 MPa and −5.3 MPa, respectively. The osmotic potentials recorded in this study were lower than results observed by Mitlöhner et al. (1997b) in Binh Chau Natural Reserve, Vietnam; those authors indicated that the values of the midday osmotic potential of 13 species in evergreen and deciduous forests varied from −0.5 to −2.1 MPa.
The 20 native tree species show their differences in osmotic potential because each tree species reaction in different ways to water stress. No correlation between the predawn leaf water potential and leaf osmotic potential at saturated was found for all native tree species under field conditions, with an exception for Syzygium wightiamum (R2 = 0.85, p < 0.05). The results in this study were accordant with Abrams’ 1990 conclusion that no correlation between predawn water potential and leaf osmotic potential at full tugor of several Oak species under field condition, and Parker et al. (1982) for Q. alba trees species.
Water stress tolerance usually involves the development of low osmotic potentials which characterize many plant species found in more arid environments. When the soil dries, live cells may adjust their water potential by osmotically accumulating active compounds to decrease their osmotic and water potentials (Lambers et al., 2008) . However, low osmotic potential values are indicators of soil salinity associated with drought tolerance (Mitlöhner & Köpp, 2007) . In this study, all 20 tree species demonstrated the lowest osmotic values around midday, when soil water availability was low and solute concentration was assumed to be higher. In actuality, the low osmotic values could be due to either solute concentration (as a result of cell dehydration) or solute accumulation (in response to stress) (Morgan, 1984) . Because osmotic adjustment often takes several days or weeks to develop, the diurnal fluctuations in osmotic potential may be largely due to changes in tissue water content (Abrams, 1988) . The results showed that the decrease in osmotic potential at midday is consistent with the decrease in the leaf water content; however, the diurnal osmotic potential change in this study is minimal, which may indicate that active osmotic adjustment is an important adaptation to drought or salinity (Munns, 1988) . From the results, it is clear that higher leaf osmotic adjustments have higher dry matter and/or grain yield under water-limited conditions (Morgan & Condon, 1986) .
3.4. Species and Site Characterization
The ecological niche preference of specific species is shown in Table 4 as a reference of soil water availability and the solute concentration in the root zone. These species, including Diospyros susarticulata, Burretiodendron hsienmu, Glycosmis cymosa, Homalium myriandrum, Gironniera subaequalis, Alphonsea philastreana, and Hydnocarpus ilicifolia, demonstrated high adaptability to soil water and osmotic stress. Moreover, they had lower osmotic and water potential values than did the other 13 of the 20 studied species. Of these, the first three species in particular are drought-tolerant species that usually grow in soil developed on limestone mountains (Chinh et al., 1996) . These characteristics may explain their distribution in limestone areas where there is significant water stress during the dry season.
Aglaia gigantean, Garcinia fagraeoides, Chukrasia tabularis, Goniothalamus macrocalyx, Syzygium wightianum, Streblus ilicifolius, and Streblus macrophyllus exhibited high predawn leaf water potentials but low leaf osmotic potentials; they may have easy access to water and high solute concentrations and as such were higher in species dominance in the study sites. As evidenced by the highest predawn leaf water and osmotic potential values, Canthium dicoccum, Deutzianthus tonkinensis, Sterculia nobilis, Turpinia montana, Vitex trifolia, and Litsea monopetala preferred to grow on deep and moist soil. The values of their predawn and osmotic potentials did not explain their drought tolerance, but rather, their preference for deep and well-drained soils (Chinh et al., 1996) .
Table 4. The classification of temperament classes based on the species adaptability to soil water deficit (predawn Yw) and osmotic stress (saturated Yp) in limestone forests in Ba Be National Park, Vietnam. “x” means tree species belonging to different groups of soil water deficit (predawn Yw) and osmotic stress (saturated Yp).
4. Conclusion
In Ba Be National Park, soil water availability in limestone forests seems to be the most limiting factor in the dry season. The Scholander apparatus and freezing point osmometry were used in order to measure plant water (Ψw) and osmotic (Ψπ) potentials, respectively in this study. All 20 native tree species’ responses to water soil availability varied among and within them; the maximum leaf water potential was not affected by any anticipated sources of variation; the minimum water potential of indigenous species, however, showed significant variation to soil water stress. The results emphasized the importance of water factors in influencing tree species distribution. The differences in water relations obtained among the 20 native tree species reflected their niche preferences. In terms of silviculture aspect, the results have implications on their distribution in case of choosing site conditions for enrichment planting in restoration zones. Choosing and utilizing the most suitable plant species to grow in the limestone stand and repair severely degraded forests are the primary options for developing and maintaining the limestone forest. Native tree species (including Diospyros susarticulata, Burretiodendron hsienmu, Glycosmis cymosa, Homalium myriandrum, Gironniera subaequalis, Alphonsea philastreana, Streblus ilicifolius, Garcinia fagraeoides and Hydnocarpus ilicifolia) highly adaptable to soil water stress should be taken into account when designing reforestation strategies in Ba Be National Park and may be thus good candidates for reforestation in limestone areas.
Acknowledgements
We acknowledge support by the Open Access Publication Funds of the Göttingen University. We wish to extend special thanks to all members of the management board who allowed us to conduct our field research in Ba Be National Park. We would like profoundly thank the forest rangers, Mr. Nam and Mr. Huynh, who worked with us under difficult conditions in the field. The authors would also like to thank Mr. Trinh Ngoc Bon who identified tree species. Thanks also go out to Dr. Cao Thi Thu Hien who provided us with statistical advice.
Cite this paper
LeVan Binh,Nguyen VanThinh,ReinhardKöpp,Vo DaiHai,RalphMitlöhner, (2015) Responses of Native Tree Species to Soil Water Stress in a Tropical Forest on Limestone, Vietnam. Open Journal of Forestry,05,711-722. doi: 10.4236/ojf.2015.57063
References
- 1. Abrams, M. D. (1988). Sources of Variation in Osmotic Potentials with Special Reference to North American Tree Species. Forest Science, 34, 1030-1046.
- 2. Anh, V. T. Q. (2006). Floristic Composition and Growth Dynamics of Riparian Forests in North-East Vietnam. Gottingen: Cuvillier.
- 3. Anon (1996). Red Data Book of Vietnam—Plants. Hanoi: Science and Technology Publishing House. (In Vietnamese)
- 4. Anonymous (2012). A Report on the Results of the Distribution of Rare Tree Species in Ba Be National Park. Project on Forestry Development, Unpublished Report. (In Vietnamese)
- 5. Bhuyan, P., Khan, M., & Tripathi, R. (2003). Tree Diversity and Population Structure in Undisturbed and Human-Impacted Stands of Tropical Wet Evergreen Forest in Arunachal Pradesh, Eastern Himalayas, India. Biodiversity & Conservation, 12, 1753-1773.
http://dx.doi.org/10.1023/A:1023619017786 - 6. Chan, L. T. (2006). Limestone Ecosystems in Vietnam. Journal of Agriculture and Rural Development, 7, 15-29.
- 7. Chinh, N. N., Cao, T., Vu, V., Dung, N. X., Dung, V. V., Nguyen, K., Hop, T., Tran, T., Nguyen, B., & Nguyen, N. (1996). Vietnam Forest Trees. Hanoi: Forest Inventory and Planning Institute.
- 8. Clements, R., Sodhi, N. S., Schilthuizen, M., & Ng, P. K. (2006). Limestone Karsts of Southeast Asia: Imperiled Arks of Biodiversity. Bioscience, 56, 733-742.
http://dx.doi.org/10.1641/0006-3568(2006)56[733:LKOSAI]2.0.CO;2 - 9. Dang, N., Mai, T., Chu, H., Huy, T., & Kinh, N. (2001). Forestry in Vietnam (1945-2000), Development Progress and Experienced Lessons. Ha Noi: Agricultural Publishing House.
- 10. Dung, V. V. (2001). The Limestone Forests in Vietnam—Strategy for Management, Protection and Development. Journal of Agriculture and Rural Development, 7, 14-18.
- 11. Dzung, N. H., Tuong, H. M., Soriaga, R., & Walpole, P. (2004). The Return of Limestone Forests, Northeastern Viet Nam. Research Network Report No. 11, Tagbilaran City: Asia Forest Network.
- 12. FIPI (1995). Handbook of Forest Inventory and Planning. Ha Noi: Forest Inventory and Planning Institute.
- 13. Furey, N. M., Mackie, I. J., & Racey, P. A. (2010). Bat Diversity in Vietnamese Limestone Karst Areas and the Implications of Forest Degradation. Biodiversity and Conservation, 19, 1821-1838.
http://dx.doi.org/10.1007/s10531-010-9806-0 - 14. Gebrekirstos, A., Teketay, D., & Mitlohner, R. (2014). Responses of Dobera glabra and Eight Co-Occurring Species to Drought and Salinity Stress at a Savanna-Scrub Ecotone: Implications in the Face of Climate Change. Open Journal of Forestry, 4, 327-337.
http://dx.doi.org/10.4236/ojf.2014.44039 - 15. IUCN (2006). The IUCN—Red List of Threatened Species. Gland: World Conservation Union (IUCN).
- 16. Khaing, N. (2013). Structure and Site Conditions of Dry Deciduous Forests in Central Myanmar. Gottingen: University of Gottingen.
- 17. Kreeb, K. H. (1990). Methoden zur Pflanzenoekologie und Bioindikation. Stuttgart: Fischer.
- 18. Lambers, H., Chapin III, F. S., & Pons, T. L. (2008). Plant Water Relations. In H. Lambers, F. S. Chapin III, & T. L. Pons (Eds.), Plant Physiological Ecology (pp. 163-223). New York: Springer.
http://dx.doi.org/10.1007/978-0-387-78341-3_5 - 19. MARD (2004). Report on Ba Be/Na Hang Conservation Complex-Ministry of Agriculture and Rural Development. Hanoi: MARD.
- 20. Mitlohner, R. (1997a). Using Trees as Indicators of Environmental Conditions by Measuring Their Internal Water Status. Plant Research and Development, 45, 33-50.
- 21. Mitlohner, R. (1998). Pflanzeninterne Potentiale als Indikatoren fur den tropischen Standort. Aachen: Shaker.
- 22. Mitlohner, R., & Kopp, R. (2007). Bioindicator Capacity of Trees towards Dryland Salinity. Trees, 21, 411-419.
http://dx.doi.org/10.1007/s00468-007-0133-3 - 23. Mitlohner, R., Tam, T. Q., & Weidelt, H. J. (1997b). Waldtypenbildung und Wasserverfugbarkeit im Monsunwald des sudostlichen Vietnam. Forstarchiv, 68, 244-250.
- 24. Morgan, J. M. (1984). Osmoregulation and Water Stress in Higher Plants. Annual Review of Plant Physiology, 35, 299-319.
http://dx.doi.org/10.1146/annurev.pp.35.060184.001503 - 25. Morgan, J., & Condon, A. (1986). Water Use, Grain Yield, and Osmoregulation in Wheat. Functional Plant Biology, 13, 523-532.
http://dx.doi.org/10.1071/pp9860523 - 26. Munns, R. (1988). Why Measure Osmotic Adjustment. Functional Plant Biology, 15, 717-726.
http://dx.doi.org/10.1071/pp9880717 - 27. Nghia, N. H. (2005). Results from Research on Conservation of Forest Plant Genetic Resources. In MARD (Ed.), Proceedings of the Conference of Forest Science and Technology for 20 Years under Renovation (pp. 2-12). Ha Noi: MARD.
- 28. Peque, D., & Holscher, D. (2014). The Abundance of Rare Tree Species in Remnant Forests across the Visayas, Philippines. Biodiversity and Conservation, 23, 1-18.
http://dx.doi.org/10.1007/s10531-014-0714-6 - 29. Pielou, E. C. (1966). Species-Diversity and Pattern-Diversity in the Study of Ecological Succession. Journal of Theoretical Biology, 10, 370-383.
http://dx.doi.org/10.1016/0022-5193(66)90133-0 - 30. Polak, M. (2000). The Botanical Diversity in the Ayawasi Area, Irian Jaya, Indonesia. Biodiversity & Conservation, 9, 1345-1375.
http://dx.doi.org/10.1023/A:1008974730159 - 31. Proctor, J., Anderson, J., Chai, P., & Vallack, H. (1983). Ecological Studies in Four Contrasting Lowland Rain Forests in Gunung Mulu National Park, Sarawak: I. Forest Environment, Structure and Floristics. The Journal of Ecology, 71, 237-260.
http://dx.doi.org/10.2307/2259975 - 32. Qin, X., Zhang, R., & Xing, F. (2012). A Study on the Flora and Vegetation of Cat Dua Island, Norteastern Vietnam. Pakistan Journal of Botany, 44, 1229-1232.
- 33. Rana, C. S., & Gairola, S. (2009). Forest Community Structure and Composition along an Elevational Gradient of Parshuram Kund Area in Lohit District of Arunachal Pradesh, India. Nature and Science, 1, 44-52.
- 34. Ranlund, Â. (2011). Structure and tree Diversity of Lowland Limestone Forest on Seram Island, Indonesia. Master’s Thesis, Stockholm: Swedish University of Agricultural Sciences.
- 35. Scholander, P. F., Bradstreet, E. D., Hemmingsen, E., & Hammel, H. (1965). Sap Pressure in Vascular Plants; Negative Hydrostatic Pressure Can Be Measured in Plants. Science, 148, 339-346.
http://dx.doi.org/10.1126/science.148.3668.339 - 36. Shannon, C. E. (1948). A Mathematical Theory of Communications. Bell System Technical Journal, 27, 379-423.
http://dx.doi.org/10.1002/j.1538-7305.1948.tb01338.x - 37. Slatyer, R. (1957). The Significance of the Permanent Wilting Percentage in Studies of Plant and Soil Water Relations. The Botanical Review, 3, 585-636.
http://dx.doi.org/10.1007/BF02870151 - 38. Toai, P. M. (2012). Structure and Regeneration of Lowland Tropical Moist Evergreen Forests in North and Central Vietnam. Gottingen: Institute of Tropical Silviculture, University of Gottingen.
- 39. Trung, T. V. (1998). Ecosystems of Tropical Forests in Vietnam. Ha Noi: Science and Techniques Publishing House.
- 40. Tuyet, D. (2001). Characteristics of Karst Ecosystems of Vietnam and Their Vulnerability to Human Impact. Acta Geologica Sinica—English Edition, 75, 325-329.
http://dx.doi.org/10.1111/j.1755-6724.2001.tb00539.x - 41. Veríssimo, A., Barreto, P., Mattos, M., Tarifa, R., & Uhl, C. (1992). Logging Impacts and Prospects for Sustainable Forest Management in an Old Amazonian Frontier: The Case of Paragominas. Forest Ecology and Management, 55, 169-199.
http://dx.doi.org/10.1016/0378-1127(92)90099-U - 42. Vermeulen, J., & Whitten, T. (1999). Biodiversity and Cultural Property in the Management of Limestone Resources-Lessons from East Asia. Washington DC: World Bank.
http://dx.doi.org/10.1596/0-8213-4508-7 - 43. Whitmore, T. (1984). Tropical Rain Forests of the Far East (2nd ed.). Oxford: Clarendon Press.
NOTES

*Corresponding author.







