Open Journal of Forestry
Vol.05 No.02(2015), Article ID:53541,10 pages
10.4236/ojf.2015.52019
Spatial Distribution of Regenerated Woody Plants in Alnus hirsuta (Turcz.) var. sibirica Stand in Japan
Hiroyuki Tobita1,2, Satoshi Nanami3, Shigeaki F. Hasegawa4, Kenichi Yazaki1, Masabumi Komatsu1, Mitsutoshi Kitao1
1Present Address: Department of Plant Ecology, Forestry and Forest Products Research Institute, Tsukuba, Japan
2Previous Address: Laboratory of Forest Ecology, Graduate School of Agriculture, Kyoto University, Kyoto, Japan
3Graduate School of Science, Osaka City University, Osaka, Japan
4Institute of Low Temperature Science, Hokkaido University, Sapporo, Japan
Email: tobi@ffpri.affrc.go.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 January 2015; accepted 23 January 2015; published 27 January 2015
ABSTRACT
The role of N2 fixation in structuring plant communities and influencing ecosystem function will be potentially large. In previous study, we investigated nodule biomass and activity, and calculated the amount of N2 fixation in a naturally established 18-year-old alder (Alnus hirsute (Turcz.) var. sibirica) stand following disturbance by road construction in Takayama, central Japan. In this study, to estimate the facilitation effects by alder on the spatial distribution of the regenerated tree species, we examined the distribution pattern of the regenerated tree species in this naturally established 18-year-old alder stand. The distribution pattern of alder and the regenerated woody species was analyzed in terms of spatial point processes and the regenerated species tended to distribute near the alder site. In particular, bird-dispersed tree species (endozoochory species) with relatively high shade tolerance showed a significant attraction to alder. These results suggest that alder will be used as roost trees and play the role of mother trees for these regenerated species at the degraded site. It was also suggested that the endozoochory species, which occupy 13 of 26 regenerated species in this stand, might regenerate faster than other species at this alder stand.
Keywords:
Bird-Dispersal, N2 Fixation, Roost Trees, Spatial Distribution, Succession

1. Introduction
Given the virtually ubiquitous limitation of plant growth by N supply (Vitousek & Howarth, 1991) , the role of N2 fixation in structuring plant communities and influencing ecosystem function is potentially large (Chapin et al., 1994; Thomas & Bowman, 1998) . Nitrogen accretion is accelerating due to N2 fixation during community development, facilitating invasion by later successional species and accelerating the rate at which succession proceeds (Thomas & Bowman, 1998) . Interspecific facilitation by plants may be more important in structuring plant communities and the ecosystem function than previously thought (Hunter & Aarssen, 1988; Callaway & Walker, 1997) . For example, alpine Trifolium species have high rates of symbiotic N2 fixation, which influenced the abundance, and growth of nearby plant species growing (Thomas & Bowman, 1998) . The abundance of some species was positively associated with the presence of Trifolium, though other species were less abundant. These results suggest that N2 fixing species may exert both facilitative and inhibitive effects on the abundance and growth of plant species growing near them and, in the process, substantially influence the spatial heterogeneity in community structure and primary production.
Species of actinorhizal Alnus that fix N through the metabolic activity of the filamentous bacterial symbiont Frankia, play an important role in the N cycle of temperate forest ecosystems (Tjepkema et al.,1986; Tobita et al., 2013b) . An interest in these Frankia-Alnus systems has increased as their value in the revegetation of deteriorated wildlife habitats and the rehabilitation of N-deficient disturbed areas has become apparent (Sharma, 1988; Baker & Schwintzer, 1990; Zitzer & Dawson, 1992; Tobita et al., 1993; Chapin et al., 1994; Enoki et al., 1997) . In the mixed Alnus-conifer young-growth stands, Alnus species appear to provide much more productive understory vegetation and wildlife habitat than similar-aged pure conifer stands (Hanley et al., 2006) . Alnus species often regenerate naturally at disturbed sites by road constructions or natural soil slide, and so on (Tobita et al., 2010) . If there are facilitative effects on plant abundance by the N2-fixer, Alnus species, the distribution pattern of regenerated plants may be also influenced by the distribution of alder.
Dispersal mechanisms promoting seed arrival from distant sources are key in primary succession (Finegan, 1984; Walker & Chapin, 1987) . As plants are sessile, both the initial spatial pattern of offspring (Houle, 1992) and the spatial population structure (Armest et al., 1991) are determined by the location of parent plants and their seed dispersal ability (Nanami et al., 1999) . In addition, stands established on degraded soils will have no mother trees of other tree species at an early stage of stand development. Large trees of other tree species, roost trees for the birds, act as foci for depositing and recruiting of bird-dispersed, endozoochory, plants (Hatton, 1989; Maltez-Mouro et al., 2007) . In the temperate zones, many fleshy-fruited plants rely on migrating birds to disperse their seeds (Johnson et al., 1985; Nakanishi, 1996) . If Alnus species may perform as roost trees for birds and play a role as mother trees, endozoochory tree species might distribute around the Alnus trees as shown in Maltez-Mouro et al. (2007) .
Alnus hirsuta var. sibirica is a deciduous early successional tree species that is widely distributed in northern districts and highlands of Japan. We investigated nodule biomass and the amount of N2 fixation in a naturally established 18-year-old stand of A. hirsuta var. sibirica in areas degraded by road construction in Takayama, central Japan (Tobita et al., 2010, 2013a) . We found the horizontal distribution of nodules in A. hirsuta var. sibirica varied among tree sizes, and in particular, for trees with smaller dbh, there was a concentration of nodule density near the stem (Tobita et al., 2010) . In addition, the N2 fixation rate in this A. hirsuta var. sibirica stand was estimated at 56.4 kg∙ha−1∙year−1, which corresponded to 66.4% of the N content in leaf litter in a year (Tobita et al., 2013a) . These results suggested that the N2 fixation of A. hirsuta var. sibirica contributed to rapid N accumulation into the soil. In this study, we tried to clarify the effects of the Alnus species on the spatial distribution of regenerated tree species at this naturally established 18-year-old stand of A. hirsuta var. sibirica.
Of course, many processes, such as the germination and survival of seeds and seedlings, growth of saplings, competition between herbaceous species, light environment, soil water content, and litter as physical obstructions, should contribute to the present distribution of each species (Hatton, 1989) . In this study, we consider the potential impact of the presence of Alnus species as one of many factors of determining for the spatial distribution of the regenerated woody plants.
2. Materials and Methods
2.1. Study Site
The study site was at an altitude of approximately 1100 m on Mt. Norikura in the eastern part of Takayama city, Gifu prefecture, central Japan (36˚9'N, 137˚15'E). A study plot of 30 ´ 35 m was set up in an alder (Alnus hirsuta Turcz. var. sibirica (Fischer) C.K. Schn.) stand (in detail: Tobita et al., 2010 ). Alder regenerated naturally after the disturbance when a road was built through the site in 1975. Alders are a deciduous and early successional species, widely distributed in the northern districts and highlands of Japan. These trees are used to improve the growth of mixed conifer plantations, produce logs and revegetate degenerated soil. In our study site, all the canopy trees were alders. The tree height was about 15 m, and the canopy of this stand was almost closed (Hasegawa & Takeda, 2001; Tobita et al., 2010) . The mean (± SD) stem diameter at breast height (dbh) of trees was 12.4 (±3.8) cm in April 1995 and the frequency distribution of dbh was unimodal, indicating that this stand comprised trees of similar age (Tobita et al., 2010) . Because several trees died during the study, the stand density varied from 1114 ha−1 in April 1995 to 1038 ha−1 in May 1996. Although the site floor was densely covered in herbaceous plants, regenerating specimens of several species of trees and shrubs were also present. The neighboring forest stand was used for coppicing, and has been dominated by Pinus thunbergii, Quercus mongolica, Betula platyphylla var. japonica, Prunus grayana, Lindera obtusiloba, and Euptelea polyandra.
2.2. Regenerated Woody Plants
From June to November 1996, naturally regenerated woody species in the study site were mapped to analyze the pattern of spatial distribution and the height and positions of the stem base were measured. All regenerated species was divided into three groups by seed-dispersal type; namely as bolochory species by gravity, anemochory species by wind, and endozoochory species by birds.
2.3. Data Analysis of Spatial Distribution of Regenerated Woody Plants
The alder population, including trees which died before April 1995, was reconstructed using data on detectable fallen trees, dieback trees and stumps (Tobita et al., 2010) . Subsequently, we constructed the five populations of alder; 1) Live trees in April 1995 and dead trees before April 1995; 2) live trees in April 1995; 3) live trees in April 1996; 4) live trees in April 1997 and 5) dead trees before April 1996. To analyze of the spatial interactions with the regenerated species, two populations of alder were used; live trees in April 1996 and dead trees before April 1996. We understand that the number of dead trees before April 1995 would be underestimate because we counted only those detectable, such as fallen trees, dieback trees, or stump.
The spatial pattern of regenerated woody species (all species and the major seven species, for which a relatively larger number of individuals emerged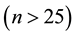 ) and alder populations was analyzed using the function
) and alder populations was analyzed using the function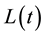 , a transformation of Ripley’s
, a transformation of Ripley’s 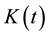 function
(Ripley, 1977)
, as suggested by
Besag, (1977)
. The function
function
(Ripley, 1977)
, as suggested by
Besag, (1977)
. The function 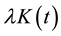
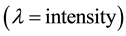 is defined as the expected number of plants within distance t of an arbitrarily chosen plant. The unbiased estimate of
is defined as the expected number of plants within distance t of an arbitrarily chosen plant. The unbiased estimate of 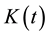 is defined as:
is defined as:
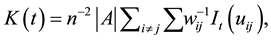 (1)
(1)
where  is the number of plants in a plot
is the number of plants in a plot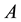 ;
; 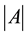 denotes plot area;
denotes plot area; 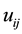 is the distance between ith and jth plants in
is the distance between ith and jth plants in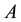 ;
; 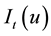 is equal to 1 if
is equal to 1 if 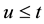 and 0 otherwise;
and 0 otherwise; 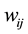 is the proportion of the circumference of a circle, centered at the ith plant and radius
is the proportion of the circumference of a circle, centered at the ith plant and radius  that lies within
that lies within


A value of 



The spatial interaction between two groups of plants was also analyzed using the bivariate function


The function 





3. Results
3.1. Spatial Distribution Pattern of Alnus hirsuta var. sibirica
The alder populations showed significant clumped distribution (Figure 1); live and dead trees in April 1995 at 0.5 m (P < 0.01), live trees in 1996 at 9.5 - 10.0 m (P< 0.05) and 0.5 m (P < 0.05), and live trees in 1997 at 0.5 - 1.0 m (P < 0.05). The magnitude of departure from randomness at large distance declined from 1995 to 1997.
3.2. Species Composition
In this stand, 23 woody species with 389 individuals were regenerated (Table 1) and the plant density of all regenerated plants was 0.37 plants m−2 in this stand. These regenerated species included 14 tree species with 229 individuals, and 9 shrub species with 160 individuals. 13 endozoochory plants with 239 individuals, 7 anemochory plants with 106 individuals, and 3 bolochory plants with 44 individuals comprising these regenerated plants. In terms of tree species, Prunus incisa, Cornus controversa, Juglans mandshurica, and Acer rufinerve were the major tree species, while Weigela hortensis, Sambucus racemosa, and Aralia elata were the major shrub species. There was no seedling of alder at the forest floor. The frequency of height of all regenerated woody species showed the mode at 0 - 0.5 m (Table 1). Except for Salix bakko, almost all individuals of each tree species were less than 4 m in height, while the height of the shrub species was less than 3 m, but the portion of taller individuals exceeded that of tree species.
Figure 1. 

Table 1. Number and height of regenerated woody species in this Alnus hirsuta var. sibirica stand. Species are shown in order by life form; tree species and shrub species. The seed dispersal type was shown as bolochory (B), endozoochory (E), and anemochory (A).
3.3. Spatial Distribution Pattern of Regenerated Woody Species
The population of all regenerated tree species was significantly clumped at all distances (scales), and was maximally (without distinct peaks) clumped at 1.5 m (P < 0.01; Figure 2(A)). The population of bolochory species (including 3 species) was significantly clumped at scales ranging from 1.5 to 2 m (P < 0.05; Figure 2(B)). The population of endozoochory species (include 13 species) was also significantly clumped at all distances, 0.5 - 10 m (P < 0.01; Figure 2(C)). The population of anemochory species (include 7 species) was significantly clumped at 0.5 - 5.5 m (P < 0.01) and 6 m (P < 0.05), and maximally clumped at 0.5 m (Figure 2(D)).
The major regenerated tree species, which appeared for more than 25 plants in this study plot, were seven species, including Juglans mandshurica of bolochory species, Acer rufinerve and Weigela hortensis of anemo-
Figure 2. 





chory species, and Cornus controversa, Prunus incisa, Sambucus racemosa, and Aralia elata of endozoochory species. Acer rufinerve, Sambucus racemosa, and Aralia elata were significantly clumped at all distances (P < 0.05 or 0.01) (Figure 3(B), Figure 3(F) and Figure 3(G)). One of the shrub species, Weigela hortensis, was also significantly clumped at almost all distance except 10 m (P < 0.05 or 0.01) (Figure 3(C)). The shrub species showed significant aggregation at a wider range than tree species. Within endozoochory species, the two tree species, Cornus controversa and Prunus incisa, showed different distribution pattern from the two shrub species, Sambucus racemosa, and Aralia elata. Cornus controversa was significantly clumped at 0.5 - 2 m (P < 0.01) and 2.5 m (P < 0.05) (Figure 3(D)). Prunus incisa were significantly clumped at 0.5 - 3.5 m (P < 0.01) (Figure 3(E)). Juglans mandshurica showed no significant departure from randomness except at 0.5, 1.5, and 7.5 m (P < 0.05) (Figure 3(A)). Aralia elata showed a distinct peak at 3 m (Figure 3(G)).
3.4. Spatial Interaction between Regenerated Woody Species and Alnus hirsuta var. sibirica
The population of all regenerated tree species showed a significant attraction to alder (live trees in 1996) at 0.5 - 2 m (P < 0.01) and at 2.5 m (P < 0.05) (Figure 4(A)). As the results of analysis among seed dispersal patterns, the population of bolochory and anemochory species showed no significant departure from the independence of alder population (live trees in 1996) (Figure 4(B) and Figure 4(D)). The population of endozoochory species, which included 13 species, also showed a significant attraction to alder (live trees in 1996) at 0.5 - 1.5 m (P < 0.01) and at 2 m (P < 0.05) (Figure 4(C)).
As the results of the analysis between each species and alder, Juglans mandshurica, Acer rufinerve, and Aralia elata showed no significant departure from the independence of alder population (live trees in 1996) (Figure 5(A), Figure 5(B) and Figure 5(G)). Weigela hortensis showed a significant repulsion from the alder population (live trees in 1996) at 4.5 m (P < 0.05) (Figure 5(C)). Prunus incisa showed a significant attraction to alder (live trees in 1996) at 0.5 - 2.5 m (P < 0.01) (Figure 5(E)). Sambucus racemosa showed a significant attraction to alder (live trees in 1996) at 2 - 2.5 and 5.5 - 7.5 m (P < 0.05) (Figure 5(F)). Cornus controversa showed no significant departure from the independence of alder population (live trees in 1996) (Figure 5(D)), but showed a significant attraction to alder (live and dead trees in 1995) at 0.5 - 1.5 m (P < 0.01) and at 2 - 2.5 (P < 0.05) (data not shown).
Aralia elata showed a significant attraction to dead alder trees (dead trees as of 1996) at 0.5 m (P< 0.05) and 1.5 m, 3.5 - 6.0 m (P < 0.01) (Figure 6(C)). Weigela hortensis also showed a significant attraction to alder (dead trees as of 1996) at 2 - 2.5 m (P < 0.05) (Figure 6(B)). Acer rufinerve showed a significant repulsion from alder population (dead trees as of 1996) at 0.5 m (P < 0.05) (Figure 6(A)).
4. Discussion
Regenerated woody species tended to distribute near the Alnus hirsuta var. sibirica. In particular, endozoochory
Figure 3. 

Figure 4. 





Figure 5. 

Figure 6. 
species with relatively high shade tolerance showed a significant attraction to alder. These results suggest alder will be used for roost trees and play a role as mother trees of these regenerated endozoochory tree species. It was suggested that endozoochory species, which occupied 13 of 26 regenerated species in this stand, might regenerate faster than species with other types of seed dispersal at the naturally regenerated stands of Alnus species after the soil disturbance.
The population of all regenerated tree species showed a significant attraction to alder (Figure 4(A)), but differed in terms of spatial interaction with Alnus species among the regenerated woody species (Figure 5). As predicted, some endozoochory species, such as Prunus incisa and Sambucus racemosa, showed significant attraction to alder (Figure 5(E) and Figure 5(F)). Viburnum tinus, which has bird-dispersal seeds, were clumped at the site without their mature trees on the upper trees (Maltez-Mouro et al., 2007) . They discussed that one of the reasons for the aggregation pattern of the Viburnum tinus was because the seeds of Viburnum tinus were dispersed under those of other species, which were used as roosted trees by birds. In the case of herbaceous species with bird-dispersal seeds, they showed a strong aggregation pattern under large trees, which would act as roosting trees for birds and foci for to deposit and recruit endozoochory plants (Hatton, 1989) . The number of birds in the plantations of Alnus species usually exceeds that of Eucalyptus and Pinus species, because the number of soil animals, which can be bird-fed, will increase with the soil fertilization (Carlson & Dawson, 1985) . In this study, we have no data on the number of birds and seed falls, but observed the gathering of birds in the crown of this alder stand during daytime. In this study plot, it was suggested that Alnus hirsuta var. sibirica was used as roost trees and mother trees for endozoochory species.
However, two of the four endozoochory species, Cornus controversa and Aralia elata, showed independent spatial distribution with alder (Figure 5(D) and Figure 5(G)). Cornus controversa showed attraction when we analyzed spatial interaction including dead alder trees (data not shown). These results indicated that the attractive distribution pattern between Cornus controversa and alder became unclear with increasing dead alder trees. Conversely, Aralia elata attractively distributed relative to the population of dead alder trees (Figure 6(C)). These results indicated that it may be necessary for Aralia elata to improve the light conditions in addition to seed supply to regenerate in this alder stand, because Aralia elata is a species requiring significant light (Tobita et al., 1993) .
Weigela hortensis, which is an anemochory species, showed a significant attraction with the population of dead alder trees (Figure 6(B)), and a significant repulsion from the population of live alder trees (Figure 5(C)). Thomas & Borman (1998) reported the similar results; namely that some anemochory species showed repulsion from a leguminous species, Trifolium. However, Acer rufinerva, which is also an anemochory species, showed a significant repulsion with the population of dead alder trees (Figure 6(A)), and showed an independent distribution from the live alder population (Figure 5(B)). Though Acer rufinervais an intolerant understory species (Masaki et al., 1992) , these results suggest that Weigela hortensis may be more light demanding than Acer rufinerva (Katsuta et al., 1998) . However, Weigela hortensis showed vegetative reproduction in several cases, which might affect the analytical results of the spatial distribution of this species.
The distribution pattern of bolochory species depends on the behavior of mammal species as well as the location of the mother trees. For example, the location of the mother trees alone could not explain the distribution pattern of the seedlings in Quercus serrata which were regenerated under the plantation of Pinus thunbergii (Tobita et al., 1993) . Though Juglans mandshurica, a bolochory species, showed no significant departure from the independence of the alder population (Figure 5(A)), this species was observed to grow close to the alders. These results led to speculation of secondary dispersal by mammals.
The horizontal spatial pattern of individuals in a plant community may reflect many factors (Maltez-Mouro et al., 2007) and has been interpreted in terms of wide-ranging of processes, including mortality due to herbivores or pathogens, gap disturbance, competition, microhabitat variability, and limited dispersal range from adults (Hatton, 1989) . The presence of canopy trees, regardless of N2-fixing or otherwise, will promote a change in the physical properties on the forest floor (Maltez-Mouro et al., 2007) . In this study, we cannot determine the factors mainly affecting the distribution pattern of the regenerated plants at this alder stand. However, it was suggested that the presence of alder might promote the regeneration of woody species as one of many factors determining for the spatial distribution. It was also suggested that the N2 fixation ability of the alder (Tobita et al., 2013a) may not only help improve soil fertility but also promote vegetative regeneration by used as roost and mother trees for these regenerated species at degraded site.
This study demonstrated only the results at one stand of A. hirsuta var. sibirica at one time. In the future research, it will be necessary to confirm the species composition and successional proceeding after several decades at this alder stand, and also to verify the facilitative effects on distribution pattern of regenerated plants at several stands with different successional stages and with different dominant Alnus species.
5. Conclusion
Regenerated woody species, in particular, endozoochory species with relatively high shade tolerance, tended to show a significant attraction to Alnus hirsuta var. sibirica. These results suggest that alder will be used for roost trees and play a role as mother trees of these regenerated endozoochory tree species at the naturally established stands of Alnus species after the soil disturbance. This study also suggested that the symbiotic N2 fixation of A. hirsuta var. sibirica might affect the distribution pattern of regenerated tree species as well as improving soil fertility.
Acknowledgements
We thank Drs. Iwatsubo, Takeda, Kawaguchi, Osawa, Tokuchi, Kitayama and the members of the Laboratory of Forest Ecology, Kyoto University, for their valuable suggestions. We are also grateful to Dr. Ando and Messrs. Kurumado and Saito for their pleasant acceptance and offer of the study site and experimental office, and Dr. Tian for his help with the fieldwork. This study was financially supported by JSPS KAKENHI Grant Numbers 91567, 24580230.
References
- Armest, J. J., Pickett, S. T. A., & McDonnell, M. J. (1991). Spatial Heterogeneity during Succession: A Cyclic Model of Invasion and Exclusion. In J. Kolasa, & S. T. A. Pickett (Eds.), Ecological Heterogeneity (pp. 256-269). New York: Springer-Verlag. http://dx.doi.org/10.1007/978-1-4612-3062-5_13
- Baker, D. D., & Schwintzer, C. R. (1990). Introduction. In C. R. Schwintzer, & J. D. Tjepkema (Eds.), The biology of Frankia and Actinorhizal Plants (pp. 1-13). Tokyo: Academic Press. http://dx.doi.org/10.1016/B978-0-12-633210-0.50006-X
- Besag, J. (1977). Contribution to the Discussion on Dr. Ripley’s Paper. Journals of the Royal Statistical Society, B39, 193- 195.
- Callaway, R. M., & Walker, L. R. (1997). Competition and Facilitation: A Synthetic Approach to Interactions in Plant Communities. Ecology, 78, 1958-1965. http://dx.doi.org/10.1890/0012-9658(1997)078[1958:CAFASA]2.0.CO;2
- Carlson, P. J., & Dawson, J. O. (1985). Soil Nitrogen Changes, Early Growth, and Response to Soil Internal Drainage of a Plantation of Alnus jorullensis in the Colombian Highlands. Turrialba, 35, 141-150.
- Chapin III, F. S., Walker, L. R., Fastie, C. L., & Sharman, L. C. (1994). Mechanisms of Primary Succession Following De- glaciation at Glacier Bay, Alaska. Ecological Monographs, 64, 149-175. http://dx.doi.org/10.2307/2937039
- Diggle, P. J. (1983). Statistical Analysis of Spatial Point Pattern (pp. 148). London: Academic Press.
- Enoki, T., Kawaguchi, H., & Iwatsubo, G. (1997). Nutrient-Uptake and Nutrient-Use Efficiency of Pinus thunbergii Parl. along a Topographical Gradient of Soil Nutrient Availability. Ecological Research, 12, 191-199. http://dx.doi.org/10.1007/BF02523784
- Finegan, G. B. (1984). Forest Succession. Nature, 312, 109-114. http://dx.doi.org/10.1038/312109a0
- Hanley, T. A., Deal, R. L., & Orlikowska, E. H. (2006). Relationships between Red Alder Composition and Understory Vegetation in Young Mixed Forests of Southeast Alaska. Canadian Journal of Forest Research, 36, 738-748. http://dx.doi.org/10.1139/x05-290
- Hasegawa, S., & Takeda, H. (2001). Functional Specialization of Current Shoots as a Reproductive Strategy in Japanese Alder (Alnus hirsuta var. sibirica). Canadian Journal of Botany, 79, 38-48. http://dx.doi.org/10.1139/b00-143
- Hatton, T. J. (1989). Spatial Analysis of a Subalpine Heath Woodland. Australian Journal of Ecology, 14, 65-75. http://dx.doi.org/10.1111/j.1442-9993.1989.tb01009.x
- Houle, G. (1992). Spatial Relationship between Seed and Seedling Abundance and Mortality in a Deciduous Forest of North-Eastern North America. Journal of Ecology, 80, 99-108. http://dx.doi.org/10.2307/2261066
- Hunter, A. F., & Aarssen, L. W. (1988). Plants Helping Plants. BioScience, 38, 34-40. http://dx.doi.org/10.2307/1310644
- Johnson, R. A., Willson, M. F., Thompson, J. N., & Bertin, R. I. (1985). Nutritional Values of Wild Fruits and Consumption by Migrant Birds. Journal of Ecology, 66, 819-827. http://dx.doi.org/10.2307/1940543
- Katsuta, M., Mori, T., & Yokoyama, T. (1998). Seeds of Woody Plants in Japan. Angiospermae. Tokyo: Japan Forest Tree Breeding Association. (In Japanese).
- Lotwick, H. M., & Silverman, B. W. (1982). Methods for Analysing Spatial Processes of Several Types of Points. Journals of the Royal Statistical Society, 44, 406-413.
- Maltez-Mouro, S., Garcia, L. V., Maranon, T., & Freitas, H. (2007). Recruitment Patterns in a Mediterranean Oak Forest: A Case Study Showing the Importance of the Spatial Component. Forest Science, 53, 645-652.
- Masaki, T., Suzuki, W., Niiyama, K., Iida, S., Tanaka, H., & Nakashizuka, T. (1992). Community Structure of a Species- Rich Temperate Forest, Ogawa Forest Reserve, Central Japan. Vegetatio, 98, 97-111. http://dx.doi.org/10.1007/BF00045549
- Nakanishi, H. (1996). Fruit Color and Fruit Size of Bird-Disseminated Plants in Japan. Vegetatio, 123, 207-218. http://dx.doi.org/10.1007/BF00118272
- Nanami, S., Kawaguchi, H., & Yamakura, T. (1999). Dioecy-Iduced Spatial Patterns of Two Codominant Tree Species. Podocarpus nagi and Neolitsea aciculata. Journal of Ecology, 87, 678-687. http://dx.doi.org/10.1046/j.1365-2745.1999.00392.x
- Peterson, C. J., & Squiers, E. R. (1995). An Unexpected Change in Spatial Pattern across 10 Years in an Aspen-White-Pine Forest. Journal of Ecology, 83, 847-855. http://dx.doi.org/10.2307/2261421
- Ripley, B. D. (1977). Modeling Spatial Patterns. Journals of the Royal Statistical Society, 39, 172-212.
- Sharma, E., & Ambasht, R. S. (1988). Nitrogen Accretion and Its Energetics in the Himalayan Alder. Functional Ecology, 2, 229-235. http://dx.doi.org/10.2307/2389699
- Thomas, B. D., & Bowman, W. D. (1998). Influence of N2-Fixing Trifolium on Plant Species Composition and Biomass Production in Alpine Tundra. Oecologia, 115, 26-34. http://dx.doi.org/10.1007/s004420050487
- Tjepkema, J. D., Schwintzer, C. R., & Benson, D. R. (1986). Physiology of Actinorhizal Nodules. Annual Review of Plant Physiology and Plant Molecular Biology, 37, 209-232. http://dx.doi.org/10.1146/annurev.pp.37.060186.001233
- Tobita, H., Enoki, T., & Kawaguchi, H. (1993). Effects of Site Conditions on Natural Regeneration in a Pinus thunbergii Plantation on Mt. Tanakami. Bulletin of the Kyoto University Forest, 65, 50-62. (In Japanese)
- Tobita, H., Hasegawa, F. S., Komatsu, M., & Kitao, M. (2013a). Growth and N2 Fixation in an Alnus hirsuta (Turcz.) var. sibirica Stand in Japan. Journal of Bioscience, 38, 761-776. http://dx.doi.org/10.1007/s12038-013-9369-9
- Tobita, H., Hasegawa, F. S., Tian, X., Nanami, S., & Takeda, H. (2010). Interactive Effects of Elevated CO2, Phosphorus Deficiency, and Soil Drought on Nodulation and Nitrogenase Activity in Alnus hirsuta and Alnus maximowiczii. Symbiosis, 50, 59-69. http://dx.doi.org/10.1007/s13199-009-0037-7
- Tobita, H., Kucho, K., & Yamanaka, T. (2013b). Abiotic Factors Influencing Nitrogen-Fixing Actinorhizal Symbioses. In A. Ricardo (Ed.), Symbiotic Endophytes (pp. 103-122). New York: Springer-Verlag. http://dx.doi.org/10.1007/978-3-642-39317-4_6
- Vitousek, P. M., & Howarth, R. W. (1991). Nitrogen Limitation on Land and in the Sea: How Can It Occur? Biogeochemistry, 13, 87-115. http://dx.doi.org/10.1007/BF00002772
- Walker, L. W., & Chapin III., F. S. (1987). Interactions among Processes Controlling Successional Change. Oikos, 50, 131-135. http://dx.doi.org/10.2307/3565409
- Zitzer, S. F., & Dawson, J. O. (1992). Soil Properties and Actinorhizal Vegetation Influence Nodulation of Alnus glutinosa and Elaeagnus angustifolia by Frankia. Plant and Soil, 140, 197-204. http://dx.doi.org/10.1007/BF00010597








