Advances in Materials Physics and Chemistry
Vol.3 No.1A(2013), Article ID:30343,6 pages DOI:10.4236/ampc.2013.31A008
Single Crystal X-Ray Diffraction Studies on Magnetic Yb5Co4Ge10*
New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India
Email: #sebastiancp@jncasr.ac.in
Copyright © 2013 Udumula Subbarao, Sebastian C. Peter. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 15, 2013; revised March 4, 2013; accepted April 12, 2013
Keywords: X-Ray Diffraction; Single Crystal; Intermetallics; Ytterbium
ABSTRACT
The high quality single crystals of Yb5Co4Ge10 have been grown by the indium metal flux method and characterized by means of single crystal X-ray diffraction data. Yb5Co4Ge10 crystallizes in the Sc5Co4Si10 structure type, tetragonal space group P4/mbm and lattice constants are a = b = 12.6369(18) Å and c = 4.1378(8) Å. Crystal structure of Yb5Co4Ge10 composed of three-dimensional [Co4Ge12] network having five, six and eight membered rings. The three non-equivalent Yb atoms are sandwiched in three different channels created by the [Co4Ge12] network. Based on the bond length analysis from the crystallographic information, we confirmed that Yb1 and Yb2 atoms are in the trivalent magnetic state and Yb3 is in the divalent non-magnetic state.
1. Introduction
The ternary intermetallic compounds of general formula RE5T4X10 (RE = rare earths; T = Co, Ni, Rh, Ir and Os and X = Si, Ge, Sn) have been reported mostly for their interesting physical properties [1-22]. These compounds crystallize in the tetragonal Sc5Co4Si10 structure type having P4/mbm space group [1]. As per Pearson crystallographic database [23] and inorganic crystal structure database [24], there are 37 compounds reported within the family of RE5T4X10 compounds and are listed in Table 1. Among them only three were studied for the crystal structure from the single crystal XRD data, including our recent discovery of Yb5Ni4Ge10 mixed valent Yb atoms [2]. However, the majority of these compounds were studied for their physical properties. For examples, the non-magnetic RE5T4X10 (RE = rare earths; T = Co, Rh, Ir, and Os; X = Ge, Si) compounds were reported as superconductors at low temperatures [5,12,17-19]. The RE5Rh4X10 (RE = Gd, Tb, Dy, Ho, Er, Tm; X = Si, Ge, Sn) and Dy5Ir4Si10 compounds were reported for their interesting magnetic properties and all of them order either ferromagnetically or antiferromagnetically at low temperature [11,18,20,21].
Katoh et al. synthesized the Yb5Co4Ge10 compound in the polycrystalline nature using Bridgman method [22]. They have proposed the mixed valent nature of Yb in Yb5Co4Ge10 based on the magnetic susceptibility data. In order to understand the structure-property relationship, we need to study the complete crystallography analysis from a high quality single crystal. The chemical bonding nature obtained from the crystal structure can be used to explain the valency of the Yb atoms. Recently, molten metal fluxes have been used as an excellent alternative to the conventional synthetic methods for the exploratory synthesis of new rare earth intermetallic compounds, as well as single crystal growth of already reported compounds [25-39].
In this paper, we report the single crystal growth of Yb5Co4Ge10 using liquid indium metal flux technique. The crystal structure of this compound has refined from the single crystal XRD data. We also discuss the crystal structure and chemical bonding of Yb5Co4Ge10 and compared with other compounds.
2. Experimental Section
2.1. Synthesis
Table 1. The list of the RE5T4X10 (RE = Rare earth, T = Co, Rh, Ir, Os and Ni; X = Si, Ge and Sn) compounds reported.
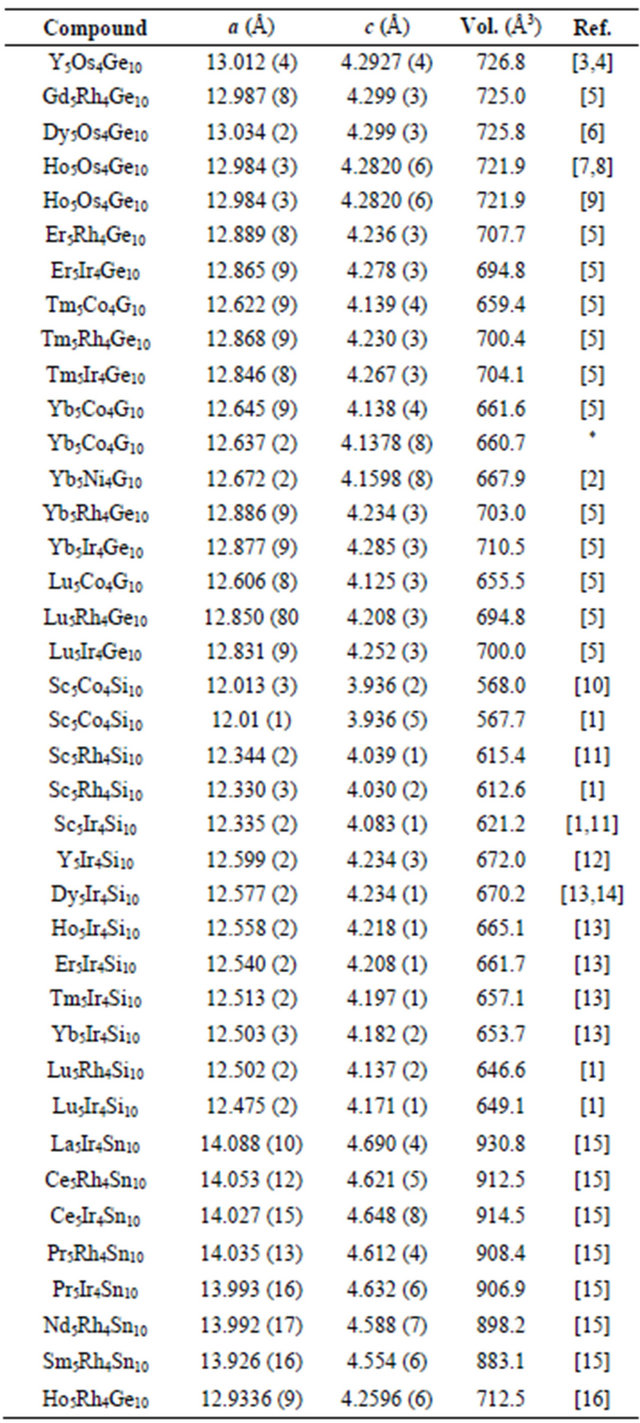
*This work.
The following reagents were used as purchased without further purification: Yb (in the form of metal pieces cut from metal chunk, 99.99% Alfa Aesar), Co (powder 99.99% Alfa Aesar), Ge (pieces, 99.999% Alfa Aesar) and In (shots, 99.99% Alfa-Aesar). High quality single crystals of Yb5Co4Ge10 were obtained by combining ytterbium metal (0.3 g), cobalt powder (0.1 g), germanium (0.3) and indium shots (2 g) in an alumina crucible. The crucible was placed in a 13 mm fused silica tube which was flame sealed under a vacuum of 10–5 torr, to prevent oxidation during heating. The reactants were then heated to 1173 K over 8 h, and maintained at that temperature for 2 h to allow proper homogenization, then cooled down to 1073 K in 2 h and kept at this temperature for 72 h. Finally, the sample was allowed to cool slowly to 303 K over 48 hrs. No reactions with the alumina crucible material could be detected. The reaction product was isolated from the excess indium flux by heating at 673 K and subsequently centrifuging through a coarse frit. Any remaining flux was removed by immersion and sonication in glacial acetic acid for 24 h. The final crystalline product was rinsed with water and dried with acetone. A few small gray rod-shaped crystals of Yb5Co4Ge10 carefully selected for the elemental analysis and single crystal XRD data collection. Yb5Co4Ge10 is stable in air and no decomposition was observed even after several months.
2.2. Elemental Analysis
Semi-quantitative microanalyses were performed on the single crystals obtained from the flux techniques using a scanning Leica 220i electron microscope (SEM) equipped with Bruker 129 eV energy dispersive X-ray analyzer (EDS). Data were acquired with an accelerating voltage of 20 kV and in 60 s accumulation time. The EDS analysis performed on visibly clean surfaces of the single crystals obtained from the flux method indicated that the atomic composition was close to 5:4:10 in good agreement with the composition obtained from the single crystal data refinement. A typical SEM image of rod-like crystals of Yb5Co4Ge10 grown from the flux synthesis is shown in Figure 1.
2.3. Single Crystal X-Ray Diffraction
Single crystal structural data of Yb5Co4Ge10 was collected at room temperature on rod shaped single crystals using a Bruker Smart-CCD diffractometer equipped with
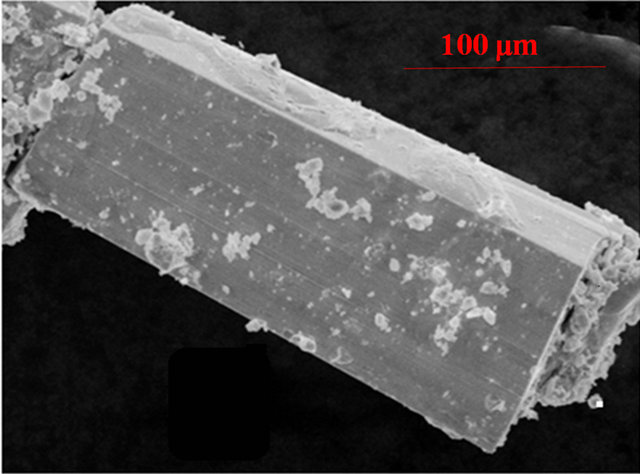
Figure 1. Typical SEM image of Yb5Co4Ge10 single crystal grown from In flux.
a normal focus, 2.4 kW sealed tube X-ray source with graphite monochromatic Mo-Kα radiation (λ = 0.71073 Å) operating at 50 kV and 30 mA, with ω scan mode. A crystal of suitable size (0.5 × 0.05 × 0.05 mm) was cut from a small rod shape crystal and mounted on a thin glass (~0.1 mm) fiber with commercially available super glue. A full sphere of 60 frames was acquired up to 57.87˚ in 2θ. The individual frames were measured with w steps of 0.50˚ and an exposure time of 20 s per frame. The programme SAINT [40] was used for integration of diffraction profiles and absorption correction was made with SADABS programme [41]. The systematic absences were leading to the centrosymmetric space group P4/mbm. However, the Platon program within WinGx system, ver. 1.80.05 [42] was used to check the additional symmetry, and this was not suggesting any additional symmetry. The structure was solved by SHELXS 97 [43] and refined by a full matrix least-squares method using SHELXL [44] with anisotropic atomic displacement parameters for all atoms. Packing diagrams were generated with Diamond [45]. In order to check for the correct composition, the occupancy parameters were refined in a separate series of least-squares cycles. All bond lengths are within the acceptable range compared to the theoretical values. Details of the crystallographic data are given in Tables 2 to 5.
Table 2. Crystal data and structure refinement for Yb5Co4Ge10.

aR = Σ||Fo| − |Fc||/Σ|Fo|, wR = {Σ[w(|Fo|2 − |Fc|2)2]/Σ[w(|Fo|4)]1/2 and calc w = 1/[σ2(Fo2) + (0.0318P)2 + 111.1197P] where P = (Fo2 + 2Fc2)/3.
Table 3. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for Yb5Co4Ge10 at 296(2) K with estimated standard deviations in parentheses.
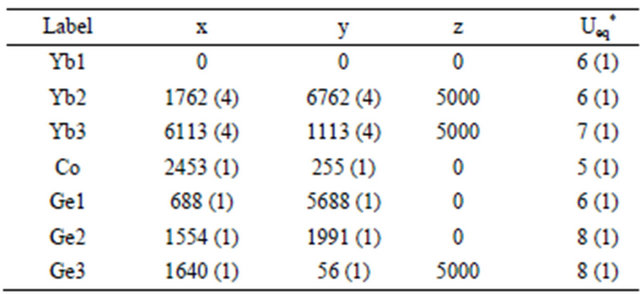
*Ueq is defined as one third of the trace of the orthogonalized Uij tensor.
Table 4. Anisotropic displacement parameters (Å2 × 103) for Yb5Co4Ge10 with estimated standard deviations in parentheses.
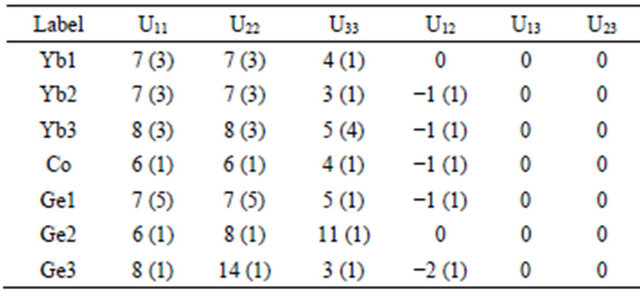
The anisotropic displacement factor exponent takes the form: −2π2[h2a*2U11 + ∙∙∙ + 2hka*b*U12].
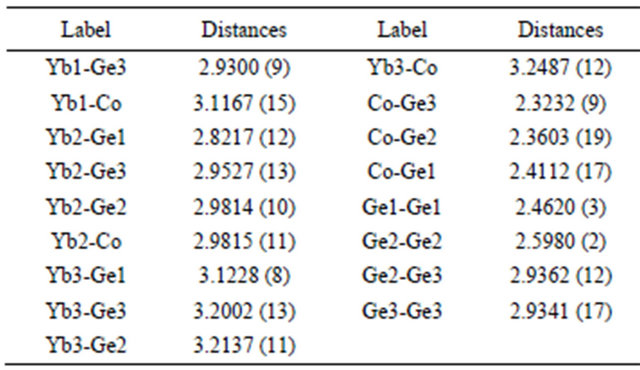
Table 5. Selected bond lengths [Å] for Yb5Co4Ge10 at 296(2) K with estimated standard deviations in parentheses.
3. Results and Discussion
3.1. Structure Refinement of Yb5Co4Ge10
The atomic parameters of Sc5Co4Si10 [1] were taken as starting parameters and the structure was refined using SHELXL-97 (full-matrix least-squares on F2) [43] with anisotropic atomic displacement parameters for all atoms. As a check for the correct composition, the occupancy parameters were refined in a separate series of leastsquares cycles. Our data confirms the previous X-ray powder data [5], but the atomic positions have been refined with higher precision. Single crystals of Yb5Co4Ge10 from different synthesis batches were used for the data collection. In the first step of refinement on the single crystal data collected shows that Yb5Co4Ge10 crystallizes in a primitive tetragonal lattice (P4/mbm) within the Sc5Co4Si10 type structure and lattice constants are a = b = 12.6369 (18) Å and c = 4.1378 (8) Å. This refinement resulted in seven crystallographical positions (three Yb, one Co and three Ge) in the Yb5Co4Ge10 structure. The data collection and structure refinement for Yb5Co4Ge10 are listed in Table 2.
The standard atomic positions and isotropic atomic displacement parameters of this compound are collected in Table 3. The anisotropic displacement parameters and important bond lengths are listed in Tables 4 and 5, respectively. Further information on the structure refinements is available from: Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany), by quoting the Registry Nos. CSD-425832.
3.2. Crystal Chemistry of Yb5Co4Ge10
The crystal structure of Yb5Co4Ge10 along c-axis is shown in Figure 2.
Yb5Co4Ge10 crystallizes in the primitive tetragonal Sc5Co4Si10 type structure (space group P4/mbm). Crystal structure of Yb5Co4Ge10 is composed of a complex [Co4Ge12] polyanion network with three types of one dimensional channels, showed along the c-axis (Figure 2), in which Yb atoms are embedded. The cages are shared through the corners of Co and Ge form pentagonal and hexagonal rings and resulted in a three dimensional network. The pentagonal and hexagonal rings are occupied with Yb2 and Yb3 atoms, respectively. These rings are connected through Co and Ge in ab plane and resulted in another large 8-membered ring, which is occupied by large Yb1 atoms. A detailed crystal structure description of the compounds crystallizing in the Sc5Co4Si10 was reported as an example of the Yb5Ni4Ge10 compound [2].
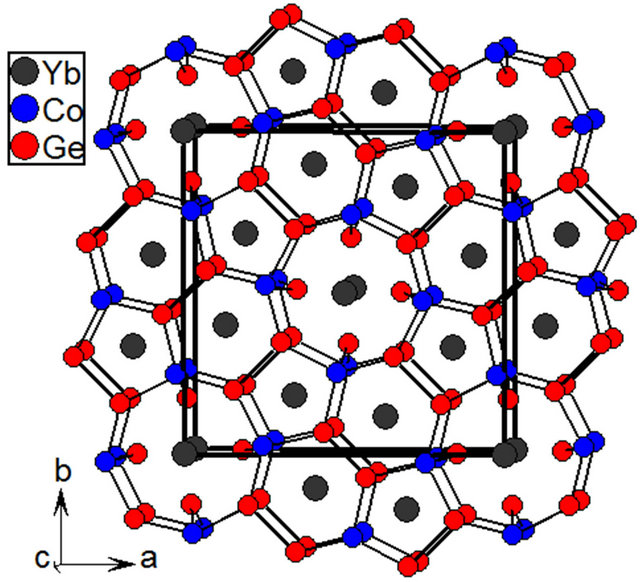
Figure 2. The crystal structure of Yb5Co4Ge10 as viewed along the c-axis; the unit cell is outlined as block solid lines.
Crystallographically there are three different Yb-atoms are present in Yb5Co4Ge10. All three Yb-coordination environments are significantly different. The Yb1 atom is located at the center of a cage consisting of 12 Ge and 4 Co atoms forming a cage as Yb[Ge12Co4], Yb2 atoms are surrounded by eight Ge and four Co forming as Yb [Ge8Co4] and Yb3 atoms are surrounded by fourteen atoms, Yb[Ge10Co4]. The Yb-Ge and Yb-Co bond lengths in Yb5Co4Ge10 are in the wide range (2.8218 (9) Å to 3.2138 (10) Å for Yb-Ge and 2.9815 (11) Å to 3.2488 (12) Å for Yb-Co) due to the different size of Yb coordination environments. The average distance obtained for Yb3-Co, 3.2488 (12) Å, is longer than the corresponding distance for Yb1-Co and Yb2-Co with 3.1167 (16) and 2.9815 (11) Å, respectively. Similarly, the average bond distance for the Yb3-Ge is 3.1754 Å, is also longer than the corresponding distance for Yb1-Ge and Yb2-Ge with 3.0175 and 2.9343 Å, respectively. Similar kinds of bonding behavior were observed in the mixed valent Yb5Ni4Ge10 as well [2]. Since Katoh et al. observed the mixed valent Yb in Yb5Co4Ge10 based on the magnetic susceptibility measurements [22], our crystallographic data suggests the Yb1 and Yb2 atoms are mostly in the trivalent state and Yb3 in the divalent state. Considering the fact that divalent Yb is larger in size (1.86 Å) [46,47] compared to trivalent Yb (1.66 Å) [46,47], Yb3 prefer to be in large cage and others (Yb1 and Yb2) are in the smaller cages.
We also compared the covalent radii and the unit cell volume four Yb based compounds reported in this family, as shown in Figure 3. The cell volume of the compounds increases as the covalent radii of the transition metals except in case of Co where there is drop in the cell volume. The drop in the cell volume for Co analogue is due to the major contribution of small size trivalent Yb atoms which was already confirmed in the magnetic susceptibility of two compounds where Yb5Ni4Ge10 has only 78% Yb3+ and Yb5Co4Ge10 has 94% Yb3+.
The comparison of covalent radii in Figure 3 also suggests that the metals like Fe, Ru, Os, Pt and Pd are
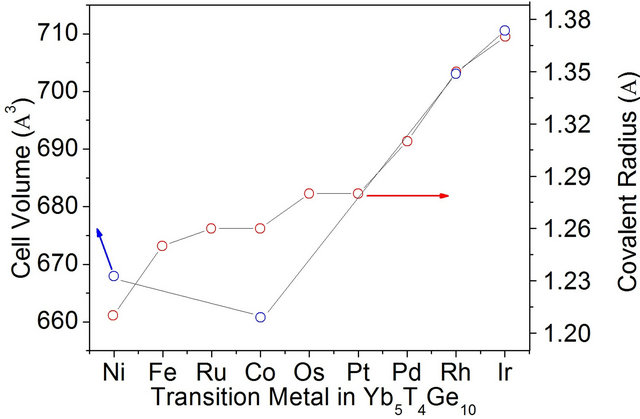
Figure 3. Comparison of the cell volume and covalent radii of transition metals in the Yb5T4Ge10 family.
suitable candidates for the formation Yb5T4Ge10 compounds because the covalent radii of these metals are within the range of the reported compounds. This assumption can also be supported by the fact that Yb has the ability to show mixed valent compounds to stabilize the compounds.
4. Concluding Remarks
High quality single crystals of Yb5Co4Ge10 were obtained from the metal flux technique and the crystal structure of Yb5Co4Ge10 was studied using single crystal X-ray diffraction. Metal flux technique has been proved as vital synthesis method to obtain high quality single crystals. An absolute structure-property relation can be established by understanding the crystal structure from the single crystal X-ray data. Our study opens a lot of scope to find the crystal structure of the compounds which have not been studied as listed in Table 1 and the synthesis of new compounds as expected based on covalent radii comparison of transition metals.
5. Acknowledgements
We thank Prof. C. N. R. Rao for his support and guidance. Financial support from JNCASR, DST and Sheikh Saqr Laboratory is gratefully acknowledged. Udumula Subbarao thanks CSIR for research fellowship and Sebastian C. Peter thanks DST for Ramanujan Fellowship.
REFERENCES
- H. F. Braun, K. Yvon and R. M. Braun, “Sc5T4Si10 (T = Co, Rh, Ir) and Y5T4Ge10 (T = Os, Ir) with a New Tetragonal Structure Type,” Acta Crystallographica Section B, Vol. 36, No. 10, 1980, pp. 2397-2399. doi:10.1107/S0567740880008825
- S. C. Peter, S. Rayaprol, M. C. Francisco and M. G. Kanatzidis, “Crystal Structure and Properties of Yb5Ni4Ge10,” European Journal of inorganic Chemistry, Vol. 2011, No. 26, 2011, pp. 3963-3968. doi:10.1002/ejic.201100350
- K. Ghosh, S. Ramakrishnan, A. D. Chinchure, K. Jonason, V. R. Marathe, G. Chandra and S. Shah, “Heat-Capacity Studies in the Y5-xDyxOs4Ge10 System,” Physical Review B, Vol. 51, No. 17, 1995, pp. 11656-11663. doi:10.1103/PhysRevB.51.11656
- K. Ghosh, S. Ramakrishnan and G. Chandra, “Magnetism in the R5Ir4Si10 (R = Ho and Er) Systems,” Physical Review B, Vol. 48, No. 6, 1993, pp. 4152-4155. doi:10.1103/PhysRevB.48.4152
- G. Venturini, M. Méot Meyer, E. McRae, J. F. Maréché and B. Roques, “Vingt Nouveaux Germaniures Ternaires TR5T4Ge10 de Metaux tr des Terres Rares et T = Co, Rh, Ir. Supraconductivite de Lu5Rh4Ge10 et Lu5Ir4Ge10,” Materials Research Bulletin, Vol. 59, No. 12, 1984, pp. 1647- 1652. doi:10.1016/0025-5408(84)90242-3
- H. F. Braun, G. Burri and L. Rinderer, “Partial Phase Diagram of the System Sc-Rh-Si,” Journal of the Less Common Metals, Vol. 68, No. 1, 1979, pp. 1-8. doi:10.1016/0022-5088(79)90281-9
- O. I. Bodak, O. L. Sologub, P. Y. Zavalii and V. E. Zavodnik, “Crystal Structure of the Compound Ho5Os4Ge10,” Russian Metallurgy (Metally), Vol. 6, 1991, pp. 156-158.
- O. I. Bodak and O. L. Sologub, “The Ho-Os-Ge System,” Russian Journal of Inorganic Chemistry, Vol. 36, 1991, pp. 1353-1355.
- O. L. Sologub, K. Hiebl, P. Rogl and H. Noël, “Magnetic Behavior of Holmium-Platinum Group Metal-Germanium Ternary Alloys,” Journal of Alloys and Compounds, Vol. 245, No. 1-2, 1996, pp. L13-L17. doi:10.1016/S0925-8388(96)02409-7
- L. S. Hausermannberg and R. N. Shelton, “Low Temperature Heat Capacity Study of Superconducting Ternary Silicides with the Sc5Co4Si10-Type Structure,” Physica B+C, Vol. 135, No. 1-3, 1985, pp. 400-404. doi:10.1016/0378-4363(85)90516-9
- N. G. Patil and S. Ramakrishnan, “Magnetism in the R5T4Sn10 (R = Ce, Pr, and Nd; T = Rh and Ir) System,” Physical Review B, Vol. 56, No. 6, 1997, pp. 3360-3371. doi:10.1103/PhysRevB.56.3360
- H. D. Yang, R. N. Shelton and H. F. Braun, “Superconductivity and Electronic Instability at High Pressure in Sc5Co4Si10-Type Compounds,” Physical Review B, Vol. 33, No. 7, 1986, pp. 5062-5065. doi:10.1103/PhysRevB.33.5062
- H. D. Yang, P. Klavins and R. N. Shelton, “Low-Temperature Physical Properties of R5Ir4Si10 (R = Dy, Ho, Er, Tm, and Yb) Compounds,” Physical Review B, Vol. 43, No. 10, 1991, pp. 7688-7694. doi:10.1103/PhysRevB.43.7688
- S. Ramakrishnan, K. Ghosh and G. Chandra, “Magnetism and Superconductivity in Sc5-xDyxIr4Si10 Alloys,” Physical Review B, Vol. 46, No. 5, 1992, pp. 2958-2963. doi:10.1103/PhysRevB.46.2958
- G. Venturini, B. Malamana and B. Roques, “New Rare Earth—Transition Metal Stannides with Sc5Co4Si10 and TiMnSi2—Type Structures. Atomic Size Effects on Their Stability,” Materials Research Bulletin, Vol. 24, No. 9, 1989, pp. 1135-1139. doi:10.1016/0025-5408(89)90071-8
- R. E. Gladyshevskii, E. Parthé, O. L. Sologub and P. S. Salamakha, “Crystal Structure of Pentaholmium Tetrarhodium Decagermanium, Ho5Rh4Ge10 with Sc5Co4Si10 Type,” Zeitschrift für Kristallographie, Vol. 203, No. 1, 1993, pp. 115-116. doi:10.1524/zkri.1993.203.Part-1.115
- L. S. Hausermannberg and R. N. Shelton, “Low-Temperature Heat-Capacity Study of Superconducting Ternary Silicides and Germanides with the Sc5Co4Si10-Type Structure,” Physical Review B: Condensed Matter, Vol. 35, No. 13, 1987, pp. 6659-6664. doi:10.1103/PhysRevB.35.6659
- H. F. Braun and C. U. Segre, “The Superconductivity of Sc5T4Si10 (T = Co, Rh, Ir) and Isomorphous Compounds,” Solid State Communications, Vol. 35, No. 10, 1980, pp. 735-738. doi:10.1016/0038-1098(80)91065-0
- N. G. Patil and S. Ramakrishnan, “Magnetism and Superconductivity in M5Rh4Ge10 (M = Gd, Tb, Dy, Ho, Er, Tm, Lu, and Y),” Physical Review B, Vol. 59, No. 14, 1999, pp. 9581-9589. doi:10.1103/PhysRevB.59.9581
- M. Kolenda, M. Hofmann, J. Leciejewicz, B. Penc and A. Szytula, “Neutron-Diffraction Studies of R5Rh4Ge10 (R = Tb, Ho, Er) Compounds,” Applied Physics A, Vol. 74, No. 1, 2002, pp. S769-S771. doi:10.1007/s003390201582
- S. Ramakrishnan, K. Ghosh and G. Candra, “Antiferromagnetism in the Dy5Ir4Si10 System,” Physical Review B, Vol. 45, No. 18, 1992, pp. 10769-10770. doi:10.1103/PhysRevB.45.10769
- K. Katoh, T. Tsutsumi, K. Yamada, G. Terui, Y. Niide and A. Ochiai, “Magnetic and Transport Properties of Yb5T4Ge10 (T = Co, Rh, Ir),” Physica B: Condensed Matter, Vol. 373, No. 1, 2006, pp. 111-119. doi:10.1016/j.physb.2005.11.098
- P. Villars and K. Cenzual, “Pearson’s Crystal Data-Crystal Structure Database for Inorganic Compounds,” ASM International, Materials Park, 2010.
- Inorganic Crystal Structure Database, “Fachinformationszentrum Karlsruhe GmbH: Eggenstein-Leopoldshafen,” Karlsruhe, 2012.
- B. Kindler, D. Finsterbusch, R. Graf and F. Ritter, “MixedValence Transition in YbInCu4,” Physical Review B, Vol. 50, No. 2, 1994, pp. 704-707. doi:10.1103/PhysRevB.50.704
- E. Bauer, “Anomalous Properties of Ce-Cuand Yb-CuBased Compounds,” Advances in Physics, Vol. 40, No. 4, 1991, pp. 417-534. doi:10.1080/00018739100101512
- P. Wachter, “Handbook on the Physics and Chemistry of Rare Earths,” Elsevier Science, Amsterdam, 1994, p. 177.
- Y. Matsumoto, S. Nakatsuji, K. Kuga, Y. Karaki, N. Horie, Y. Shimura, T. Sakakibara, A. H. Nevidomskyy and P. Coleman, “Quantum Criticality without Tuning in the Mixed Valence Compound Β-YbAlB4,” Science, Vol. 331, No. 6015, 2011, pp. 316-319. doi:10.1126/science.1197531
- U. Subbarao and S. C. Peter, “Crystal Structure of YbCu6In6 and Mixed Valence Behavior of Yb in YbCu6–XIn6+X (X = 0, 1, and 2) Solid Solution,” Inorganic Chemistry, Vol. 51, No. 11, 2012, pp. 6326-6332. doi:10.1021/ic300552w
- S. Ernst, S. Kirchner, C. Krellner, C. Geibel, G. Zwicknagl, F. Steglich and S. Wirth, “Emerging Local Kondo Screening and Spatial Coherence in the Heavy-Fermion Metal YbRh2Si2,” Nature, Vol. 474, 2011, pp. 362-366. doi:10.1038/nature10148
- U. Subbarao and S. C. Peter, “Crystal Growth and Properties of YbCuGa3: First Monoclinic System in the RETX3 Family,” Crystal Growth & Design, Vol. 13, No. 2, 2013, pp. 953-959. doi:10.1021/cg301765f
- U. Subbarao, M. Gutmann and S. C. Peter, “New Structure Type in the Mixed-Valent Compound YbCu4Ga8,” Inorganic Chemistry, Vol. 52, No. 4, 2013, pp. 2219- 2227. doi:10.1021/ic302688n
- S. C. Peter, S. M. Disseler, J. N. Svensson, P. Carretta and M. J. Graf, “Yb4LiGe4—A Yb Mixed Valent Zintl Phase with Strong Electronic Correlations,” Journal of Alloys and Compounds, Vol. 516, No. 516, 2012, pp. 126- 133. Doi: 10.1016/J.Jallcom.2011.11.148
- S. C. Peter and M. G. Kanatzidis, “The New Binary Intermetallic YbGe2.83,” Journal of Solid State Chemistry, Vol. 183, No. 9, 2010, pp. 2077-2081. doi: 10.1016/J.Jssc.2010.06.022
- S. C. Peter, J. Salavador, J. B. Martin, W. Wong-Ng and M. G. Kanatzidis, “New Intermetallics YbAu2In4 and Yb2Au3In5,” Inorganic Chemistry, Vol. 49, No. 22, 2010, pp. 10468-10474. doi:10.1021/ic101502e
- M. Chondroudi, S. C. Peter, C. D. Malliakas, M. Balasubramanian, Q. Li and M. G. Kanatzidis, “Yb3AuGe2In3: An Ordered Variant of the YbAuIn Structure Exhibiting Mixed-Valent Yb Behavior,” Inorganic Chemistry, Vol. 50, No. 4, 2011, pp. 1184-1193. doi:10.1002/ejic.201100350
- S. C. Peter, S. Rayaprol, M. C. Francisco and M. G. Kanatzidis, “Crystal Structure and Properties of Yb5Ni4Ge10,” European Journal of Inorganic Chemistry, Vol. 2011, No. 26, 2011, pp. 3963-3968. doi:10.1021/ja204971n
- S. C. Peter, M. Chondroudi, C. D. Malliakas, M. Balasubramanian and M. G. Kanatzidis, “Anomalous Thermal Expansion in the Square-Net Compounds RE4TGe8 (RE = Yb, Gd; T = Cr-Ni, Ag),” Journal of the American Chemical Society, Vol. 133, No. 35, 2011, pp. 13840-13843. doi:10.1021/ja204971n
- S. C. Peter, S. Sarkar and M. G. Kanatzidis, “Metallic Yb2AuGe3: An Ordered Superstructure in the AlB2-Type Family with Mixed-Valent Yb and a High-Temperature Phase Transition,” Inorganic Chemistry, Vol. 51, No. 20, 2012, pp. 10793-10799. doi:10.1021/ic301197w
- SAINT, “Bruker AXS,” 6.02 ed., SAINT, Madison, 1999.
- G. M. Sheldrick, SADABS, “Empirical Absorption Correction Program,” University of Göttingen, Göttingen, 1997.
- L. J. Farrugia, “WinGX Suite for Small-Molecule SingleCrystal Crystallography,” Journal of Applied Crystallography, Vol. 32, No. 4, 1999, pp. 837-838. doi:10.1107/S0021889899006020
- G. M. Sheldrick, “A Short History of SHELX,” Acta Crystallographica Section A, Vol. A64, No. 1, 2008, pp. 112-122. doi:10.1107/S0021889899006020
- SHELXTL 5.10, “Bruker Analytical X-Ray Systems,” Madison, 1997.
- Crystal Impact, “Crystal Impact GbR,” Version 3.g, Crystal Impact, Bonn, 2011.
- R. T. Sanderson, “Electronegativity and Bond Energy,” Journal of the American Chemical Society, Vol. 105, No. 8, 1983, pp. 2259-2261. doi:10.1021/ja00346a026
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, “Table of Bond Lengths Determined by X-Ray and Neutron Diffraction,” Journal of the American Chemical Society, Perkin Transactions 2, Vol. 12, 1987, pp. S1-S19. doi:10.1039/p298700000s1
NOTES
*Crystallographic information files (CIF).
#Corresponding author.

