Open Journal of Stomatology
Vol.3 No.2(2013), Article ID:32049,5 pages DOI:10.4236/ojst.2013.32030
SEM evaluation of apical intraradicular dentine cleanliness and degree of erosion after the application of three irrigating solutions
![]()
Department of Restorative Dentistry and Endodontics, University of Rome “Tor Vergata”, Rome, Italy
Email: manuele.mancini@tiscali.it
Copyright © 2013 Manuele Mancini, Luigi Cianconi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 19 March 2013; revised 25 April 2013; accepted 8 May 2013
Keywords: EDTA; Nitric Acid; Phosphoric Acid; Irrigation; Smear Layer
ABSTRACT
Aims: The aim of the study was to compare the efficacy of 37% phosphoric acid, 12% nitric acid, 17% EDTA in endodontic smear layer removal and degree of erosion in the apical third of endodontic canals. Methods and Material: One hundred and four extracted single-rooted human teeth were randomized into four groups (n = 26) and instrumented using ProTaper Universal Ni-Ti rotary instruments. Each canal was irrigated with one of the following solutions: 37% phosphoric acid, 12% nitric acid, 17% EDTA, 5.25% NaOCl (control). All specimens were then irrigated with 5 mL distilled water and dried with sterile paper points. Kruskal-Wallis and Mann-WhitneyU statistical tests were used. Results: Scanning electron microscopy (SEM) evaluation showed no significant differences among test reagents in smear layer removal. However, the efficacy of 12% nitric acid and 17% EDTA in removing the smear layer was significantly greater than 5.25% NaOCl (control). Degree of erosion could not be evaluated. Conclusion: Protocols used in this study were not sufficient to completely remove the smear layer in the apical third of shaped root canals.
1. INTRODUCTION
Mechanical shaping of root canals was shown by many authors to create a smear layer covering the endodontic walls [1,2]. Smear layer contains inorganic and organic material1. Despite controversy over maintaining the smear layer, it was shown that the smear layer itself may contain bacteria and protect the bacteria within the dentinal tubules [3]. It has also been demonstrated that smear layer forms a barrier between the filling material and sound dentin that inhibits the penetration of irrigants into dentinal tubules, increases microleakage with commonly used sealers, and decreases the bond strength of resin based materials [4-6]. Currently, a final irrigation with chemicals such as ethylenediaminetetraacetic acid (EDTA) and sodium hypochlorite (NaOCl) is recommended to remove the inorganic and organic components of the smear layer [1,4,7]. However, there is concern that this combined irrigation regime causes inadvertent erosion of the intraradicular dentin [8-11]. Phosphoric acid is used to remove smear layer, both in restorative dentistry and endodontics [12-14]. A similar chelating agent, nitric acid, has been studied by few authors under its capacity to remove restorative dentistry smear layer [15]. Despite the relevant literature available concerning the effect of these agents on the smear layer removal, the small number of studies with similar parameters, such as time intervals, concentrations and methodologies limits the ability of valid comparisons between different treatment protocols. Therefore, the aim of this study was to assess the smear layer removal ability and the erosive effects of 37% phosphoric acid, 12% nitric acid, 17% EDTA through scanning electron microscopy (SEM) digital image analysis of the apical third of instrumented root canals.
2. METHODS AND MATERIAL
2.1. Tooth Selection
One hundred and four periodontally involved human maxillary central incisors with single straight root canal extracted from 28- to 64-year-old patients were selected, with the approval of the Ethics in Research Committee. The teeth were devoid of caries, cracks, endodontic treatments or restorations. Only teeth with intact and mature root apices were selected. After extraction, teeth were stored in 2% thymol solution at room temperature, and used within 1 week.
2.2. Root Canal Preparation
The teeth were decoronated to standardized root length of 15 mm and randomly divided into one of four groups (n = 26). The working lengths were measured by deducting 1 mm from lengths recorded when the tips of #10 or #15 K-files (Dentsply Maillefer, Ballaigues, Switzerland) were visible at the apical foramina. Hot glue was used to seal apexes of all teeth to prevent the flow of irrigants through them. The specimens were shaped using ProTaper Universal Ni-Ti rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland) according to the manufacturer’s instructions until ProTaper F4 file reached the working length. Each instrument was only used for the preparation of four teeth. After using each file and before proceeding to the next, canals were irrigated with 2 mL of 5.25% NaOCl 37˚C (Chematek SpA, Rome, Italy). After instrumentation, all teeth underwent final irrigation as follows: (1) phosphoric acid Group, 1 mL of 37% phosphoric acid for 1 minute followed by 3 mL of 5.25% NaOCl 37˚C; (2) nitric acid Group, 1 mL of 12% nitric acid (Chematek SpA, Rome, Italy) for 1 minute followed by 3 mL of 5.25% NaOCl 37˚C; EDTA Group, 1 mL of 17% EDTA (Chematek SpA, Rome, Italy) for 1 minute followed by 3 mL of 5.25% NaOCl 37˚C; and (4) control Group, 1 mL of 5.25% NaOCl 37˚C for 1 minute followed by 3 mL of 5.25% NaOCl 37˚C. The irrigating solutions were delivered via a sterile 30-gauge NiTi needle (Stropko NiTi Flexi-Tip, SybronEndo, Orange, USA), which penetrated to within 1 to 2 mm of the working length. The root canals then were irrigated with 5 mL of distilled water and dried with sterile paper points.
2.3. Specimen Preparation
Scanning electron microscopy was used to evaluate endodontic smear layer removal (“cleanliness”) and erosion in the apical third of the instrumented root canals. To prepare the samples for imaging, the teeth/roots were usually split longitudinally in the bucco-lingual plane. To facilitate fracture into two halves, all roots were grooved longitudinally on the external surface with a diamond disc, avoiding penetration of the root canals. The roots were then split in two halves with a chisel. For each root, the half containing the most visible part of the apex was conserved and coded. The coded specimens were secured on metal stubs, desiccated, sputter-coated with gold, and viewed with SEM (Digital scanning microscope, DSM 950, Carl Zeiss, Oberkochen, Germany).
2.4. Scanning Electron Microscope Preparation
The cleanliness and degree of erosion was evaluated at 2 mm from the apical foramen of each canal wall and photographed at 2000× magnification. The views were divided into sixteen subareas by overlaying a grid. Two observers performed blind evaluation independently after the examination of 26 specimens jointly for calibration purposes. Intraexaminer and interexaminer reliability for the SEM assessment was verified by the Kappa test. Cleanliness was scored according to the following criteria [16]: score 0 = No smear layer (no smear layer on the surface of the root canals with all tubules clean and open); score 1 = Moderate smear layer (no smear layer on the surface of root canals, but tubules contain debris); score 2 = Heavy smear layer (smear layer covers the root canal surface and the tubules). The same observers scored the degree of erosion of dentinal tubules as follows: score 0 = No erosion (all tubules look normal in appearance and size); score 1 = Moderate erosion (peritubular dentin is eroded); score 2 = Severe erosion (intertubular dentin is destroyed, and tubules are connected to each other). Data were analyzed using Kruskal-Wallis and Mann-WhitneyU tests (software used: XLSTAT ver. 2012. 6.08); p values were computed and compared with statistical significance at the p = 0.05 level.
3. RESULTS
Kappa test results, with a significance set at 0.5, showed good intraexaminer and interexaminer agreement with values ranging from 0.90 and above for the different groups. Table 1 shows cleanliness and degree of erosion findings. Specimens treated with 17% EDTA (EDTA group) showed a thick smear layer at the apical third; virtually no erosion was seen in any specimen of the EDTA Group (Figure 1(a)). Samples treated with 37% phosphoric acid (phosphoric acid group) showed a heavy smear layer in the apical third similar to the control group (Figure 1(c)). Samples treated with 12% nitric acid (nitric acid group) showed presence of a smear layer (Figure 1(b)). The degree of erosion could not be statistically evaluated because of the few areas devoid of smear layer among the specimens. Samples in the 5.25% NaOCl 37˚C (control group) showed a heavy smear layer (Figure 1(d)).
Statistical Analysis
Table 1 shows results of the statistical comparison between groups for cleanliness. Significant values were between p < 0.05 and p < 0.0001. No significant difference in cleanliness was found between the control group
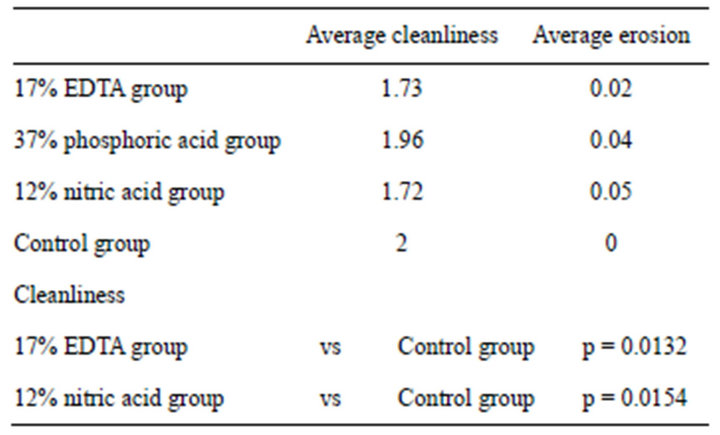
Table 1. Statistical analysis on presence/absence of smear layer and degree of erosion.
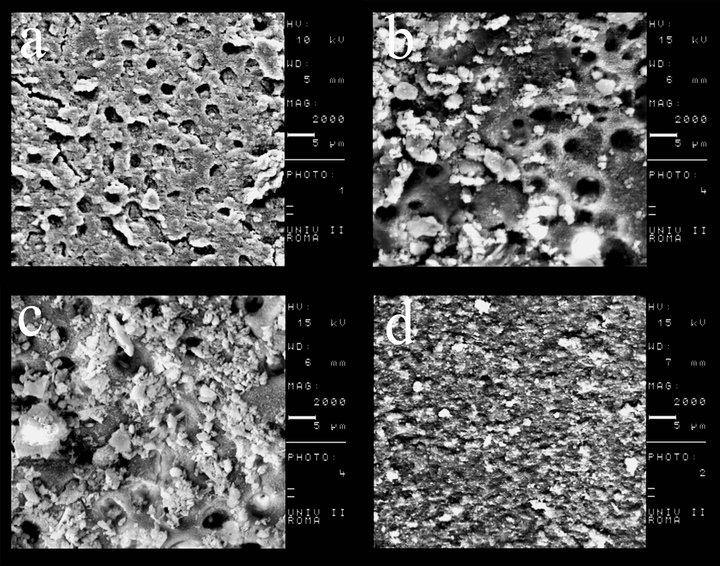
Figure 1. (a) 17% EDTA at the apical third, 2000×; (b) 12% nitric acid at the apical third, 2000×; (c) 37% phosphoric acid at the apical third, 2000×; (d) control group at the apical third, 2000×.
and phosphoric acid group, while the EDTA group and nitric acid group showed significant differences with control (both p < 0.05). The EDTA and nitric acid groups exhibited more efficient removal of the smear layer than the control group. The statistical analysis on the degree of erosion could not be performed due to the small number of specimens with evaluable areas.
4. DISCUSSION
The main purpose of this investigation was twofold: 1) evaluation of the effectiveness of three acidic solutions (37% phosphoric acid, 12% nitric acid, 17% EDTA) in removing the smear layer in the apical third of shaped canals, and 2) evaluation of the degree of erosion caused by these solutions. Removal of smear layer in the apical third has always been a challenge; therefore the experimental process was focused on this area. Within the study, phosphoric acid, a strong acid routinely used in dentistry to remove the smear layer and smear plugs formed during coronal cavity preparations [17], nitric acid, a very low pH acidic molecule, used in restorative dentistry and EDTA, a well-known chelating agent widely used to remove inorganic components of the smear layer [8,9], were analysed. Although some studies on the ability of phosphoric acid in removing the smear layer from root canals are available in the literature [18], the concentrations used are rather low (below 5% and 24%) compared with the ones used to remove the smear layer from coronal dentin. As far as we are concerned, there is no study in the literature comparing phosphoric acid, nitric acid and EDTA, at the same concentrations as those used in the present study. The use of a high concentration of phosphoric acid may carry a higher risk of cytotoxicity, especially when used in the apical third of the root canal. Therefore, the use of gel might be preferred than the liquid form although no study evaluating this effect in the periapical tissue was found in the literature. To minimize the destructive effects on dentin reported by some investigators [9,16], we used a low volume (1 ml) of chelating agents for a short application time (1 minute). The effectiveness of endodontic files, rotary instrumentation, irrigating solutions, and chelating agents to clean, shape, and disinfect root canals influence mostly the success, longevity, and reliability of modern endodontic treatments. Nevertheless, controversy still exists regarding the effectiveness of the myriad of file systems, ultrasonic irrigation, irrigating solutions, and chelating agents used to accomplish the chemomechanical cleansing of the root canal system [19]. ProTaper F4 was used because, it has been shown that larger canal diameter exposes the dentin to a higher volume of irrigants, allowing a better flow of the solution and, hence, further improving the efficiency of smear layer removal [16,20]. Within the limits of our ex-vivo study, the following conclusions can be drawn: 1) 12% nitric acid, 17% EDTA or 37% phosphoric acid did not cleanse endodontic walls in the apical third, and 2) the evaluation of the erosion in the apical third was not possible because none of the irrigants was able to completely remove the smear layer from the endodontic walls. Because the goal of the present work was restricted to a limited area of the three-dimensional endodontic system, the application of these results to the clinical situation is not straightforward. Sodium hypochlorite solutions remain the most widely recommended irrigant in endodontics on the basis of its unique capacity to dissolve necrotic tissue remnants and excellent antimicrobial potency [21]. However, in this study, the specimens of the control group showed the presence of the smear layer from at apical third of the canals, which is consistent with results previously reported by some authors [18,22]. In addition to NaOCl, the use of a chelating agent has been advocated to rid the root canal system of the smear layer. It is believed that removing this layer could dissolve attached microbiota and their toxins from root canal walls, improve the seal of root canal fillings, and reduce the potential of bacterial survival and reproduction [2,3]. However, the results from the present study demonstrated that treatment with 1 mL 17% EDTA 5.25% NaOCl 37˚C failed to clean the root canal system (Figure 1(a)), and left remnants of the smear layer in the apical third. This finding is essentially in agreement with previous studies indicating that this irrigating combination is less effective in the apical third of canals [11, 14,22]. Khedmat and Shokouhinejad [18] and Saito et al. [23] showed results that are in accordance with ours, using similar volume, concentration and time of application of EDTA at the apical third level. In contrast with our results, Baumgartner and Mader [2], and Calt and Serper [9], found that the combination of 17% EDTA and 5% NaOCl is an effective irrigating solution in removing the smear layer in the apical third of instrumented canals. These different results may be explained by the different volume of irrigants used (from 3 to 10 mL). Lui et al. [24] found that a 1-minute irrigation with 17% EDTA followed by a final flush of NaOCl successfully achieved smear-free walls in instrumented root canals. This result might be attributable to the fact that the Authors activated the irrigant solutions with an ultrasonic tip to within 1 to 2 mm of the root apex. In our study, 12% nitric acid did not remove the smear layer from the apical third of the canals. This finding cannot be compared with previous papers, due to the lack of studies on this acid used as irrigating solutions in endodontics. Evaluation of the degree of erosion subsequent the action of irrigants on dentinal walls was one goal of our investigation. Due to the heavy smear layer, only very few specimens were analysed for erosion. Moreover, small areas of free-smearlayer specimens were evaluable, insufficient to perform a statistical analysis of degree of erosion. Therefore, we could conclude that none of the irrigating solutions showed erosion at the apical third although erosive effects of EDTA and phosphoric acid have been reported in several studies [9,25].
5. CONCLUSION
Based on the results of this study, the application of 1 mL of 37% phosphoric acid, 12% nitric acid, 17% EDTA, or 5.25% NaOCl 37˚C for 1 minute followed by 3 mL of 5.25% NaOCl was not sufficient to completely remove the smear layer in the apical third. Erosive effects of irrigants could not be analyzed. Further methodologically sound in vitro investigations are needed to evaluate irrigant activation systems, influence on adhesion, and cytotoxicity of tested solution in order to enable an appropriate evaluation of cleanliness and erosion of endodontic canals.
REFERENCES
- McComb, D. and Smith, D.C. (1975) A preliminary scanning electron microscopic study of root canals after endodontic procedures. Journal of Endodontics, 1, 238-242. doi:10.1016/S0099-2399(75)80226-3
- Mader, C.L., Baumgartner, J.C. and Peters, D.D. (1984) Scanning electron microscopic investigation of the smeared layer on root canal walls. Journal of Endodontics, 10, 477-483. doi:10.1016/S0099-2399(84)80204-6
- Torabinejad, M., Handysides, R., Khademi, A.A. and Bakland, L.K. (2002) Clinical implications of the smear layer in endodontics: A review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology Endodontics, 94, 658-666. doi:10.1067/moe.2002.128962
- Clark-Holke, D., Drake, D., Walton, R., et al. (2003) Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. Journal of Dentistry, 31, 275-281. doi:10.1016/S0300-5712(03)00032-0
- Shahravan, A., Haghdoost, A.A., Adl, A., et al. (2007) Effect of smear layer on sealing ability of canal obturation: A systematic review and meta-analysis. Journal of Endodontics, 33, 96-105. doi:10.1016/j.joen.2006.10.007
- Economides, N., Liolios, E., Kolokuris, I., et al. (1999) Long-term evaluation of the influence of smear layer removal on the sealing ability of different sealers. Journal of Endodontics, 25, 123-125. doi:10.1016/S0099-2399(99)80010-7
- Mello, I., Robazza, C.R., Antoniazzi, J.H., et al. (2008) Influence of different volumes of EDTA for final rinse on smear layer removal. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology Endodontics, 106, 40-43. doi:10.1016/j.tripleo.2008.06.019
- Baumgartner, J.C. and Mader, C.L. (1987) A scanning electron microscopic evaluation of four root canal irrigation regimens. Journal of Endodontics, 13, 147-157. doi:10.1016/S0099-2399(87)80132-2
- Calt, S. and Serper, A. (2002) Time-dependent effects of EDTA on dentin structures. Journal of Endodontics, 28, 17-19. doi:10.1097/00004770-200201000-00004
- Niu, W., Yoshioka, T., Kobayashi, C. and Suda, H. (2002) A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. International Endodontic Journal, 35, 934-939.
- Torabinejad, M., Cho, Y., Khademi, A.A., Bakland, L.K. and Shabahang, S. (2003) The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. Journal of Endodontics, 29, 233-239. doi:10.1097/00004770-200304000-00001
- Jacques, P. and Hebling, J. (2005) Effect of dentin conditioners on the microtensile bond strength of a conventional and a self-etching primer adhesive system. Dental Materials, 21, 103-109. doi:10.1016/j.dental.2003.12.004
- Miyasaka, K. and Nakabayashi, N. (1999) Combination of EDTA conditioner and phenyl-P/HEMA self-etching primer for bonding to dentin. Dental Materials, 15, 135- 157. doi:10.1016/S0300-5712(99)00025-1
- Pérez-Heredia, M., Ferrer-Luque, C.M. and GonzálezRodríguez, M.P. (2006) The effectiveness of different acid irrigating solutions in root canal cleaning after hand and rotary instrumentation. Journal of Endodontics, 32, 993-997. doi:10.1016/j.joen.2006.05.016
- Eick, J.D., Robinson, S.J., Byerley, T.J., Chappell, R.P., Spencer, P. and Chappelow, C.C. (1995) Scanning transmission electron microscopy/energy-dispersive spectroscopy analysis of the dentin adhesive interface using a labeled 2-hydroxyethylmethacrylate analogue. Journal of Dental Research, 74, 1246-1252. doi:10.1177/00220345950740060201
- Torabinejad, M., Khademi, A.A., Babagoli, J., et al. (2003) A new solution for the removal of the smear layer. Journal of Endodontics, 29, 170-175. doi:10.1097/00004770-200303000-00002
- Ayad, M.F. (2001) Effects of rotary instrumentation and different etchants on removal of smear layer on human dentin. Journal of Prosthetic Dentistry, 85, 67-72. doi:10.1067/mpr.2001.112792
- Khedmat, S. and Shokouhinejad, N. (2008) Comparison of the efficacy of three chelating agents in smear layer removal. Journal of Endodontics, 34, 599-602. doi:10.1016/j.joen.2008.02.023
- Crumpton, B.J., Goodell, G.G. and McClanahan, S.B. (2005) Effects on smear layer and debris removal with varying volumes of 17% REDTA after rotary instrumentation. Journal of Endodontics, 31, 536-538. doi:10.1097/01.don.0000148871.72896.1d
- Teixeira, C.S., Felippe, M.C. and Felippe, W.T. (2005) The effects of application time of EDTA and NaOCl on intracanal smear layer removal: An SEM analysis. International Endodontic Journal, 38, 285-290. doi:10.1111/j.1365-2591.2005.00930.x
- Zehnder, M. (2006) Root canal irrigants. Journal of Endodontics, 32, 389-398. doi:10.1016/j.joen.2005.09.014
- Ciucchi, B., Khettabi, M. and Holz, J. (1989) The effectiveness of different endodontic irrigation procedures on the removal of the smear layer: A scanning electron microscopic study. International Endodontic Journal, 22, 21-28. doi:10.1111/j.1365-2591.1989.tb00501.x
- Saito, K., Webb, T.D., Imamura, G.M. and Goodell, G.G. (2008) Effect of shortened irrigation times with 17% ethylene diamine tetra-acetic acid on smear layer removal after rotary canal instrumentation. Journal of Endodontics, 34, 1011-1014. doi:10.1016/j.joen.2008.05.014
- Lui, J.N., Kuah, H.G. and Chen, N.N. (2007) Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. Journal of Endodontics, 33, 472- 475. doi:10.1016/j.joen.2006.12.007
- De-Deus, G., Paciornik, S., Pinho Mauricio, M. and Prioli, R. (2006) Real-time atomic force microscopy of root dentin during demineralization when subjected to chelating agents. International Endodontic Journal, 39, 683-692. doi:10.1111/j.1365-2591.2006.01128.x

