Open Journal of Nursing
Vol.4 No.1(2014), Article ID:42290,6 pages DOI:10.4236/ojn.2014.41008
Feasibility of bioelectric impedance as a measure of muscle mass in mechanically ventilated ICU patients
![]()
Symptom Management College of Nursing, The Ohio State University, Newton Hall, Columbus, USA
Email: Chlan.1@osu.edu
Copyright © 2014 Linda L. Chlan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Linda L. Chlan. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 8 November 2013; revised 21 December 2013; accepted 14 January 2014
KEYWORDS
Mechanical Ventilation; Bioimpedance; Muscle Mass; ICU-Acquired Weakness
ABSTRACT
Background: Critically ill patients receiving prolonged mechanical ventilatory support are at risk for loss of muscle mass and muscle strength from immobility. Feasible, non-invasive methods are needed to accurately obtain data on markers of muscle mass to design effective interventions and monitor patient progress during recovery from critical illness. Bioelectric impedance has been used in other settings to obtain data on body composition and muscle mass. Purpose: The aims of this study were to determine the feasibility of bioelectric impedance as a marker of muscle mass in a sample of mechanically ventilated patients and to assess data trends in these obtained values. Methods: A descriptive design was used to obtain standard bioelectric impedance parameters (total body resistance, legs resistance, and percent lean body mass) over 4 days from eligible patients already enrolled in a randomized clinical trial. Results: Bioimpedance parameters were readily obtained over 4 days in a sample of 43 patients (age 59 + 15.7 years, 56% male) receiving prolonged ventilatory support (mean 9.4 + 10.4 days) due to respiratory failure. Reasons for not obtaining impedance measures included skin impairment, monitoring devices, or presence of implantable cardiac defibrillator or pacemaker. Average total body impedance was 464.3 + 117.1 ohms, while average impedance of legs was 479.1 + 146.4 ohms. Lean body mass was 68.4% (+10.8). Conclusions/Implications for Practice: With carefully trained staff and a standardized measurement protocol, bioimpedance is a feasible method to obtain body composition data reflective of muscle mass in mechanically ventilated patients. Further research will determine the utility of bioimpedance to monitor recovery and effectiveness of interventions to restore function after prolonged periods of ventilatory support and immobility in mechanically ventilated patients.
1. INTRODUCTION
In the United States, more than 5 million persons admitted annually to intensive care units (ICU) receive mechanical ventilation and approximately 34% of these patients require prolonged ventilatory support (PVS), defined as longer than 48 hours [1]. Critically ill patients receiving PVS display a variety of muscle wasting syndromes labeled collectively as ICU-acquired weakness (ICU-AW) [2]. ICU-AW is a very common problem, with a prevalence of 58% - 96% in mechanically ventilated patients [3]. Factors such as systemic inflammation, corticosteroids and neuromuscular blocking agents, electrolyte disturbances, and immobility have been implicated in the pathogenesis of ICU-AW [2]. Respiratory muscle weakness is associated with peripheral limb weakness, which significantly delays weaning from ventilatory support [4]. Reduced quality of life in patients receiving PVS has been documented primarily in the physical domains and is associated with pulmonary symptoms, abnormal pulmonary function, and persistent muscle wasting and weakness [5]. Skeletal muscle atrophy from disuse is of particular concern and significantly affects recovery in elderly ICU patients receiving mechanical ventilation [6].
Immobility and prolonged bed rest result in loss of muscle strength and muscle mass, contributing significantly to ICU-AW [7]. Interventions are needed to combat this ICU problem. Feasible, non-invasive methods to measure muscle mass and strength are needed to monitor recovery from critical illness in response to interventions to restore functional status. While previous investigations have reported on assessment of muscle strength [7-10], there is a paucity of information on methods for measuring muscle mass. To begin to fill this gap, this paper describes bioelectric impedance as a measure of body composition and its potential as a marker of skeletal muscle mass in patients receiving prolonged mechanical ventilatory support (PVS). The purpose of this descriptive study was to determine the feasibility of obtaining bioimpedance measures in critically ill patients receiving PVS and to assess for any discernible data trends in these obtained values.
2. BIOELECTRIC IMPEDANCE AS A MEASURE OF BODY COMPOSITION AND MUSCLE MASS
The basic principle underlying bioelectric impedance is that if an alternating current is applied to a substance, energy will be dissipated as it travels through the substance, producing a measurable voltage [11]. Bioimpedance measurements are obtained by applying an alternative electrical current of a known frequency, amplitude, and timing to a substance. As the electrical current travels through the substance, it loses energy due to the substance’s inherent resistance, thus reducing its amplitude [11]. Bioimpedance analysis is the assessment of change in electrical tissue conductivity that indicates altered body composition [12].
Bioelectric impedance measurements have been widely used to determine body composition in non-ICU settings [13-15] and have been used to document loss of skeletal muscle mass associated with functional impairment and disability in older adults [16]. The measurements are usually made between a wrist and a foot or between the feet. A small, high frequency current (typically 50 kHz) is applied to one pair of electrodes and the resulting voltage on the skin is measured from another pair of electrodes. This approach eliminates skin resistance from the measurement [17]. The current is conducted in the limbs by intraand extra-vascular fluids and the skeletal muscles. Fat and bone do not contribute significantly to the measurement because of their high resistivity. The commonly reported impedance parameters are the magnitude of the impedance (the ratio of voltage/current) also referred to as the resistive component, and the phase angle or reactance, measured in ohms. The phase angle is a measure of the delay between the measured voltage and current waveforms. The phase angle results from the electrical capacitive characteristics of the tissue’s cell membranes. In the case of measurements made up mostly of peripheral tissues, the phase angle is due to muscle tissue. Body fluids do not contribute to the reactive part of an impedance measurement. The patient experiences no sensation during the impedance measurements as the muscle and nerve membranes are not depolarized by the applied low-intensity current [6]. Impedance measurements do not require any effort by the subject and have been shown to be very repeatable in assessing data trends [18].
Any atrophy that reduces the cross-sectional area of muscle tissue would increase the resistance [6]. Aaron and Shiffman [19] have reported the impedance phase angle to decrease with various muscle diseases and injury conditions. Rutkove and colleagues [18] demonstrated in persons with acute amyotrophic lateral sclerosis that there was a significant decrease in impedance phase angle and an increase in resistance as the disease progressed. While there are no reports in the literature of bioimpedance as a marker of muscle mass in mechanically ventilated patients, for this study it was expected that with changes in muscle mass and strength as a result of experiencing PVS and immobility, the phase angle would decrease and the impedance magnitude increase (resistance), reflecting changes in the muscle structure in these patients.
3. METHODS
3.1. Design
A descriptive study design was used to determine the feasibility of obtaining bioimpedance measures as a marker of muscle mass in critically ill patients receiving PVS and to examine the data for any discernible data trends. Feasibility can be defined as “capable of being done or carried out” [20]. Feasibility was operationalized for this study as whether bioimpedance measures could be reasonably done in mechanically ventilated patients.
The data reported here are from a sub-sample of mechanically ventilated patients (n = 286) already enrolled for a multi-ICU randomized clinical trial (RCT) whose aim was to test music for anxiety self-management. In the parent study, patients were randomized to one of three groups: 1) patient-initiated music listening, 2) active control of noise-cancelling headphones only, or 3) usual ICU care. Details on the parent study protocol can be found elsewhere [21].
3.2. Setting
Patients for the bioimpedance measurements were obtained from 4 of 12 Midwestern ICUs participating in the parent RCT. These sites were a combination of medical and medical-surgical ICUs, including both teaching and non-teaching hospitals. Human subjects’ approval was obtained from the parent institution and from all participating hospitals.
3.3. Sample
Any patient enrolled in the parent study from the 4 ICUs was eligible for bioimpedance measurements. Impedance was not obtained in those patients with an implantable cardiac defibrillator device, pacemaker, skin impairment or the presence of a monitoring device or catheter in any of the electrode placement areas. Bioimpedance measurements were obtained from 43 patients. Refer to Table 1 for the sample demographic characteristics.
4. BIOIMPEDANCE MEASUREMENT PROTOCOL
Bioelectric impedance measurements were made daily over 4 days during a 6-month period. This time frame was chosen to reflect the median length of time patients were maintained on protocol for the parent study and to minimize any data collection burden. Research nurses were trained in obtaining bioimpedance measures as outlined below; refer to Table 2 for details.
Both global (total body and lean muscle mass) and local measurements (legs) were obtained. The resistive and reactive impedance was measured at 50 kHZ using the Biodynamics Bioimpedance Analyzer (Model 450) with the patient in a supine position. The electrodes were placed on the dorsum of both the right foot and hand. The arms and legs were abducted to prevent them from touching either the trunk or each other. To obtain local muscle measurements of the legs, electrodes were placed over the tibialis anterior muscle which directly reflected changes in a muscle minimizing other tissue or fluid volume changes.
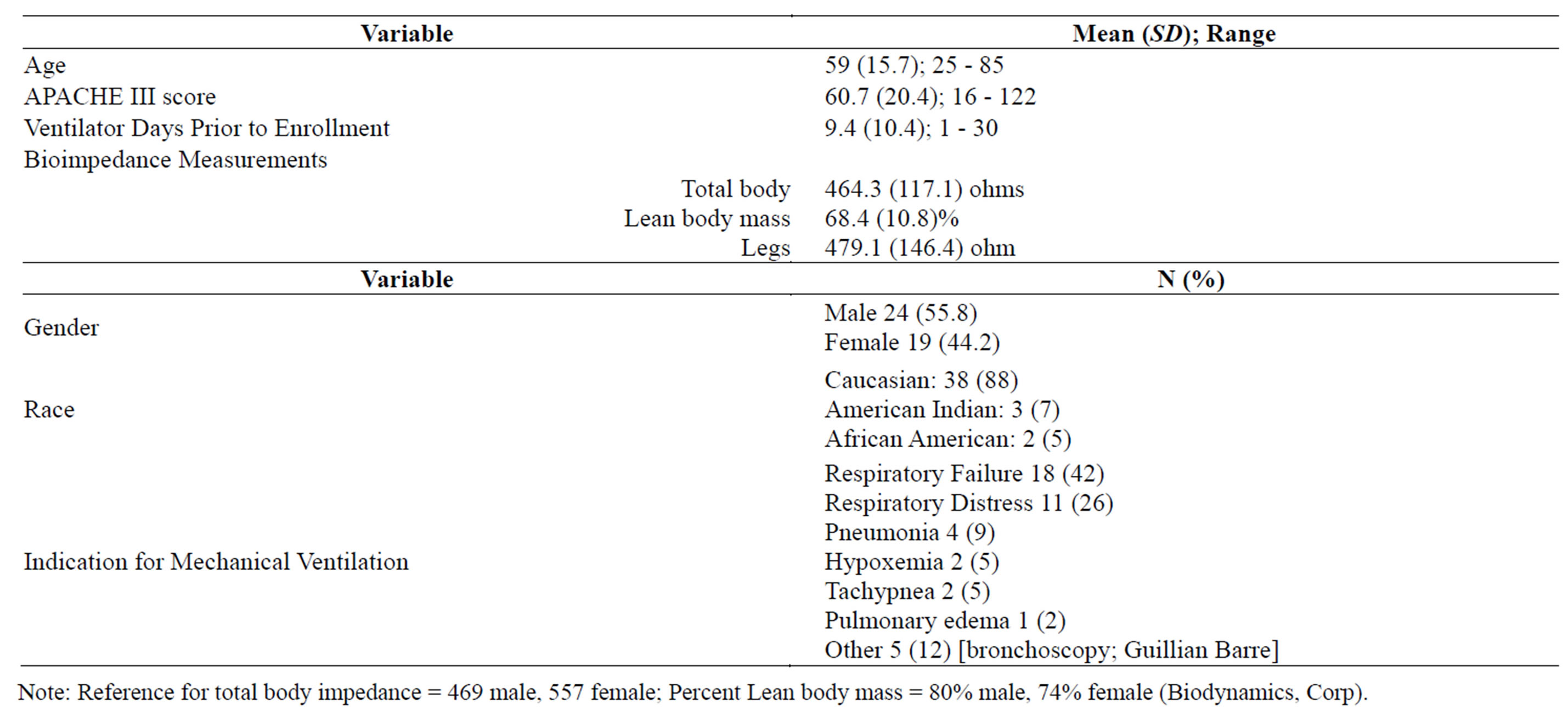
Table 1. Demographic Characteristics of Study Sample (n = 43).

Table 2. Bioimpedance Measurement Protocol.
Analysis
Descriptive statistics and graphing of bioimpedance data were used to address the study aims and assess for any data trends.
5. RESULTS
The purpose of this descriptive study was to determine the feasibility of obtaining serial bioimpedance measurements and to assess for any data trends in this sample of patients.
Study entry mean resistance and lean body mass measurements are reported in Table 1. These parameters are within normal range for adults [12], except for the percent lean body mass. Percent lean body mass in this sample of patients is lower at 64% than the normal adult values of 80% for males and 74% for females [12].
5.1. Feasibility of Obtaining Bioimpedance Measurements
Feasibility was operationalized as whether bioimpedance measures could reasonably be done in mechanically ventilated patients. Based on the findings obtained, bioimpedance indeed is feasible as measures were obtained in a select sample of patients.
While obtaining bioimpedance parameters was feasible, there are a number of measurement challenges with this and any biophysiological measure in critically ill patients that is not unique to impedance. Given the critical nature of patients admitted, the pool of eligible patients for bioimpedance measurements was limited due to our exclusion criteria and the inability to place the 7 electrodes. Reasons measurements were not obtained from other patients enrolled in the parent RCT included: compression stockings/devices in place that were not to be removed (36%); patient request (11%); patient up in chair (13%); skin impairment, breakdown or rash (9%); patient in procedure/off unit (6%); nurse requested to not disturb patient (5%); other requests such as family, patient weaning, unstable or invasive catheter or monitoring device present (18%).
Once patients were eligible for impedance data collection, the measurements were obtained without incidence and in an efficient manner. However, the skin consistency or surface area for some patients prevented the electrodes from firmly adhering to the skin, despite the research nurse’s best effort. This proved to be frustrating for both research staff and patients.
While the actual time to obtain the impedance values is a few minutes, the time it takes to prepare and position the patient, including precise placement of the 7 electrodes, can be laborious. Patients need to be in bed in a supine position with no contact of the legs or arms to one another. If a patient was up in the chair or had just been re-positioned by the bed side nurse, the patient’s position was not changed to accommodate the impedance measurements.
5.2. Assessment of Data Trends: Pattern of Impedance Parameters over Study Period
A second purpose of this study was to discern any data trends. Figures 1-3 depict graphic presentation of data trends from the individual impedance parameters (resistance) and lean body mass in this sample of mechanically ventilated patients over a period of 4 days. The heavy black line indicates the mean value for each impedance measure. For total body impedance, the average for this sample of available patients trends upward at day 3 and day 4, which may suggest an increase in resistance and loss of muscle mass (Figure 1). The percent lean body mass remains fairly consistent over the study period in those patients who provided impedance measures (Figure 2). Of interest is the average resistance in the legs; impedance values in the available patient sample appear to trend upwards after day 2 and continue to rise at day 3 and day 4. In the sample of available patients, this may suggest loss of muscle mass in the legs (Figure 3).
6. DISUCSSION
This paper reports some of the first data on serial bioimpedance measurements obtained from a small sample of patients receiving PVS for an average of 9 days. A majority of patients in this feasibility study were male over the age of 60 years. Regardless, patients had similar total body muscle mass as indicated by impedance resistance values close to healthy adults. This sample of patients had slightly lower percent lean body mass as compared to healthy adult normals. Given that four data collection points were not available from all patients in this study, further discussion of data trends is not possible.
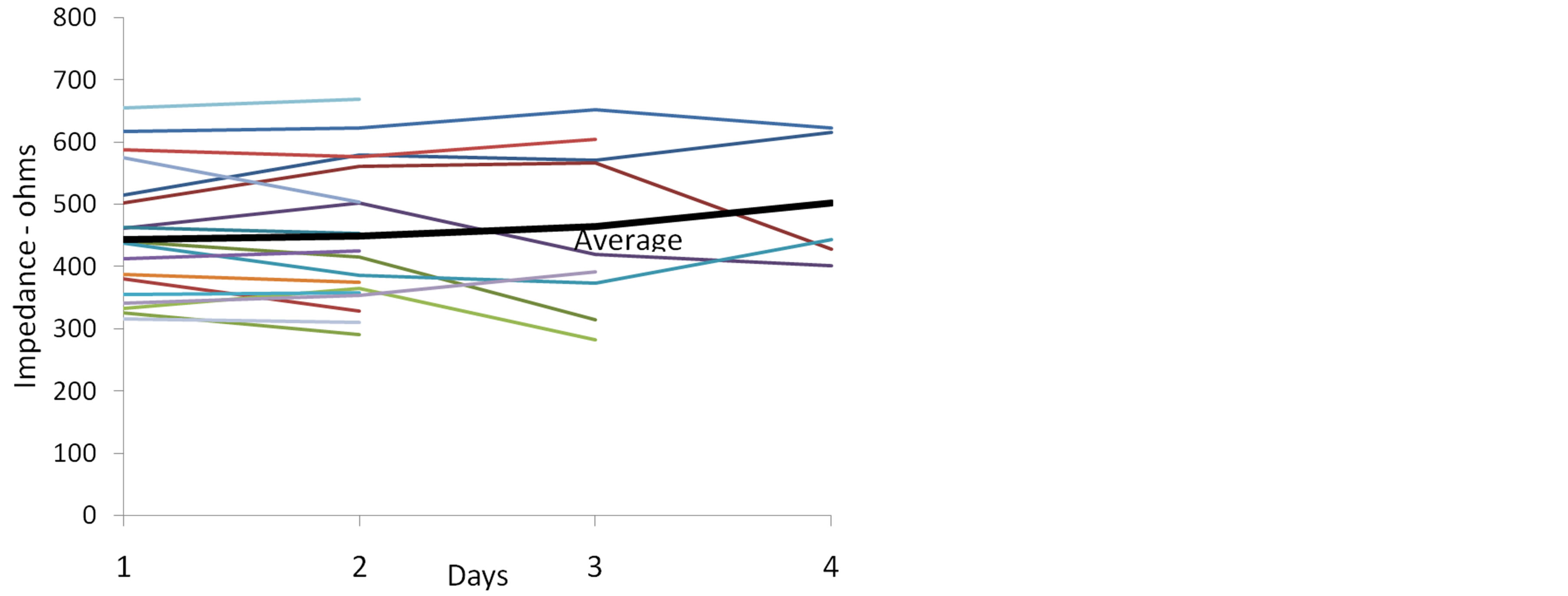
Figure 1. Total Body Impedance (right leg + trunk + right arm) over Study Period (resistance).
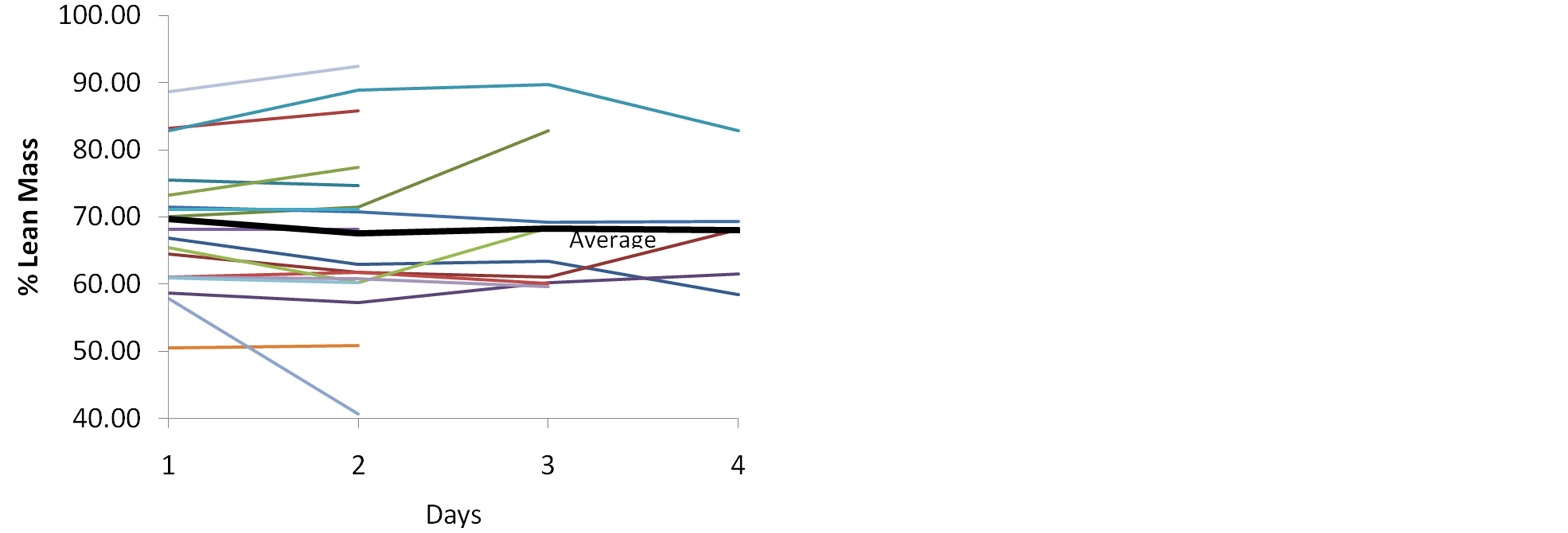
Figure 2. Percent Lean Body Mass over Study Period.
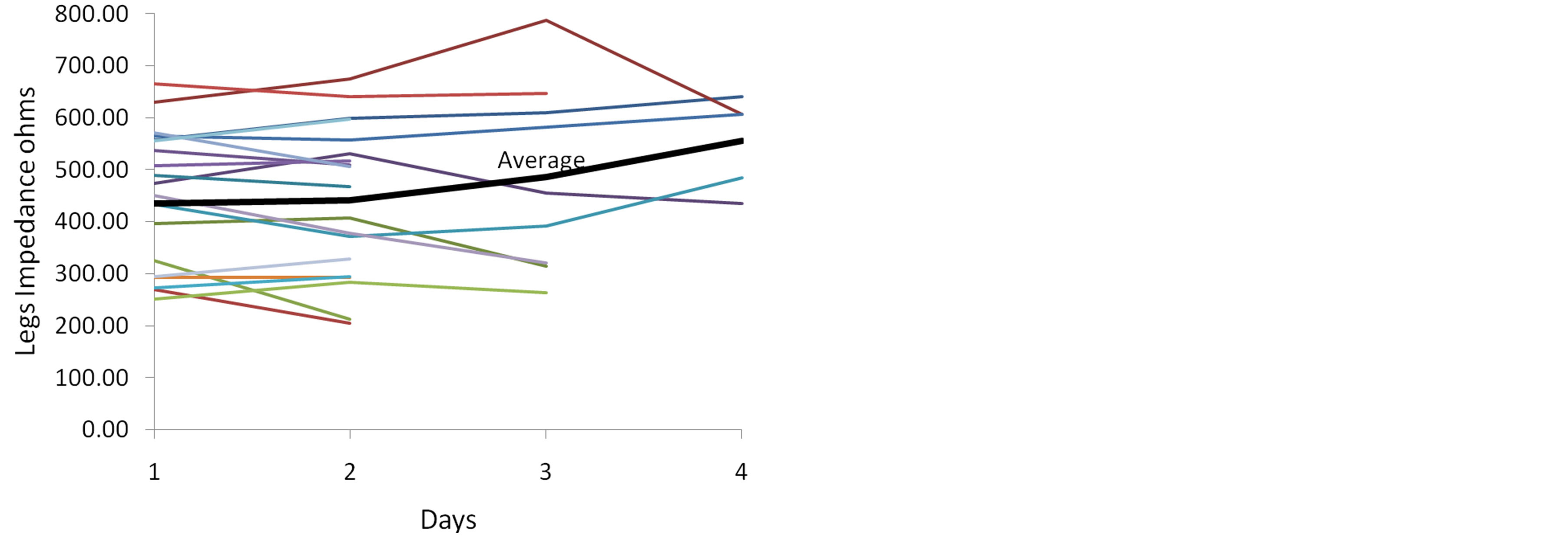
Figure 3. Impedance of the Legs over Study Period (resistance).
6.1. Limitations
There are several limitations to this study, including a small sample of only 43 patients with 4 serial assessments. Further, data were not obtained from each of the 43 patients each of the 4 days, which limits the interpretation of the data trends discussion. The patient position in bed needs to be supine for measurements, which was not always possible if the patient was sitting up in the chair or had just been repositioned by the nurse. In addition, we were not able to obtain impedance data from all patients enrolled in the parent study due to placement of monitoring lines and other devices for patient care, which limited our sample size. However, for the first assessment of the feasibility of bioimpedance in this challenging patient population, it is important to adhere to the measurement protocol and obtain quality data. Lastly, we did not obtain proxy evaluation from family as to the patient’s functional status prior to ICU admission. This evaluation may have shed some light on the impedance data, most prominently the leg resistance results.
6.2. Implications for Further Research and Clinical Practice
As with any clinical research study, it can be a challenge to obtain data on biophysiological parameters in critically ill patients. Investigators are advised to include as many patients as possible in any further research using impedance in the ICU. It is important to monitor impedance values over time to obtain assess data trends as isolated values are not meaningful. Likewise, correlating body composition measures, proxy report of functional status prior to ICU admission, and laboratory data such as protein and albumin levels may provide insight into these findings and suggest areas for future investigation. Impedance values could be obtained in patients when they are first admitted to the ICU to provide baseline data on body composition as it is a non-invasive measurement that does not require any volitional effort. These data can be used to monitor patients’ body composition as they recover from critical illness, and may be used to eventually predict which patients may require extensive postICU care and rehabilitation. Interventions are needed to promote activity and weaning as early as possible to minimize decrements in muscle mass and strength. Activity and muscle preservations exercises need to be incorporated early into routine ICU care.
While respiratory muscle strength was not assessed in this study, other investigators have suggested a link between decreased muscle mass and strength with difficulty weaning from ventilatory support due to respiratory muscle weakness and fatigue [7]. Every day that a patient is mechanically ventilated is a day that may go by without weight-bearing exercises or mobility to prevent further loss of muscle. Future research is needed before the bioimpedance can be recommended as a useful measure to monitor innovative interventions to minimize loss of muscle mass and strength in patients receiving PVS.
ACKNOWLEDGEMENTS
Supported in part by a grant from the National Institutes of Health, National Institute of Nursing Research (1R01 NR009295) and from a seed grant from the Center for Excellence in Critical Care, Academic Health Center, University of Minnesota.
REFERENCES
- Cox, C., Carson, S., Govert, J., Chelluri, L. and Sanders, G. (2007) An economic evaluation of prolonged mechanical ventilation. Critical Care Medicine, 35, 1918-1927. http://dx.doi.org/10.1097/01.CCM.0000275391.35834.10
- Schweikert, W. and Hall, J. (2007) ICU-acquired weakness. CHEST, 131, 1541-1549. http://dx.doi.org/10.1378/chest.06-2065
- Chelluri, L., Im, K. and Belle, S. (2004) Long-term mortality and quality of life after prolonged mechanical ventilation. Critical Care Medicine, 32, 61-69. http://dx.doi.org/10.1097/01.CCM.0000098029.65347.F9
- De Jonghe, B., Bastuji-Garin, S. and Durand, M. (2007) Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Critical Care Medicine, 35, 2007-2015. http://dx.doi.org/10.1097/01.ccm.0000281450.01881.d8
- Jackson, J., Mitchell, N. and Hopkins, R. (2009) Cognitive functioning, mental health, and quality of life in ICU survivors: An overview. Critical Care Clinics, 25, 615- 628. http://dx.doi.org/10.1016/j.ccc.2009.04.005
- Taruli, A.W., Duggal, N., Esper, G.J., Garmirian, L.P., Fogerson, P.M., Lin, C.H., et al. (2009) Electrical impedance myography in the assessment of disuse atrophy. Archives of Physical Medicine and Rehabilitation, 90, 1806- 1810. http://dx.doi.org/10.1016/j.apmr.2009.04.007
- Fan, E., Zanni, J., Dennison, C., Lepre, S. and Needham D. (2009) Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit. AACN Advanced Critical Care, 20, 243-253. http://dx.doi.org/10.1097/NCI.0b013e3181ac2551
- Ali, N., O’Brien, J. and Hoffman, S. (2008) Acquired weakness, handgrip strengths, and mortality in critically ill patients. American Journal of Respiratory and Critical Care Medicine, 178, 261-268. http://dx.doi.org/10.1164/rccm.200712-1829OC
- Hodgin, K., Nordon-Craft, A., McFann, K., Mealer, M. and Moss, M. (2009) Physical therapy utilization in intensive care units: Results from a national survey. Critical Care Medicine, 37, 561-568. http://dx.doi.org/10.1097/CCM.0b013e3181957449
- Schweikert, W., Pohlman, M., Pohlman, A. and Kress, J.P. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet, 373, 1874-1882. http://dx.doi.org/10.1016/S0140-6736(09)60658-9
- Rutkove, S.B. (2009) Electrical impedance myography: Background, current state, and future directions. Muscle and Nerve, 40, 936-946. http://dx.doi.org/10.1002/mus.21362
- Biodynamics Corporation. (2004-2009) Quick start guide for the BIA 450. www.biodyncorp.com
- Lukaski, H.C. and Bolunchuk, W.W. (1988) Estimation of body fluid volumes using tetrapolar bioelectrical impedance measurements. Aviation, Space and Environmental Medicine, 59, 1163-1169.
- Lukaski, H., Johnson, P., Bolunchuk, W.W. and Lykken, G. (1985) Assessment of fat free mass using bioelectrical measurements of the human body. American Journal of Clinical Nutrition, 41, 810-817.
- Mattsson, S. and Thomas, B. (2006) Development of methods for body composition studies. Physical Medicine and Biology, 51, R203-R228. http://dx.doi.org/10.1088/0031-9155/51/13/R13
- Janssen, I., Heymsfield, S. and Ross R. (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society, 50, 889-896. http://dx.doi.org/10.1046/j.1532-5415.2002.50216.x
- Patterson, R. (2000) Bioelectric Impedance Measurements. In: Bronzio, J.D., Ed., Biomedical Engineering Handbook (Vol. 1), CRC Press and IEEE Press, 731-735.
- Rutkove, S.B., Zhang, H., Schoenfeld, D.A., Raynor, E.M., Shefner, J.M., Cudkowicz, M.E., et al. (2007) Electrical impedance myography to assess outcome in amyotrophic lateral sclerosis clinical trials. Clinical Neurophysiology, 118, 2413-2418. http://dx.doi.org/10.1016/j.clinph.2007.08.004
- Arron, R. and Shiffman, C.A. (2000) Using localized impedance measurements to study muscle changes in injury and disease. Annals of the New York Academy of Sciences, 904, 171-180. http://dx.doi.org/10.1111/j.1749-6632.2000.tb06443.x
- Merriam-Webster Dictionary. http://www.merriam-webster.com/dictionary/feasible
- Chlan, L., Weinert, C., Heiderscheit, A., Tracy, M.F., Skaar, D.J., Guttormson, J. and Savik, K. (2013) Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: A randomized clinical trial. JAMA: The Journal of the American Medical Association, 309, 2335-2344. http://dx.doi.org/10.1001/jama.2013.5670

