Open Journal of Immunology
Vol.2 No.4(2012), Article ID:26300,8 pages DOI:10.4236/oji.2012.24024
Memory and effector cells in children with bacterial infections of the gastrointestinal and respiratory tracts
![]()
1Department of Health Sciences, Autonomus Metropolitan University, Mexico City, Mexico; monikapm@hotmail.com, mcgt@xanum.uam.mx, leor@xanum.uam.mx
2Faculty of Medicine, National Autonomous University of Mexico, Mexico City, Mexico; mapr@servidor.unam.mx
3Maternal Pediatric of Government Hospital, Mexico City, Mexico; consuelocarmen@yahoo.com, fer.vach@gmail.com
4Department of Agricultural and Animal Production, Autonomus Metropolitan University, Mexico City, Mexico; dmartin@correo.xoc.uam.mx
5Department of Health Care, Autonomus Metropolitan University, Mexico City, Mexico;
*Corresponding Author: onajera@correo.xoc.uam.mx
Received 29 September 2012; revised 22 October 2012; accepted 30 October 2012
Keywords: Memory Cells; Effector Cells; Bacterial Infection; Immune Response
ABSTRACT
Infections in infants and children under five years of age are a public health in México and are one of the major causes of death. Methods In this study, lymphocyte immunophenotyping for CD3+ (T-cells), CD3+CD4+, CD3+CD8+, CD3+CD19+, CD3+CD16/56+, CD45RA+, CD45RO+, CD62L− and CD28− were determined in the whole blood of gastrointestinal and respiratory bacterial infected children, using a fourcolor flow cytometry technique. Results: Our data showed that the percentages and the absolute numbers of monocytes and granulocytes are increased in infected children, when compared to the control group. Similarly, we observed increases in the percentages of B lymphocytes, CD8+ cells, memory T cells (CD4+CD45RO+ and CD8+CD45RO+) and effector lymphocytes (CD4+CD62L− and CD8+CD28−) in infected children compared with the control group. In contrast, naive T cells were decreased in the bacterial infected children relative to the control group. Additionally, we used ELISA assays to identify the pathogen agent in gastrointestinal and respiratory infection. Comparing different types of infection, we found that the children with respiratory bacterial infections had higher percentages of B lymphocytes, and cytotoxic lymphocytes (CD8+CD28−); and the children with gastrointestinal infections had higher percentages of CD3+ lymphocytes and effector cells (CD4+CD62L−). Conclusions The increase in B lymphocytes and CD8+CD28− cells in the children with respiratory infections and the increase of T lymphocytes and CD4+CD62L− cells in the children with gastrointestinal bacterial infections indicate that both cellular and humoral responses coincide, and both responses are necessary for eliminating the pathogen.
1. INTRODUCTION
Gastrointestinal and respiratory infections are a public health problem in Mexico because of their high incidence rates and high morbidity and mortality in infants and children under five years of age [1].
Bacterial infections are common, and the resolution of these infections requires the activation of the innate and adaptive immune systems [2]. The adaptive immune system is composed of multiple, functionally distinct cell types, including CD3+CD4+CD8− and CD3+CD4−CD8+ lymphocytes [3]. The cellular subpopulations express the common lymphocyte antigen CD45 on their cell surface. The CD45 antigen is a membrane glycoprotein with two isoforms, CD45RA and CD45RO. CD45RA is expressed in naïve cells that have not encountered an infectious agent, whereas CD45RO is expressed in memory cells that have previously responded to an infectious agent [4, 5].
T lymphocytes also express phenotypic markers that are related to their effector functions, such as the surface markers CD28 and CD62L (L-selectin). The loss of these markers denotes the differentiation process into CD28- and CD62L− cells. CD28− cells exhibit cytotoxic functions and produce cytokines (IFN-γ and TNFα) [6,7]. CD62L− cells are effector memory cells that migrate towards sites of inflammation and are rapidly stimulated by pathogens to execute effector functions, including the production of cytokines, such as IFN-γ, IL-4 and IL-5. CD62L− cells are particularly abundant in the lung, liver and intestine [8-10].
CD62L is expressed on lymphocytes and is involved in regulating cellular trafficking by binding to its ligands on the vascular endothelium [8-10]. CD28 is a costimulatory molecule that binds to its ligand, CD86, on antigen-presenting cells (APCs) and provides the second signal required for T cell activation [11]. The enumeration of lymphocyte subsets provides important information for the diagnosis and monitoring of a variety of conditions affecting the immune system.
In this study, we evaluated the changes in leukocyte, naïve and memory (CD45RA+ and CD45RO+, respectively) and effector (CD4+CD62L− and CD8+CD28−) cell populations due to serious respiratory or gastrointestinal bacterial infection.
2. MATERIALS AND METHODS
2.1. Patients and Blood Samples
Peripheral blood samples were obtained from pediatric patients at the Xochimilco Maternal-Pediatric Government Hospital of Mexico City. The samples were collected from 30 uninfected children and 30 children with serious bacterial infection that required hospitalization. The exclusion criteria for all children were: viral diseases, tuberculosis, congenital diseases, autoimmune and heart diseases, birth complications, preterm births and recent blood transfusions. The bacterial infections were diagnosed rigorously based on clinical data and routine laboratory tests. In the infected group comprising 12 girls and 18 boys, 9 children presented with gastrointestinal bacterial infections and 21 presented with respiratory bacterial infections. The ages of the children ranged from 6 months to 5 years, and the weights and heights of the children were within the normal range for their age based on the values established for Mexican children [12]. In the uninfected group of children, there were 9 girls and 21 boys.
The uninfected children had sought medical attention for outpatient surgeries for the treatment of phimosis in the boys and hernias in the girls. The children with bacterial infection of the respiratory tract presented with fever, cough and varying degrees of respiratory failure. The children with bacterial infection of the gastrointestinal tract presented with diarrhea, fever and different degrees of dehydration.
The Medical Committee on Ethics of the General Direction of Medical Services of the GDF, Mexico City, approved this study.
2.2. Phenotypic Staining of Cells from Whole Blood
The blood samples were collected in tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.) with anti-coagulant EDTA and processed the same day. The following combinations of conjugated antibodies were used in this study: FITC-anti-CD45/PE-anti-CD14; FITC-anti-CD3/PE-anti-CD16+anti-CD56/PerCP-anti-CD19; FITC-anti-CD45RA/PE-anti-CD45RO/PerCP-anti-CD3/APC-anti-CD4; FITC-anti-CD45RA/PerCP-anti-CD45RO/PE-anti-CD3/APC-anti-CD8; FITC-anti-CD4/PE-anti-CD62L/PerCP-anti-CD3; and FITC-anti-CD8/PE-anti-CD28/PerCP-anti-CD3 (Becton Dickinson, San Jose, CA). Each antibody combination (20 ml) was incubated with 100 µl of whole blood for 20 minutes in the dark at room temperature. After incubation, a lysis solution was added to each tube to eliminate the erythrocytes, and the cells were washed with PBS. The cells were fixed using 1% paraformaldehyde.
2.3. Flow Cytometry Analysis
The data were obtained and analyzed using the CellQuest II software and a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA) calibrated using CaliBRITE beads. For each sample, 10,000 cells were analyzed. The assessment of the leukocyte proportions and different lymphocyte subpopulations was performed based on forward-scatter and FL-1-, FL-3- and FL-4-scatter fluorescence parameters, depending on the specific subpopulation. The data were analyzed using dual fluorescence dot plots (FL-1 vs. FL-2) (Figure 1). The absolute numbers of cells in the different populations were calculated by multiplying the percentage of the selected subpopulation/100 by the total lymphocyte count of the hematic biometry/100 by the leukocyte number of the hematic biometry.
2.4. Determination of the Presence of Antibodies against Infectious Agents by ELISA
Using plasma from the pediatric patients, we determined the presence of antibodies against five infectious agents by enzyme-linked immunosorbent assay (ELISA). This method is sensitive, extremely versatile and easy to handle in research and diagnostic laboratories. The samples from the patients with gastrointestinal infections

Figure 1. (a) and (b) The lymphocyte gate set in the FL-1-SSC, FL-3 or FL-4-SSC distribution (R1), the gates were selected to restrict the an alysis of CD3+, CD4+ and CD8+ positive cells. c) Dot plot FL-1-FL-2 distribution to define native (CD45RA+), memory (CD45RO+) and Ddull (CD45RA+CD45RO+) cells. (d) and (e) Dot plot FL-1-FL-2 distribution, showed CD4+CD62L− and CD8+CD28− effector cells in lower right quadrant.
were tested to determine the presence of antibodies against antigens in the outer membrane proteins of the Escherichia coli strains EHEC (enterohemorrhagic E. coli) and EPEC (enteropathogenic E. coli) and Salmonella typhi. The samples from the patients with respiretory infections were tested to determine the presence of antibodies against Staphylococcus aureus and Klebsiella pneumoniae.
2.5. Statistical Analysis
The mean percentages, mean absolute numbers and standard errors were determined for each group and each cell population using the JMP software, and the statistical significance between groups was determined using the Student’s t-test. A p value of 0.05 or less was considered significant. To determine the bacterial antigens, a cutoff was established based on a method described by Kurstak [13] in which the mean and standard deviation of both subsets (infected and uninfected children) were used to establish a range of minimum and maximum values for uninfected children. The patients above the maximum value of the range were considered positive for the ELISA test.
3. RESULTS
3.1. Lymphocyte Subpopulations
The flow cytometry analysis of the different types of leukocytes demonstrated that the average percentage of total lymphocytes in the peripheral blood was lower in the bacteria-infected children than in the uninfected children (43.1% ± 3.3% vs. 57.5% ± 3.6%, p < 0.006). In contrast, the percentage of granulocytes (47.1% ± 3.2% vs. 35.4% ± 3.6%, p < 0.02) and monocytes (9.4% ± 1.3% vs. 4.0% ± 1.4%, p < 0.008) was significantly higher in the bacteria-infected children compared with the uninfected children. A significant difference was also observed between the uninfected and bacteria-infected children in the absolute numbers of lymphocytes, granulocytes and monocytes in the peripheral blood (p < 0.05); fewer lymphoid cells but more granulocytes and monocytes were observed in the bacteria-infected patients compared with the uninfected patients (Table 1).

Table 1. Percentages and absolute numbers of peripheral blood leukocytes, lymphocytes subsets, helper lymphocytes (CD4+CD62L−) and cytotoxic cells (CD8+CD28L−) in uninfected and infected children.
Statistically significant differences were not observed between the study groups in either the percentage or the absolute numbers of T lymphocytes (CD3+) or NK cells (CD16+CD56+). A higher percentage of B lymphocytes (CD19+) was observed in the infected children compared with the uninfected children (24.2% ± 2.2% vs. 16.6% ± 2.7%, p < 0.03), and a similar trend was observed for the absolute number of B lymphocytes (p < 0.05) (Table 1).
3.2. Effector Cells
In the bacteria-infected children, we observed a higher percentage of CD3+CD62L− effector cells in the total lymphocyte population compared with the uninfected children (35.7% ± 5.5% vs. 24.5% ± 3.6%, p < 0.02). Higher percentages of helper cells and cytotoxic cells were also observed in the infected children compared with the uninfected children; increases were observed in both the CD4+CD62L− (23.8% ± 3.5% vs. 11.3% ± 3.3%, p < 0.01) and CD8+CD28− subsets (30.2% ± 3.1% vs. 20.0% ± 3.1%, p < 0.02). With respect to the latter two subsets, the differences in the absolute cell numbers between the infected and uninfected children demonstrated similar trends as the cell percentages and were statistically significant (p < 0.02 and p < 0.04, respectively) (Table 1).
3.3. Memory Cells
The percentage of CD3+ T lymphocytes was lower in the bacteria-infected children than in the uninfected children (49.1% ± 4.5% vs. 70.1% ± 4.5%, p < 0.001), and a similar trend was observed for the absolute numbers of CD3+ T lymphocytes (p < 0.0001). Statistically significant differences in the percentage and absolute numbers of naive T cells (CD3+CD45RA+) were not observed between the bacteria-infected and uninfected groups. In contrast, a higher percentage of memory T cells (CD3+CD45RO+) was observed in the bacteria-infected children compared with the control group (25.4% ± 1.9% vs. 17.1% ± 1.9%, p < 0.0001), and the absolute number of cells was higher (p < 0.01). There were significantly lower percentages of naïve cells in the infected children compared with the uninfected children (53.6% ± 2.5% vs. 69.9% ± 2.4% for the CD4+CD45RA+ cells, p < 0.0001, and 54% ± 3.1% vs. 73.6% ± 3.0% for the CD8+CD45RA+ cells, p < 0.001). A similar trend was observed for the absolute numbers of these two subpopulations, and the differences between the bacteria-infected and uninfected children were statistically significant (p < 0.007 and p < 0.01, respectively) (Table 2).
A higher percentage of CD8+ T lymphocytes was observed in the peripheral blood of the bacteria-infected

Table 2. Percentages and absolute numbers of peripheral blood naïve (CD45RA+), memory (CD45RO+) and Ddull lymphocyte subsets (CD45RA+CD45RO+) in uninfected and infected children.
children compared with the uninfected children (31.9% ± 2.1% vs. 20.1% ± 2.0%, p < 0.003), and the absolute numbers of CD8+ T lymphocytes demonstrated a similar trend (p < 0.05). The percentages of CD4+CD45RO+ and CD8+CD45RO+ memory cells were also higher in the infected children compared with the uninfected children (35.5% ± 1.9% vs. 16.7% ± 1.8% for the CD4+CD45RO+ cells, p < 0.0001, and 34.0% ± 2.1% vs. 11.3% ± 2.0% for the CD8+CD45RO+ cells, p < 0.0001). The absolute numbers of these two subsets of cells demonstrated a similar trend in the infected and uninfected children and were significantly different (p < 0.002 and p < 0.05, respectively). In addition, the percentage of doublepositive cells (CD4+CD45RA+CD45RO+) was higher in the bacteria-infected children than in the uninfected children (11.0% ± 0.7% vs. 8% ± 0.7%, p < 0.01), and the absolute number of these cells showed a similar trend (p < 0.01) (Table 2).
3.4. Analysis of Children with Gastrointestinal Infection vs. Children with Respiratory Infection
Comparisons between the children with bacterial infections of the gastrointestinal tract and of the respiratory tract did not demonstrate statistically significant differences in the proportions of the different leukocyte cell types. We observed a higher mean percentage of B lymphocytes in the children with respiratory infections (28.3% ± 2.4%) compared with the children with gastrointestinal infections (14.9% ± 3.6%) (p < 0.0001). In contrast, a higher percentage of T lymphocytes was observed in the children with gastrointestinal bacterial infections compared with the children with respiratory bacterial infections (62.8% ± 5.4% vs. 49.8% ± 3.4%, p < 0.05).
Additionally, the children with gastrointestinal bacterial infections showed higher percentages of CD4+CD62L− cells compared with the children with respiratory bacte

Figure 2. Percentages of peripheral blood effectors lymphocytes according to infection type. Dates are expressed as mean ± standard error. n: 30 uninfected. n: 9 gastrointestinal infection. n: 21 respiratory infection. *Significant difference between gastrointestinal and respiratory infection: CD3+ p < 0.0008; CD4+CD62L− p < 0.0002; CD8+CD28− p < 0.04.
rial infections (34.6% ± 6.8% vs. 20.3% ± 3.1%, p < 0.002). In contrast, the children with respiretory bacterial infections exhibited a higher percentage of CD8+CD28− cells compared with the children with gastrointestinal bacterial infections (33.6% ± 3% vs. 23% ± 5.4%, p < 0.04) (Figure 2). When we compared the subpopulations of naïve and memory T lymphocytes, we found that the children with respiratory bacterial infections demonstrated a higher percentage of double-positive CD4+CD45RA+CD45RO+ lymphocytes than the children with gastrointestinal bacterial infections (12.7% ± 0.9% vs. 8.7% ± 1.0%, p < 0.001). The percentage of CD4+CD45RA+ cells was lower in the children with respiratory bacterial infections than in the children with gastrointestinal infections (48.5% ± 4.2% vs. 59.0% ± 3.9%, p < 0.001) (Figure 3).
The ELISA analysis to detect antibodies against specific pathogens demonstrated that 5 of the 9 patients with symptoms of a gastrointestinal infection were positive for E. coli antigens. Of these 5 patients, 3 were positive for the E. coli enterohemorrhagic antigen (EHEC 0157: H7) (0.453 ± 0.139 vs. 0.052 ± 0.300 g/dl, p < 0.05), and 2 were positive for the EPEC antigens (0.400 ± 0.111 vs. 0.286 ± 0.05 g/dl, p < 0.05) compared with the individuals who did not exhibit symptoms of infection. None of the 9 patients with gastrointestinal infections was positive for antibodies against the Salmonella typhi outer membrane proteins.
The results of the ELISA demonstrated that of the 21 patients exhibiting symptoms of a respiratory infection, 14 were positive for Staphylococcus aureus antigens (0.969 ± 0.228 vs. 0.510 ± 0.05 g/dl, p < 0.05) and 4 were positive for Klebsiella antigens (0.632 ± 0.249 vs. 0.310 ± 0.05 g/dl, p < 0.05) compared with uninfected patients. Only 3 patients were negative for the bacterial antigens analyzed in this study.
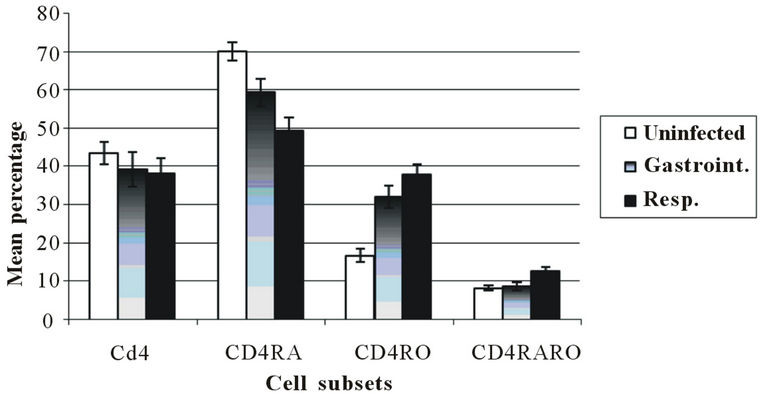
Figure 3. Percentages of peripheral blood naïve (CD45RA+), memory (CD45RO+) and Ddull (CD45RA+CD45RO+) lymphocytes subset according to infection type. Dates are expressed as mean ± standard error. n: 30 uninfected; n: 9 gastro-intestinal infection; n: 21 respiratory infection. *Significant difference between gastrointestinal and respiratory infection: CD4+CD45RA+ p < 0.001; CD4+CD45RA+RO+ p < 0.001.
We did not observe differences in the proportion of leukocytes, memory cells and effectors cells associated with the type of infectious agent.
4. DISCUSSION
Respiratory and gastrointestinal bacterial infections are public health problems that affect children under five years of age in developing countries. Thus, the goal of our study was to evaluate the changes in the proportions of leukocytes, effector cells and naïve and memory cells caused by the common pathogens responsible for severe respiratory and gastrointestinal bacterial infections in Mexican children.
This study is important because bacterial infections were considered as disease entities. Indeed, a number of papers have evaluated the immunological response against specific bacterial agents, such as E. coli and Coxiella burnetii [14,15]. In the current study, we detected E. coli in a number of patients with gastrointestinal infections, and Staphylococcus aureus and Klebsiella were found in patients with respiratory infections. The latter pathogens have not been widely studied.
We observed higher percentages and absolute numbers of granulocytes, monocytes and B lymphocytes in the peripheral blood from the bacteria-infected children compared with the uninfected children. This finding suggests that these cells were proliferating at the time of the sample collection. Granulocytes and monocytes participate in the innate immune response, a critical component of the response early in infection, and act as phagocytic cells and mediators of the inflammatory response [2,16]. In contrast, B lymphocytes, which are part of the adaptive immune system, produce antibodies and under certain circumstances, act as APCs [17]. In addition, because they produce large amounts of secretory IgA, B lymphocytes play an important role in intestinal mucosal immunity [18]. In patients infected with encapsulated bacteria, such as Haemophilus influenzae and Streptococcus pneumoniae, it has been observed that the absolute numbers of peripheral B lymphocytes are increased; the bacterial capsule is a potent immunogenic component that stimulates the synthesis of IgM and other Ig isotypes in B lymphocytes [19].
The percentages and absolute numbers of cells with effector functions (CD3+CD62L−, CD3+CD28−, CD4+CD62L− and CD8+CD28−) were higher in the bacteria-infected children compared with the uninfected children. Effector cells play an important role in controlling infections because they produce many proteins that eliminate pathogens. The functional cytotoxic T lymphocytes (CTLs) produce cytokines and granzymes that kill pathogen-infected cells and control the infection [20]. However, these effector T cells have a short lifespan and die following infection, whereas a smaller subset of cells differentiates into memory cells [21,22].
An increase in CD4+CD62L− cells has been linked to the migration of such cells out of the blood stream and into inflammatory sites, as well as to the subsequent production of cytokines, such as IFN-γ, IL-4 and IL-5. This event is important because a predominance of IFN-γ or IL-4 can direct the immune response towards either a cellular or a humoral response, respectively [23]. Moreover, CD4+ helper T cells can influence effector and memory CD8+ T cell development [20]. Previous studies have shown that the levels of helper (CD4+CD62L−) and cytotoxic (CD8+CD28−) cells are increased in children with gastrointestinal and respiratory bacterial infections compared with uninfected and malnourished children [24]. The increased number of cytotoxic CD8+CD28− cells has been linked to the stimulation of the IL-2 and IL-6 receptors, resulting in the activation and proliferation of the cells and the production of perforin and granzymes, which damage the bacterial membranes and ultimately kill the bacteria by osmotic shock or apoptosis [25,26]. The present study shows the changes in the lymphocyte subpopulations that are associated with the respiratory and gastrointestinal bacterial infections observed most frequently in Mexican children.
Previous studies have demonstrated the changes in the proportion of effector or memory cells in children during bacterial infections; Mycobacterium tuberculosis induces a significant increase in the proportion of effector T cells (CD8+CD45RO−CD62L−CD28−CD27−) [27], and Helicobacter pylori produces an increased percentage of memory/effector CD4+ T cells [28].
In contrast, in the current study, the percentages and absolute numbers of CD45RO+ T cells (CD3+, CD4+ and CD8+) in patients with bacterial infections were higher. The memory T cell population is heterogeneous and consists of multiple subsets exhibiting different characteristics, including effector and proliferative functions. These cells can persist for very long periods of time in the absence of antigen, and their response to reinfections is faster [20]. CD4+ memory T cells recruit and activate both innate (dendritic and NK cells) and adaptive (T and B lymphocytes) immune cells. Together, these cells promote the elimination of infectious organisms, such as bacteria and viruses [29,30]. Memory CD8+ T cells produce cytokines, such as IFN-γ and IL-2. These cytokines contribute to the efficient elimination of infectious pathogens by activating macrophages and NK cells. The cytokines also promote the activation and proliferation of T cells and memory cells, which play an important role in the enhanced response against pathogens that occurs during subsequent infections [20,31].
In the children with respiratory infections, a higher percentage of B lymphocytes and a lower percentage of T lymphocytes were observed in comparison with the children with gastrointestinal bacterial infections. This observation may indicate that respiratory infections trigger a humoral response, whereas gastrointestinal infections trigger a cellular response. Microorganisms exhibit marked structural differences in the cell wall, and these differences are the basis for classifying the bacteria into two main groups, Gram-positive and Gram-negative bacteria. Gram-positive bacteria contain a thick peptidoglycan wall, whereas Gram-negative bacteria have an outer membrane composed of lipopolysaccharides and lipoproteins [32].
Additionally, we observed a higher percentage of helper (CD4+CD62L−) cells in the children with gastrointestinal bacterial infection than in the children with respiratory bacterial infection; these cells are associated with a Th2-type humoral response [8,33]. In contrast, we observed a higher percentage of cytotoxic cells (CD8+CD28−) in the patients with respiratory bacterial infections; these cells are closely related to a Th1-type cellular immune response [34]. These data indicate that gastrointestinal and respiratory bacterial infections induce a systemic cellular and humoral (Th1/Th2) immune response. The cellular and humoral immune responses are multifactorial and result from the interactions of a large number of cells and cytokine patterns that collaborate to maintain an organism in a homeostatic state [35,36].
It is known that the pattern of cytokines secreted by immune cells determines whether a Th1 or Th2 phenotype predominates in the response. Specifically, the cytokines that predominate in a cellular or Th1 response are IFN-γ, TNF-α and IL-2. In contrast, the cytokines that predominate in a humoral or Th2 response are IL-4, IL-6 and IL-10 [35,36]. It should be emphasized that these types of responses are not mutually exclusive. In this study, according to the cell types observed, the humoral and cellular immune responses coexist. It will be important for future studies to characterize the patterns of cytokines and other cells and molecules to understand the mechanisms that govern the response to bacterial infections.
We observed that the different pathogens identified in this study were not associated with specific changes in the proportion of leukocytes, effector cells, naïve cells and memory cells, likely because the number of samples evaluated was small.
5. CONCLUSION
In conclusion, this study demonstrates the importance of utilizing a sensitive four-color immunophenotyping method to assess the changes in lymphocyte subpopulations in bacteria-infected children. Our data demonstrate that in Mexican children, respiratory and gastrointestinal bacterial infections modify the percentages of the host immune cells. A higher percentage of effector and memory cells were found in the infected children. In particular, the respiratory bacterial infections induced an increase in the percentage of B lymphocytes and cytotoxic cells, indicating that the humoral and cellular responses cooperate to eliminate the etiological agent. We observed a similar pattern in the gastrointestinal infections; the data demonstrated an increase in T lymphocytes and CD4+CD62− in the infected children. The results indicate that in the respiratory and gastrointestinal infections, the cellular and humoral responses coincide, and both responses are necessary for eliminating the pathogen and for reducing the severity of the disease.
6. ACKNOWLEDGEMENTS
This study was supported in part by CONACyT, Mexico grants 311030 and 47905 (for Monika Palacios Martínez). PhD. Edith Cortés Barbarena thanks by mounting the cytometry technique.
REFERENCES
- C Instituto Nacional de Estadística y Geografía (2005) Infant mortality. http://www.inegi.gob.mx
- Turvey, S.E. and Broide, D.H. (2010) Innate immunity. Journal of Allergy Clinical Immunology, 125, S24-S32. doi:10.1016/j.jaci.2009.07.016
- Koretzky, G. (2010) Multiple roles of CD4 and CD8 in T cell activation. Journal of Immunology, 185, 2643-2644. doi:10.4049/jimmunol.1090076
- Dawes, R., Petrova, S., Liu, Z., Wraith, D., Beverley, P.C.L. and Tchilian, E.Z. (2006) Combinations of CD45 isoforms are crucial for immune function and disease. Journal of Immunology, 176, 3417-3425.
- Jackson, S.M., Harp, N., Patel, D., Zhang, J., Willson, S., Kim, Y.J., Clanton, C. and Capra, J.D. (2007) CD45RO enriches for activated, highly mutated human germinal center B cells. Blood, 110, 3917-3925. doi:10.1182/blood-2007-05-087767
- Mertens, J., Fabri, M., Zingarelli, A., Kubacki, T., Meemboor, S., Groneck, L., Seeger, J., Bessler, M., Hafke, H., Odenthal, M., Bieler, J.G., Kalka, C., Schneck, J.P., Kashkar, H. and Kalka-Moll, W.M. (2009) Streptococcus pneumoniae serotype 1 capsular polysaccharide induces CD8+CD28-regulatory T lymphocytes by TCR crosslinking. PLoS Pathogy, 5, 1-15. doi:10.1371/journal.ppat.1000596
- Uda, H., Mima, T., Yamaguchi, N., Katada, Y., Fukuda, M., Fujii, N., Nakamura, K. and Saiki, O. (2002) Expansion of a CD28 intermediate subset among CD8 T Cells in patients with Infectious mononucleosis. Journal of Virology, 76, 6602-6608. doi:10.1128/JVI.76.13.6602-6608.2002
- Pepper, M. and Jenjins, M.K. (2011) Origins of CD4+ effector and central memory T cells. Nature Immunology, 12, 467-471. doi:10.1038/ni.2038
- Sallusto, F., Geginat, J. and Lanzavecchia, A. (2004) Central memory and effector memory T cell subsets: Function, Generation, and Maintenance. Annual Review Immunology, 22, 745-763. doi:10.1146/annurev.immunol.22.012703.104702
- Unsoeld, H. and Pircher, H. (2005) Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. Journal of Virology, 79, 4510-4513. doi:10.1128/JVI.79.7.4510-4513.2005
- Weng, N., Akbar, A.N. and Goronzy, J. (2009) CD28 T cells: Their role in the age associated decline of immune function. Trends in Immunology, 30, 306-312. doi:10.1016/j.it.2009.03.013
- Ramos-Galván, R. (1975) Pediatric somatometry: A semilongitudinal study on children in Mexico City. Archivos de Investigación Médica, 6, 383-396.
- Kurstak, E. (1985) Progress in enzyme immunoassays: Production of reagents, experimental design, and interpretation. Bull World Health Organ, 63, 793-811.
- Li, Y., Frey, E. and Mackenzie, A.M.R. (2000) Finlay BB. Human response to Escherichia coli O157:H7 infection: Antibodies to secreted virulence factors. Infection and Immunology, 68, 5090-5095. doi:10.1128/IAI.68.9.5090-5095.2000
- Honstettre, A., Meghari, S., Nune’s, J.A., Lepidi, H., Raoult, D., Olive, D. and Mege, J.L. (2006) Role for the CD28 Molecule in the Control of Coxiella burnetii Infection. Infection and Immunology, 74, 1800-1808. doi:10.1128/IAI.74.3.1800-1808.2006
- Segal, A.W. (2005) How neutrophils kill microbes. Annual Review Immunology, 23, 97-223. doi:10.1146/annurev.immunol.23.021704.115653
- Bonilla, F.A. and Oettgen, H.C. (2010) Adaptive immunity. Journal of Allergy and Clinical Immunology, 125, S33-S40. doi:10.1016/j.jaci.2009.09.017
- Wershil, B.K. and Furuta, G.T. (2008) Gastrointestinal mucosal immunity. Journal of Allergy and Clinical Immunology, 121, S380-S383. doi:10.1016/j.jaci.2007.10.023
- Cook, J., Hepler, R., Pancari, G., Kuklin, N., Fan, H., Wang, X.M., Cope, L., Tan, C., Joyce, J., Onishi, J., Montgomery, D., Anderson, A. and McNeely, T. (2009) Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Human Vaccine, 5, 254-263. doi:10.4161/hv.5.4.6765
- Cui, W. and Kaech, S.M. (2010) Generation of effector CD8+ T cells and their conversion to memory T cells. Immunology Review, 236, 151-166. doi:10.1111/j.1600-065X.2010.00926.x
- Joshi, N.S. (2007) Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunology, 27, 281-295.
- Rutishauser, R.L. (2009) Transcriptional repressor Blimp- 1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunology, 31, 296-308.
- Jambo, K.C., Sepako, E., Heyderman, R.S. and Gordon, S.B. (2010) Potential role for mucosally active vaccines against pneumococcal pneumonia. Trends of Microbiology, 18, 81-89. doi:10.1016/j.tim.2009.12.001
- Nájera, O., González, C., Cortés, E., Toledo, G. and Ortiz, R. (2007) Effector T lymphocytes in well-nourished and malnourished infected children. Clinical Experimental Immunology, 48, 501-506. doi:10.1111/j.1365-2249.2007.03369.x
- Voskoboinik, I., Dunstone, M.A., Baran, K., Whisstock, J.C. and Trapani, J.A. (2010) Perforin: Structure, function, and role in human immunopathology. Immunology Review, 235, 35-54.
- Waterhouse, N.J., Sutton, V.R., Sedelies, K.A., Ciccone, A., Jenkins, M., Turne, S.J., Bird, P.I. and Trapani, J.A. (2006) Cytotoxic T lymphocyte-induced killing in the absence of granzymes A and B is unique and distinct from both apoptosis and perforin-dependent lysis. Journal of Cellular Biology, 173, 133-144. doi:10.1083/jcb.200510072
- Jacobsen, M., Detjen, A.K., Mueller, H., Gutschmidt, A., Leitner, S., Wahn, U., Magdorf, K. and Kaufmann, S.H.E. (2007) Clonal Expansion of CD8+ Effector T Cells in Childhood Tuberculosis. Journal of Immunology, 179, 1331- 1339.
- Helmin-Basa, A., Michalkiewicz, J. and Gackowska, L. (2011) Pediatric Helicobacter pylori infection and circulating T-Lymphocyte activation and differentiation. Helicobacter, 16, 27-35. doi:10.1111/j.1523-5378.2010.00809.x
- McKinstry, K.K., Strutt, T.M. and Swain, S.L. (2010) The potential of CD4 T-cell memory. Immunology, 130, 1-9. doi:10.1111/j.1365-2567.2010.03259.x
- Booth, N.J., McQuaid, A.J., Soband, T., Kissane, S., Agius, E., Jackson, S.E., Salmon, M., Falciani, F., Yong, K., Rustin, M.H., Akbar, A.N. and Vukmanovic-Stejic, M. (2010) Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. Journal of Immunology, 8, 4317-4326. doi:10.4049/jimmunol.0903781
- Wong, P. and Parmer, E. (2019) CD8 T cell responses to infectious pathogens. Annual Review Immunology, 21, 29-70. doi:10.1146/annurev.immunol.21.120601.141114
- Cisternas, O. (2007) La tinción de gram como herramienta de uso diario en el diagnóstico precoz de algunos patógenos. Revista de Hospital del Niño, 23, 140-146.
- Hill, D.A. and Artis, D. (2010) Intestinal bacteria and the regulation of immune cell homeostasis. Annual Review Immunology, 28, 623-667. doi:10.1146/annurev-immunol-030409-101330
- D’Elios, M.M., Benagiano, M., Bella, Ch.D. and Amedei, A. (2011) T-cell response to bacterial agents. Journal of Infection in Developing Countries, 5, 640-645. doi:10.3855/jidc.2019
- MacDonald, A.S., Straw, A.D., Dalton, N. and Pearce, E.J. (2002) Dendritic cells: A role for CD40. Journal of Immunology, 168, 537-540.
- Coffman, R.L. (2010) The Origin of TH2 Responses. Science, 328, 1116-1117. doi:10.1126/science.1192009

