Open Journal of Animal Sciences
Vol.3 No.2(2013), Article ID:29918,6 pages DOI:10.4236/ojas.2013.32020
Presence of Actinobacillus pleuropneumoniae, Streptococcus suis, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis and Mycoplasma hyopneumoniae in upper respiratory tract of swine in farms from Aguascalientes, Mexico
![]()
1Centro de Ciencias Básicas, Universidad Autónoma de Aguascalientes, Aguascalientes, México; *Corresponding Author: alguerre@correo.uaa.mx
2Groupe de Recherche sur les Maladies Infectieuses du Porc, Faculté de Médecine Vétérinaire, Université de Montréal, St-Hyacinthe, Canada
3Laboratorio de Biología Celular y Tisular, Departamento de Morfología, Centro de Ciencias Básicas, Universidad Autónoma de Aguascalientes, Aguascalientes, México
Copyright © 2013 Abraham Loera-Muro et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 20 January 2013; revised 3 March 2013; accepted 7 April 2013
Keywords: Porcine Respiratory Disease Complex; Actinobacillus pleuropneumoniae; Streptococcus suis; Pasteurella multocida; Bordetella bronchiseptica; Haemophilus parasuis; Mycoplasma hyopneumoniae
ABSTRACT
Respiratory diseases are one of the most important health problems in pig herds. The porcine respiratory disease complex (PRDC) is the term used to describe pneumonic diseases caused by multiple infectious agents that provoke weight loss in animals or death. In the PRDC multiple pathogens (bacteria and/or viruses) work in combination to induce this respiratory disease. Within this complex, Actinobacillus pleuropneumoniae, Streptococcus suis, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis and Mycoplasma hyopneumoniae are the main bacterial pathogens involved in great economic losses to the swine industry. The aim of this work was to estimate the presence of A. pleuropneumoniae, S. suis, P. multocida, B. bronchiseptica, H. parasuis and M. hyopneumoniae in the upper respiratory tract of pigs in representative swine farms in Aguascalientes, Mexico, using PCR technique. The study was performed in 14 swine farms. We obtained a total of 212 nasal swabs. Near 20% of samples were positive for A. pleuropneumoniae (located in the 79% of farms); 17% were positive for S. suis (in 86% of farms), of these, 3% were S. suis serovar 2; 30% were positive for H. parasuis (93% of farms); 23% of the samples to P. multocida (in 79% of farms); and 19% to M. hyopneumoniae (in 64% of farms). B. bronchiseptica was not detected in this study. The results obtained show that bacterial pathogens of PRDC were present in the upper respiratory tract of pigs in all farms studied; therefore, these pathogens are widely disseminated in pig farms of Aguascalientes, Mexico.
1. INTRODUCTION
Respiratory diseases are one of the most important health problems in pig herds. Due to the multifactorial nature of these diseases, they are considered as a porcine respiratory disease complex (PRDC). The PRDC is the term used to describe pneumonic diseases is caused by multiple infectious agents, which produce weight loss in animals or death. The PRDC is a major health problem in the current production of pigs [1,2]. Within this complex, the bacteria A. pleuropneumoniae, S. suis, P. multocida, B. bronchiseptica, H. parasuis and M. hyopneumoniae are the main bacterial pathogens involved in causing great economic losses to the swine industry [3], and begin to be regarded as important zoonotic agents with significant increase of infectious in humans [4-6].
A. pleuropneumoniae causes hemorrhagic swine pleuropneumonia, this is a severe contagious and economically significant disease in the swine industry [7-9]. S. suis is recognized as a major respiratory pathogens of pigs and emerging zoonotic agent [4,5,10]. P. multocida caused the atrophic rhinitis in pigs [11,12], as well as B. bronchiseptica [13,14]. H. parasuis causes Glässer’s disease or polyserositis, as well as pneumonia [15-17]. M. hyopneumoniae is the primary cause of enzootic pneumonia in pigs [18-20].
The objective of this work was to estimate the presence of A. pleuropneumoniae, S. suis, P. multocida, B. bronchiseptica, H. parasuis and M. hyopneumoniae in the upper respiratory tract of asymptomatic pigs in swine farms in Aguascalientes, Mexico, using PCR technique.
2. MATERIALS AND METHODS
2.1. Sampling Procedures
For this study were selected 14 farms producing pigs in the State of Aguascalientes, Mexico, for convenience and willingness of producers. The sampling period was from June to October 2011.The total number of pigs sampled was 212 (Table 1). The sample size was calculated according to Alvarez et al. [21], for a study in the State of Yucatan, Mexico, to sought A. pleuropneumoniae and M. hyopneumoniae. The nasal swabs were taken by inserting the swab in the nasal cavity of the pig softly, rotating the swab to easily enter the cavity, and withdrawing it in the same way. The samples were store in sterile tubes. The pigs were randomly selected, ensuring sampling of pigs of different ages and weights, covering the entire farm. Each tube was labeled with the number of the farm, followed by a period and the number of tube following a sequential order. The tubes were store in a cooler until reach the laboratory. In the laboratory, the tubes were store at 4˚C until processed.
2.2. Control Strains
All strains used as positive control in this study are from laboratory of Dr. Mario Jacques. The control strains were: A. pleuropneumoniae serovar 1-4074, S. suis serovar 2-735, H. parasuis serovar 5 (Nagazaki), P. multocida 4-4056 (type D, DNT+), M. hyopneumoniae ATCC 25095 and B. bronchiseptica 276. Negative control used was Escherichia coli ATCC 25922.
2.3. DNA Isolation
DNA isolation was performed as described by Sambrook and Russell [22], with Loera et al. [23] modifications. The swabs were cut in an Eppendorf tube with tweezers and scissors sterilized by flame. Subsequently, 1ml of lysis buffer (15% agarose, 0.03 mM Tris, 0.05 M EDTA, and 0.03 mg lisozime·ml−1, pH 8) was added directly to the tubes and then they were incubated for 30 minutes at 37˚C. After, 100 ml SDS 10% (0.1 v/v), 100 ml NaCl 5M (0.1 v/v) and 5 ml Proteinase K (0.4 mg·ml−1; USB Corporation) was added, and the tubes were mixed and incubated for 1 h at 50 ˚C. Then, 500 μl of the supernatant was taken, in which 200 μl of phenol-chloroform-isoamyl alcohol (24:24:1, Gibco BRL) was added. The mixture was vortexed (Fisher) and centrifuged for 5 min at 10,000 g. The supernatant was recovered and 200 ml ammonium acetate (7.5 M) and 200 ml absolute ethanol (1 v) was added, and mixed for 5 min by inversion and allowed to precipitate overnight at 4˚C. Then, samples were centrifuged at 4˚C for 15 min at 10,000 g. The supernatant was discarded and the pellet was washed several times with 100 ml ethanol (70%). Pellets were dried at 37˚C, resuspended in distilled water and kept at −20˚C until use for the PCR reactions.
2.4. PCR Reactions
PCR amplifications were carried out in a DNA thermal cycler (TECHNE T-412). PCR against A. pleuropneumoniae was performed as described by Schaller et al. [24], with modifications for MacInnes et al. [3] and Loera et al. [23]. The PCR run conditions were: 95˚C for 1 min followed by 30 cycles of 94˚C for 30 s, 54˚C for 30 s and 72˚C for 1 min with a final elongation step at 72˚C for 5 min. Multiplex PCR for detection of S. suis was developed based on the method of Marois et al. [25]. Multiplex PCR permitted the simultaneous detection of the S. suis species (primers 16S-195 [s] and 16S-489 [as2]) and serotypes 2 and 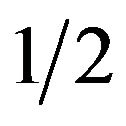 (primers cps2J-s and cps2J-as). The amplification conditions were: 1 cycle at 95˚C for 1 min, 40 cycles at 94˚C for 30 s, 60˚C for 30 s, 72˚C for 1 min and a final elongation cycle at 72˚C for 10 min. The PCR protocol for detection of H. parasuis was used like previously described Oliveira et al. [26] with modifications for MacInnes et al. [3]. The PCR run conditions were: 95˚C for 5 min followed by 30 cycles of 94˚C for 30 s, 59˚C for 30 s and 72˚C for 2 min with a final elongation step at 72˚C for 5 min. PCR against toxigenic strains of P. multocida was developed under the conditions of Kamp et al. [27]. The amplification conditions were: 1 cycle at 95˚C for 1 min, 32 cycles at 95˚C for 30 s, 65˚C for 60 s, 72˚C for 2.5 min and a final elongation cycle at 72 ˚C for 20 min. The PCR for B. bronchiseptica was performed using the method Hozbor et al. [28], with modifications by Resgister and DeJong [29]. The PCR run conditions were: 1 cycle at 95˚C for 1 min, 35 cycles at 94˚C for 60 s, 53˚C for 30 s, 72˚C for 20 s and a final
(primers cps2J-s and cps2J-as). The amplification conditions were: 1 cycle at 95˚C for 1 min, 40 cycles at 94˚C for 30 s, 60˚C for 30 s, 72˚C for 1 min and a final elongation cycle at 72˚C for 10 min. The PCR protocol for detection of H. parasuis was used like previously described Oliveira et al. [26] with modifications for MacInnes et al. [3]. The PCR run conditions were: 95˚C for 5 min followed by 30 cycles of 94˚C for 30 s, 59˚C for 30 s and 72˚C for 2 min with a final elongation step at 72˚C for 5 min. PCR against toxigenic strains of P. multocida was developed under the conditions of Kamp et al. [27]. The amplification conditions were: 1 cycle at 95˚C for 1 min, 32 cycles at 95˚C for 30 s, 65˚C for 60 s, 72˚C for 2.5 min and a final elongation cycle at 72 ˚C for 20 min. The PCR for B. bronchiseptica was performed using the method Hozbor et al. [28], with modifications by Resgister and DeJong [29]. The PCR run conditions were: 1 cycle at 95˚C for 1 min, 35 cycles at 94˚C for 60 s, 53˚C for 30 s, 72˚C for 20 s and a final
Table 1. Presence of swine respiratory pathogens in upper respiratory tract of swine and its distribution per farm.

Ap, A. pleuropneumoniae; Ss, S. suis; Ss ser. 2, S. suis serotype 2; Hp, H. Parasuis; Pm, P. multocida; Bb, B. bronchiseptica; Mh, M. hyopneumoniae.
Table 2. Primers sequences used in this study.

elongation cycle at 72˚C for 5 min. Last, PCR for detection of M. hyopneumoniae was developed using the method of Cai et al. [30]. The amplification conditions were: 1 cycle at 95˚C for 1 min, 35 cycles at 94˚C for 20 s, 60˚C for 30 s, 72˚C for 40 s and a final elongation cycle at 72˚C for 7 min. All PCR reactions contained 0.25 μl 5 U·μl−1 Taq DNA polymerase (Fermentas), and 2 µl template. All amplified products were observed by electrophoresis in 1.5% agarose gel, stain with 1 μg ethidium bromide·ml−1. Images of the gels were captured using the Chemi Doc (Bio-Rad), image analyzer and the software Quantity One (Bio-Rad, California, USA). The primer sequences are shown in Table 2.
3. RESULTS AND DISCUSSION
As a representative sample, 14 pig farms distributed in all the area of the State of Aguascalientes, Mexico, were selected for this study. This region, located at central of
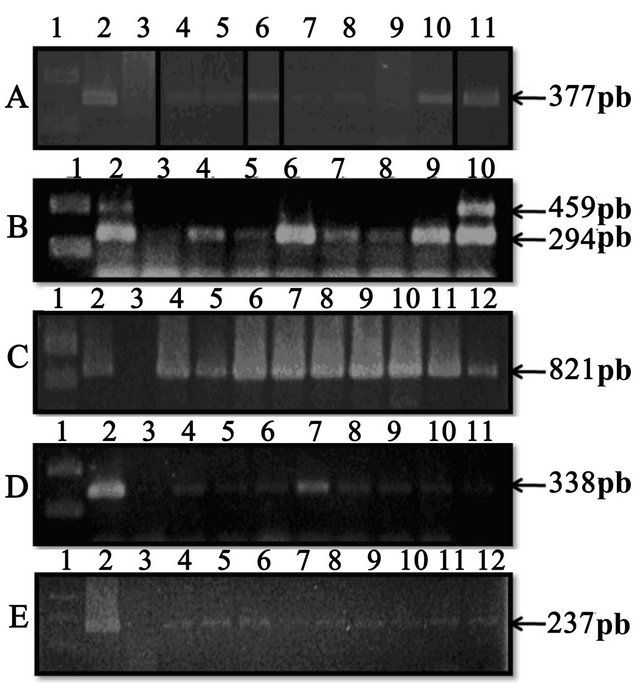
Figure 1. PCR products of the different PCR done; all were analyzed on 1.5% agarose gel electrophoresis, followed by ethidium bromide stain. (A) A. pleuropneumoniae PCR’s: Lane 1—DNA ladder (Fermentas); Lane 2—A. pleuropneumoniae S-1-4074 (positive control); Lane 3—E. coli ATCC 25922 (negative control); Lane 4 to 11—Nasal swabs samples; (B) S. suis PCR’s: Lane 1—DNA ladder (Fermentas); Lane 2—S. suis 735 serovar 2 (positive control); Lane 3—E. coli ATCC 25922 (negative control); Lane 4 to 10—Nasal swabs samples; (C) H. parasuis PCR’s: Lane 1—DNA ladder (Fermentas); Lane 2—H. parasuis serovar 5 (Nagazaki) (positive control); Lane 3—E. coli ATCC 25922 (negative control); Lane 4 to 12—Nasal swabs samples; (D) P. multocida PCR’s: Lane 1—DNA ladder (Fermentas); Lane 2—P. multocida 4- 4056 (type D, DNT+) (positive control); Lane 3—E. coli ATCC 25922 (negative control); Lane 4 to 11—Nasal swabs samples; (E) M. hyopneumoniae PCR’s: Lane 1—DNA ladder (Fermentas); Lane 2—M. hyopneumoniae ATCC 25095 (positive control); Lane 3—E. coli ATCC 25922 (negative control); Lane 4 to 11—Nasal swabs samples. No evidence of B. bronchiseptica in nasal swab samples was found in any of the farms sampled in this study (B. bronchiseptica serovar 276 was the positive control).
the country, is semi-arid (overage annual precipitation of 550 mm, 80% in the rainy season in summer), with an average annual temperature of 17˚C - 18˚C; however, in spring and summer the overage daily temperature is above 30˚C [31]. A total of 212 pigs were sampled, with around 15 pigs per farm (Table 1). Pigs were sampled randomly; they had no apparent signs of illness and were in normal production process on farms. Most pigs sampled were 1 - 2 years old; even though, reproductive sows with different ages were also sampled.
Of the 212 nasal swabs, 19.8% were positive to A. pleuropneumoniae and distributed in 78.6% of the farms sampled. Almost 17% of the samples were positive for S. suis, with a distribution in 85.7% of farms; of these, 3.3% were S. suis serovar 2 (the most virulent serovar). In the case of H. parasuis, 29.7% of the samples were positive and distributed in 92.9% of the farms. For P. multocida, 22.6% were positive, with a distribution in the 78.6% of the farms. For M. hyopneumoniae, 19.3% were positive, with a distribution in 64.3% of the farms sampled. Finally, no evidence of B. bronchiseptica in nasal swab samples was found in any of the farms studied (Table 1 and Figure 1).
Furthermore, 35.7% of farms showed the presence of five bacterial pathogens of PRDC; the same frequency was observed by the jointly presence of four of them. In 21.4% of farms were found three pathogens; and two pathogens were observed in 7.1% of farms. Farms affected by six PRDC bacterial pathogens were not observed, as well as farms free of these pathogens. Like wise, the bacterial pathogens pairs found most frequently in the same farm were the following: S. suis and H. parasuis (85.7% of farms), and with the same frequency (71.4%) the pairs A. pleuropneumoniae and H. parasuis, H. parasuis and P. multocida, and the pair P. multocida and S. suis.
In Mexico, there are several studies on different pathogens of PRDC, mainly on A. pleuropneumoniae [23,32- 39], but there are few field studies on the bacterial pathogens distribution that make up this complex [21,40], which is the biggest health problem faced by pork producers today. One of these studies, conducted by Álvarez et al. [21], estimate the frequency of A. pleuropneumoniae, M. hyopneumoniae and swine influenza virus (SIV) in the State of Yucatan, region located at the Southeast of the country. They found a frequency of 100% for A. pleuropneumoniae and M. hyopneumoniae. Although in our study we found a lower frequency of both pathogens, probably due to regional differences, it is remarkable the high percentage of farms affected by PRDC pathogens observed in both studies, which clearly show the severe respiratory health problems facing pig production in our country.
4. CONCLUSION
Bacterial pathogens of porcine respiratory disease complex were present in the upper respiratory tract of pigs in all swine farms sampled, in the State of Aguascalientes, Mexico. These results show the great respiratory health problems present in our swine farms, which is a reflection of the current situation of the Mexican field.
5. ACKNOWLEDGEMENTS
This project was supported by a grant from CONACYT, Mexico (No. 258863). We appreciate the support received by Dr. Ricardo Oropeza, Institute of Biotechnology, UNAM, Mexico, for their help in obtain the control strains. We thank Josée Labrie for critical reading of the manuscript. We also thank to Adriana C. Moreno Flores for her technical assistance.
REFERENCES
- Brogden, K.A. and Guthmiller, J.M. (2002) Polymicrobial diseases. ASM Press, Washington DC.
- Pósa, R., Donkó, T., Bogner, P., Kovács, M., Repa, I. and Magyar, T. (2011) Interaction of Bordetella bronchiseptica, Pasteurella multocida, and fumonisin B1 in the porcine respiratory tract as studied by computed tomography. Canadian Journal of Veterinary Research, 75, 176-182.
- MacInnes, J., Gottschalk, M., Lone, A., Metcalf, D., Ojha, S., Rosendal, T., Watson, S. and Friendship, R. (2008) Prevalence of Actinobacillus pleuropneumoniae, Actinobacillus suis, Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis in representative Ontario swine herds. Canadian Journal of Veterinary Research, 72, 242-248.
- Hao, N., Nga, T. and Schultsza, C. (2011) Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in Southern Vietnam. PLoS, 6, Article ID: e17943. doi:10.1371/journal.pone.0017943
- Kim, H., Lee, S., Moon, H., Kim, Y., Lee, S., Hur, M. and Yun, Y. (2011) Streptococcus suis causes septic arthritis and Bacteremia: Phenotypic characterization and molecular confirmation. The Korean Journal of Laboratory Medicine, 31, 115-117. doi:10.3343/kjlm.2011.31.2.115
- Nga, T., Nghia, H. and Schultsza, C. (2011) Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagnostic Microbiology and Infectious Disease, 70, 461- 467. doi:10.1016/j.diagmicrobio.2010.12.015
- Buettner, F., Konze, S., Maas, A. and Gerlach, G. (2011) Proteomic and immunoproteomic characterization of a DIVA subunit vaccine against Actinobacillus pleuropneumoniae. Proteome Science, 9, 23. doi:10.1186/1477-5956-9-23
- Tobias, T.J., Boum, A., Klinkenberg, D., Daemen, A.J., Stegeman, J.A., Wagenaar, J.A. and Duim, B. (2012) Detection of Actinobacillus pleuropneumoniae in pigs by real time quantitative PCR for the apxIVA gene. The Veterinary Journal, 193, 557-560. doi:10.1016/j.tvjl.2012.02.004
- Sadilkova, L., Nepereny, J., Vrzal, V., Sebo, P. and Osicka, R. (2012) Type IV fimbrial subunit protein ApfA contributes to protection against porcine pleuropneumonia. BMC Veterinary Research, 43, 2. doi:10.1186/1297-9716-43-2
- Wertheim, H., Nghia, H., Taylor, W. and Schultsz, C. (2009) Streptococcus suis: An emerging human pathogen. Emerging Infections, 48, 617- 625.
- Tang, X., Zhao, Z., Hu, J., Wu, B., Cai, X., He, Q. and Chen, H. (2009) Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. Journal of Clinical Microbiology, 47, 951-958. doi:10.1128/JCM.02029-08
- Hotchkiss, E., Hodgson, C., Lainson, A. and Zadoks, R. (2011) Multilocus sequence typing of a global collection of Pasteurella multocida isolates from cattle and other host species demonstrates niche association. BMC Microbiology, 11, 115. doi:10.1186/1471-2180-11-115
- Kaut, C., Duncan, M., Kim, J., Maclaren, J., Cochran, K. and Julio, S. (2011) A novel sensor kinase is required for Bordetella bronchiseptica to colonize the lower respiratory tract. Infection and Immunity, 79, 3216-3228. doi:10.1128/IAI.00005-11
- MacArthur, I., Jones, J., Goodlett, D., Ernst, R. and Preston, A. (2011) Role of pagL and lpxO in Bordetella bronchiseptica lipid a biosynthesis. Journal of Bacteriology, 193, 4726-4735. doi:10.1128/JB.01502-10
- Mullins, M., Register, K., Bayles, D., Dyer, D., Kuehn, J. and Phillips, G. (2011) Genome sequence of Haemophilus parasuis strain 29755. Standards in Genomic Sciences, 5, 61-68. doi:10.4056/sigs.2245029
- Zhang, N., Chu, Y., Gao, P., Zhao, P., He, Y. and Lu, Z. (2012) Immunological identification and characterization of extracellular serine protease-like protein encoded in a putative espP2 gene of Haemophilus parasuis. Journal of Veterinary Medical Science, 74, 983-987. doi:10.1292/jvms.11-0260
- Zhou, M., Zhang, Q., Zhao, J. and Jin, M. (2012) Haemophilus parasuis encodes two functional cytolethal distending toxins: CdtC contains an atypical cholesterol recognition/interaction region. PLoS One, 7, Article ID: e32580. doi:10.1371/journal.pone.0032580
- Liu, W., Feng, Z., Fang, L., Zhou, Z., Li, Q., Li, S., Luo, R., Wang, L., Chen, H., Shao, G. and Xiao, S. (2011) Complete genome sequence of Mycoplasma hyopneumoniae strain 168. Journal of Bacteriology, 193, 1016-1017. doi:10.1128/JB.01305-10
- Villarreal, I., Vranckx, K., Calus, D., Pasmans, F., Haesebrouck, F. and Maes, D. (2012) Effect of challenge of pigs previously immunized with inactivated vaccines containing homologous and heterologous Mycoplasma hyopneumoniae strains. BMC Veterinary Research, 8, 2. doi:10.1186/1746-6148-8-2
- Vranckx, K., Maes, D., Marchioro, S., Villarreal, I., Chiers, K., Pasmans, F. and Haesebrouck, F. (2012) Vaccination reduces macrophage infiltration in bronchus-associated lymphoidtissue in pigs infected with a highly virulent Mycoplasma hyopneumoniae strain. BMC Veterinary Research, 8, 24. doi:10.1186/1746-6148-8-24
- Álvarez, M., Rodríguez, J., Ciprián, A., Rodríguez, L., Ayora, G. and Segura, J. (2004) Serological profile of porcine influenza virus, Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae, in farms of Yucatán, México. Veterinaria México, 35, 296-305.
- Sambrook, J. and Rossell, D. (2001) Molecular cloning, a laboratory manual. 3rd Edition, Cold Spring Harbor Laboratory Press, New York.
- Loera, V., Jacques, M., Tremblay, Y., Avelar-González, F., Loera, A., Ramírez-López, E., Medina-Figueroa, A., González-Reynaga, H. and Guerrero-Barrera, A. (2013) Detection of Actinobacillus pleuropneumoniae in drinking water from pig farms. Microbiology, 159, 535-543. doi:10.1099/mic.0.057992-0
- Schaller, A., Djordjevic, S.P., Eamens, G.J., Forbes, W.A., Kuhn, R., Kuhnert, P., Gottschalk, M., Nicolet, J. and Frey, J. (2001) Identification and detection of Actinobacillus pleuropneumoniae by PCR based on the gene apxIVA. Veterinary Microbiology, 79, 47-62. doi:10.1016/S0378-1135(00)00345-X
- Marois, C., Bougeard, S., Gottschalk, M. and Kobisch, M. (2004) Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. Journal of Clinical Microbiology, 42, 3169-3175. doi:10.1128/JCM.42.7.3169-3175.2004
- Oliveira, S., Galina, L. and Pijoan, C. (2001) Development of a PCR test to diagnose Haemophilus parasuis infections. Journal of Veterinary Diagnostic Investigation, 13, 495-501. doi:10.1177/104063870101300607
- Kamp, E., Bokken, G., Vermeulen, T., Jong, M., Buys, H., Reek, F. and Smits, M. (1996) A specific and sensitive PCR assay suitable for large-scale detection of toxigenic Pasteurella multocida in nasal and tonsillar swabs specimens of pigs. Journal of Veterinary Diagnostic Investigation, 8, 304-309. doi:10.1177/104063879600800305
- Hozbor, D., Fouque, F. and Guiso, N. (1999) Detection of Bordetella bronchiseptica by the polymerase chain reaction. Research in Microbiology, 150, 333-341. doi:10.1016/S0923-2508(99)80059-X
- Register, K. and DeJong, K. (2006) Analytical verification of a multiplex PCR for identification of Bordetella bronchiseptica and Pasteurella multocida from swine. Veterinary Microbiology, 117, 201-210. doi:10.1016/j.vetmic.2006.05.003
- Cai, H., Dreumel, T., McEwen, B., Hornby, G., Bell-Rogers, P., McRaild, P., Josephson, G. and Maxie, G. (2007) Application and field validation of a PCR assay for the detection of Mycoplasma hyopneumoniae from swine lung tissue samples. Journal of Veterinary Diagnostic Investigation, 19, 91-95. doi:10.1177/104063870701900115
- INEGI (2012) www.inegi.org.mx
- Negrete-Abascal, E., Tenorio, V., Serrano, J.J., García, C. and de la Garza, M. (1994) Secreted proteases from Actinobacillus pleuropneumoniae serovar 1 degraded porcine gelatin, hemoglobin and immunoglobulin A. Canadian Journal of Veterinary Research, 58, 83-86.
- Negrete-Abascal, E., Tenorio, V., Guerrero, A.L., García, R.M., Reyes, M. and de la Garza, M. (1998) Purification and characterization of a protease from Actinobacillus pleuropneumoniae serovar 1, an antigen common to all the serovars. Canadian Journal of Veterinary Research, 62, 183-190.
- Negrete-Abascal, E., Reyes, M., García, R., Vaca, S., Girón, J., García, O., Zenteno, E. and de la Garza, M. (2003) Flagella and motility in Actinobacillus pleuropneumoniae. Journal of Bacteriology, 185, 664-668. doi:10.1128/JB.185.2.664-668.2003
- Enríquez-Verdugo, I., Guerrero, A.L., Serrano, J.J., Godínez, D., Rosales, J.L., Tenorio, V. and de la Garza, M. (2004) Adherence of Actinobacillus pleuropneumoniae to swine-lung collagen. Microbiology, 150, 2391-2400. doi:10.1099/mic.0.27053-0
- García-González, O., García, R.M., de la Garza, M., Vaca-Pacheco, S., Paniagua, G.L., Mejía, R., et al. (2004) Actinobacillus pleuropneumoniae metalloprotease: Cloning and in vivo expression. FEMS Microbiology Letters, 234, 81-86. doi:10.1111/j.1574-6968.2004.tb09516.x
- Hamer-Barrera, R., Godínez, D., Enríquez, V., Vaca-Pacheco, S., Martínez-Zúñiga, R., Talamás-Rohana, P., Suá- rez-Güemez, F. and de la Garza, M. (2004) Adherence of Actinobacillus pleuropneumoniae serotype 1 to swine buccal epithelial cells involves fibronectin. Canadian Journal of Veterinary Research, 68, 33-41.
- Serrano, L., Tenorio-Gutiérrez, V., Suárez, F., ReyesCortés, R., Rodríguez-Mendiola, M., Arias-Castro, C., Godínez-Vargas, D. and de la Garza, M. (2008) Identification of Actinobacillus pleuropneumoniae biovars 1 and 2 in pigs using a PCR assay. Molecular and Cellular Probes, 22, 305-312. doi:10.1016/j.mcp.2008.09.001
- Gutiérrez, L., Vargas, D., Ocampo, L., Sumano, H., Martínez, R. and Tapia, G. (2011) Plasma concentrations resulting from florfenicol preparations given to pigs in their drinking water. Journal of Animal Science, 89, 2926-2931. doi:10.2527/jas.2010-3576
- Williams, J.J., Torres-León, M., Echeverria-Coello, P. and Matos-Medina, M. (2000) Aislamiento e identificación de Actinobacillus pleuropneumoniae en pulmones de cerdos con pleuroneumonía crónica sacrificados en el rastro municipal de Mérida. Revista Biomédica, 11, 175-181.

