Advances in Biological Chemistry
Vol. 2 No. 3 (2012) , Article ID: 21809 , 6 pages DOI:10.4236/abc.2012.23032
Characterization of tectonins I and II from Physarum polycephalum
![]()
Department of Biochemistry, Faculty of Science, Okayama University of Science, Okayama, Japan
Email: *minami@dbc.ous.ac.jp
Received 9 June 2012; revised 13 July 2012; accepted 19 July 2012
Keywords: Tectonin; Physarum polycephalum; WD Repeat
ABSTRACT
Features of tectonin I and II expression in Physarum polycephalum cells were examined at both the protein and mRNA levels. Among the five cell cycle stages of P. polycephalum, the plasmodia, microplasmodia, and sclerotia contained both tectonin I and tectonin II; the spores did not contain any of these; and the amoebae contained tectonin I, but not tectonin II. When the mating of amoebae formed plasmodia, the mRNA and protein levels of tectonin II increased with the growth of zygotes. In the early stage of differentiation from plasmodia to spores, the mRNA levels of tectonins I and II decreased. Tectonins I and II were associated with the membrane fraction that precipitated at 200,000 ×g and could be released only by urea treatment of this fraction. Furthermore, when the fraction was digested with proteinase K, tectonin II completely disappeared. Immunofluoro-microscopy indicated that tectonins I and II exist in the lamellipodia of plasmodia.
1. INTRODUCTION
Physarum polycephalum, one of the true slime molds, grows under rotten trees or dead leaves in forests. Physarum has a unique life cycle that includes the diploid plasmodium, microplasmodium, and sclerotium; the haploid spore; and the haploid amoeba. Tectonin is one of the unique proteins found in the plasmodium.
Physarum has two types of tectonin: I and II [1]. As shown in Figure 1(a), the primary structure of the C-terminal region of tectonin II shows high homology (68% identity) with that of tectonin I. Tectonin I also shows 33% identity to a bacterial lipopolysaccharide-binding lectin, L-6, from horseshoe crab hemocytes [2]. Tectonin I and the C-terminal region of tectonin II contain six tandem repeat sequences similar to WD repeats that are found in diverse proteins, such as β-subunit of G proteins, and have various functions such as protein-protein interaction [3-5]. In those proteins, it is known that WD repeats form a β-propeller structure. Furthermore, to date many proteins that have a domain homologous with the primary structure of tectonin have been described [6-9]. Tectonin domains in these proteins perform various functions in cells; for example, human Tecpr1 plays an important role in the selective autophagy pathway [10].
The N-terminal region of tectonin II shows approximately 31% identity to the galactoside-binding sequence of B-chain of ricin, a toxic plant protein [11]. From these reports, it is speculated that tectonins I and II from P. polycephalum have a lectin-like function and mediate phagocytosis.
In this report, we describe the expression of tectonins I and II at each stage of the P. polycephalum cell cycle and their membrane localization and discuss the function of tectonin.
2. MATERIALS AND METHODS
2.1. Culture of P. polycephalum
Amoebae of P. polycephalum (ATCC #24466 and #24467) were grown on corn-meal agar at 24˚C. The two strains of amoebae were plated together on an SM-2 agar plate so that they fused to form plasmodia [12]. For the subcultivation of amoebae, they were grown on lawns of live Escherichia coli on SM-1 agar plates at 24˚C [13]. Microplasmodia were grown at 24˚C in liquid semi-defined medium [14]. Plasmodia were cultured on corn-meal agar plates. Alternatively, for differentiation into sclerotia, plasmodia were grown on filter papers at 24˚C. Subsequently, the filter papers crawling plasmodia were dried in desiccators [15], inducing the formation of sclerotia. Fruiting bodies were formed from starved plasmodia under light irradiation [16].
2.2. Preparation of Antibodies Specific for Tectonin I
The tectonin I sequence from the initiation to the termination codon was amplified by PCR. The PCR product was ligated into the pQE30 vector (Qiagen, CA, USA). E. coli were then transformed with the ligation product and ampicillin-resistant colonies were selected. The transformed cells were grown at 37˚C in LB medium supplemented with ampicillin until the OD600 reached nearly 0.6. The expression of recombinant proteins was derived by 1 mM isopropyl-β-D-thiogalactoside. Recombinant proteins were purified on a Ni-NTA agarose column (Invitrogen, CA, USA). The purified protein was emulsified with an equal volume of Freund’s complete adjuvant and injected into a rabbit. Blood was then collected, and the IgG fraction was prepared by ammonium sulfate fractionation and Protein G-Sepharose 4 Fast Flow (GE Healthcare UK Ltd., Buckinghamshire, UK) column chromatography according to the general method of Harlow and Lane [17].
2.3. Immunoblot Analysis
SDS-PAGE was performed in a 12.5% polyacrylamide gel. Proteins were separated on the gel and electrophoretically transferred onto a polyvinylidene fluoride membrane (Millipore, MA, USA). The proteins on the membrane were reacted with tectonin I-specific antibody, diluted 1:50,000, and then with a horseradish peroxidase-conjugated antirabbit IgG antibody (Cell Signaling Technology, Inc., MA, USA). Detection of the desired proteins was achieved with an ECL Plus kit (GE Healthcare UK Ltd.) and visualized with an LAS-1000 Plus camera system (Fujifilm, Tokyo, Japan).
2.4. Northern Blot Analysis
For RNA preparation, each stage-specific cell preparation was lyophilized. A total of 1 mg (dry weight) of each type of cell, except for spores, was suspended in 0.5 ml of RNAwiz (Applied Biosystems, CA, USA) and total RNA samples were then purified according to the RNAwiz manuscript. Spores (approximately 50 mg) were suspended in extraction solution [0.3 ml of phenol and 0.7 ml of buffer (0.1 M sodium acetate, pH 6.0, 10 mM EDTA, 1% w/v SDS, 0.1 M LiCl)] preheated to 80˚C. After the spores were destroyed using a ZircoPrep Mini (Nippon Genetics Co. Ltd., Tokyo, Japan), total RNA was purified according to the method of Melera and Rusch [18]. These RNA samples were subjected to 1% formaldehyde-MOPS agarose gel electrophoresis. RNAs were then transferred from the gel to a Hybond N+ membrane (GE Healthcare UK Ltd.). For producing the probes, the cDNA fragments for tectonin I (27 - 681 bp) and II (13 - 434 bp) were amplified by PCR and labeled using an AlkPhos Direct Kit (GE Healthcare UK Ltd.). The labeled cDNAs were hybridized to the membrane containing the RNA for 15 h according to the AlkPhos Direct kit manuscript. RNAs were detected with CDP-Star (GE Healthcare UK Ltd.) detection reagent and the signals were visualized on X-ray film.
2.5. Cell Fractionation of Tectonins I and II
Microplasmodia (approximately 2 g wet weight) were suspended in 20 ml of 20 mM Tris-HCl buffer (pH 7.5) and disrupted by sonication. The crude extract was centrifuged at 1000 × g for 15 min. The supernatant was again centrifuged at 10,000 × g for 15 min and was separated into precipitate and supernatant. This supernatant was then ultracentrifuged at 200,000 × g for 1 h. The precipitate obtained at each centrifugation was suspended in 20 ml of the same buffer. Finally, each supernatant and precipitate was analyzed by Western blotting.
2.6. Solubilization of Tectonins I and II
Microplasmodia (approximately 3 g wet weight) were disrupted by sonication as described in the preceding section. The suspension was then subjected to ultracentrifugation, and the precipitate was suspended in 40 ml of 20 mM Tris-HCl buffer (pH 7.5). The suspension was separated into eight equal quantities and 5 ml of a buffer containing NaCl (final concentration, 1 M), urea (5 M), Na2CO3 (5 M), Triton X-100 (1% v/v), proteinase K (1 mg/ml), or FeCl3 (10 mM) was added to each suspension. These suspensions were then incubated on ice for 1 h and were again subjected to ultracentrifugation. Each precipitate was suspended in 10 ml of the same buffer. All samples including the precipitates and supernatants were analyzed by Western blotting.
2.7. Immunofluorescence Microscopy
Plasmodia were placed on a glass slide and fixed with 10% (v/v) TCA as described previously [19]. After washing, the cells were incubated at room temperature for 1 h with TBS containing 1% (w/v) BSA and 1000-fold diluted anti-tectonin I antibody. They were then incubated with 2000-fold diluted Alexa Fluor 488 goat anti-rabbit IgG (H + L) (Molecular Probes, OR, USA). The immunostained cells were observed with an LSM410 confocal laser microscope (Carl Zeiss Co. Ltd., Jena, Germany). Fluorescence labeling was observed at a wavelength of 488 nm. Images were expressed as the projections of a series of 1-nm optical sections.
3. RESULTS AND DISCUSSION
3.1. Reactivity of Antibody Specific for Tectonin
Purified recombinant tectonin I that was expressed as a His-tag fusion protein in E. coli was injected into rabbits as antigen. As shown in Figure 1(b), the antibody specific for tectonin reacted with both tectonins I and II in the crude extract from plasmodia. This specific antibody was used for the subsequent experiments.
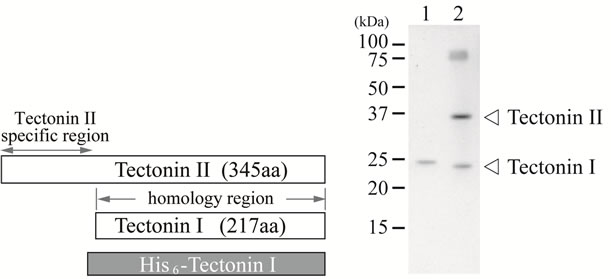 (a) (b)
(a) (b)
Figure 1. The characteristic primary structures of tectonins I and II and the reactivity of antibody specific for tectonin. (a) Tectonin I shows high homology to the C-terminal two thirds of tectonin II. A recombinant His-tag fusion tectonin I that contains the full sequence of tectonin I was expressed in E. coli; (b) Immunoblotting of tectonins I and II using anti-tectonin I antibody. Lane 1, recombinant His-tag fusion tectonin I protein. Lane 2, crude extract prepared from plasmodia.
3.2. Stage-Specific Expression of Tectonins I and II
The expression of tectonins I and II at each stage of the life cycle was detected by Western and Northern blotting (Figure 2). Tectonins I and II were detected in the plasmodia, microplasmodia cultured in liquid medium, and dehydrated, dormant-stage sclerotia. However, these proteins were not detected in spores. Interestingly, only tectonin I was expressed in haploid amoebae, indicating that tectonin II is not essential to amoebal cells and may have a different function from tectonin I.
During differentiation from haploid amoebae to plasmodia, tectonin II was detected in cells by both Western and Northern blotting (Figure 3). When small zygotes were observed after 48 h, the RNA expression of tec tonin II had already begun (Figure 3(c)). After 96 h from the start of mating, when cytoplasmic streaming in the small yellowish plasmodia was visible under microscopy, the expression of tectonin II protein could be detected in the cells. From that point onward, the tectonin proteins in the cells increased with the RNA levels. When complete plasmodia were observed after 120 h, the tectonin II content in the cells was maximal. Thus, tectonin II appears to mediate some functions in plasmodia but not in amoebae.
Neither tectonin I nor II was found in spores (Figure 2). To examine the change of tectonins I and II during differentiation from plasmodia to spores, the expression level was evaluated by Western and Northern blotting. RNA synthesis of tectonins I and II in the cells was not detected even when many protuberances in the lamellipodia of cells were seen, as shown in photograph 2 of Figure 4. However, cells at this stage were able to return to typical plasmodia by the interruption of the condition for differentiation (e.g. light, starvation, and static culture) and to recover the expression of tectonins I and II. Thus,
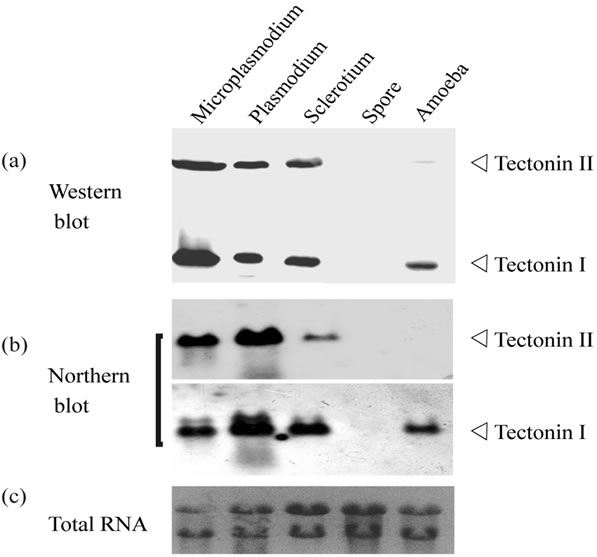
Figure 2. Stage-specific expression of tectonins I and II. (a) Western blot analysis. Each crude extract prepared from plasmodia, microplasmodia, sclerotia, amoebae, and spores was subjected to SDS-PAGE. Each lane corresponds to 0.5 mg cells (dry weight). Tectonins I and II were detected using anti-tectonin I antibody; (b) Northern blot analysis. Total RNAs from cells at five different stages were applied on the gel at 10 μg. The mRNAs for tectonin I and II were detected by each specific probe; (c) Ethidium bromide staining for total RNAs.
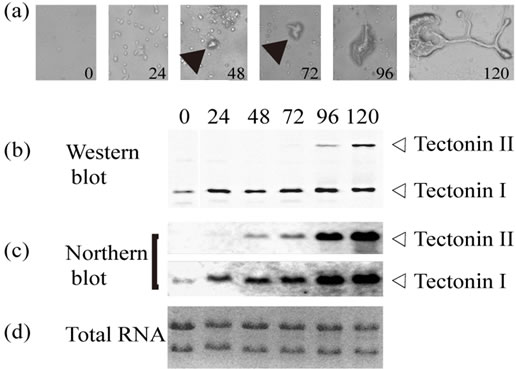
Figure 3. Change of tectonin expression during differentiation from amoebae to plasmodia. (a) Microphotographs of time course of differentiation from amoeba to plasmodia (×400). Each number shows the culture time in hours. The culture was started when amoebae (strains #24466 and #24467) were placed together on six SM-2 agar plates (1.6 × 105 cells/plate). Arrows show zygotes; (b) Western blot analysis. At each time point, all of the cells on one plate were collected and immediately suspended in 40 μl of SDS-PAGE sample buffer. After heat denaturing, the entire volume of the suspension was used for SDS-PAGE. Tectonins I and II in each lane were detected using anti-tectonin I antibody; (c) Northern blot analysis. From the cells collected from the plates at each time point, total RNAs were purified. Approximately 5 μg of total RNA per lane was subjected to electrophoresis; (d) Ethidium bromide staining for total RNAs.
although the RNA level in the cells decreased during the early stages, tectonin expression persisted in the cells until the formation of spores was complete.
3.3. Localization of Tectonins I and II
Diverse proteins that contain tectonin domains in their structures have been identified, and their functions have been reported [6-9]. However, no function has yet been assigned to the tectonin first found in P. polycephalum, and it is speculated that tectonin resides on the surface of the plasma membrane and is involved in phagocytosis [1]. We examined the association of tectonins I and II with the cellular membrane. The crude extract obtained after sonication of microplasmodia was centrifuged at 1000, 10,000, and 200,000 × g, and both tectonins I and II were detected in the precipitates obtained by centrifugation at 200,000 × g (Figure 5). This finding suggested that tectonins I and II were associated with relatively light membranes such as intracellular vesicles and were easily released from the membranes. Because hydropathy analysis revealed that both tectonins are hydrophilic (results not shown), they may be associated with the membranes through other membrane proteins.
Tectonins I and II could be released from the membrane fraction by chemical treatment. Tectonin I was released
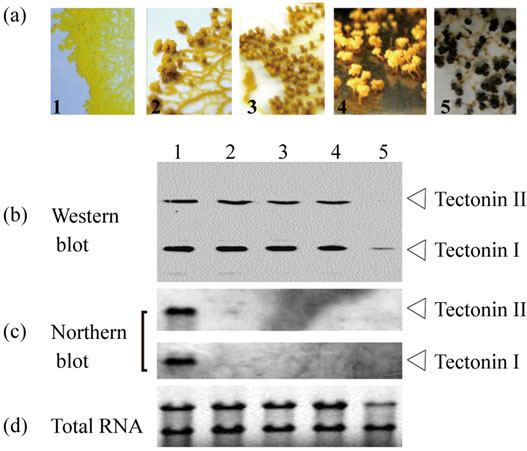
Figure 4. Change of tectonin expression during differentiation from plasmodia to spores. (a) Microphotographs (×100) show the time course of differentiation from plasmodium (panel 1) to spores (panel 5). In stage 4, the morphology of the spore is formed completely. In stage 5, the color change of whole body from yellow to black appears; (b) Western blot analysis. Tectonins I and II in crude extract prepared from cells at each stage were detected using anti-tectonin I antibody. Each lane corresponds to 0.16 mg cells (dry weight); (c) Northern blot analysis. From the cells collected from the plates at each time point, total RNAs were purified. Approximately 3 μg of total RNA per lane was subjected to agarose gel electrophoresis; (d) Ethidium bromide staining for total RNAs.
by urea, Na2CO3, or Triton X-100 treatment, but tectonin II was released only by urea (Figure 6). These results showed that tectonins I and II associate with membranes with different conformations. Further, when the membrane fraction was digested with proteinase K, tectonin II disappeared completely, whereas tectonin I remained in the membrane. This result agrees with that of Huh et al. [1], who examined the sensitivity of tectonin to trypsin digestion in microplasmodia. Tectonin II, unlike tectonin I, may thus be exposed on the membrane surface. Because it is thought that intracellular membranes form vesicles upon sonication, the orientations of tectonins I and II may be different in the vesicles.
Figure 7 shows an immunofluorescence micrograph of plasmodia reacted with anti-tectonin I antibody. The micrograph shows that tectonins I and II are located on the lamellipodium of the plasmodia. Treatment with a tectonin II-specific antibody showed that the localization of tectonin II coincided completely with that of tectonin I (data not shown). As discussed by Huh et al. [1], tectonin proteins may be involved in phagocytosis in the lamellipodia of plasmodia.
3.4. Conclusion
In this study, we could not identify clear functions or intracellular locations of tectonins I and II. However, we provide evidence that tectonins I and II have different expression patterns during the Physarum life cycle and may play different roles in the organism. It is clear that both tectonins I and II are located in the lamellipodia of plasmodia. Tectonins I and II may be associated strongly with intracellular membranes such as phagocytic vesicles through membrane-spanning proteins. Furthermore, their
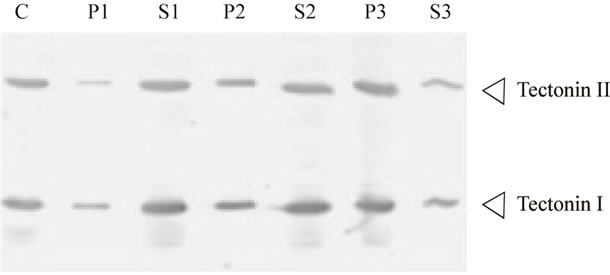
Figure 5. Cell fractionation of Tectonins I and II. The crude extract from microplasmodia was separated into supernatant and precipitate by three steps of centrifugation as described in Materials and Methods section. The crude extract, corresponding to 20 μg of protein, was subjected to Western blotting (lane C). The supernatant (lanes S1-S3) and precipitate suspended with the same volume (lanes P1-P3) at each centrifugation step were also subjected to Western blotting. Tectonins were detected using anti-tectonin I antibody. Lane P1, precipitate from centrifugation at 1000 × g; S1, supernatant from 1000 × g; P2, precipitate from 10,000 × g; S2, supernatant from 10,000 × g; P3, precipitate from 200,000 × g; S3, supernatant from 200,000 × g.
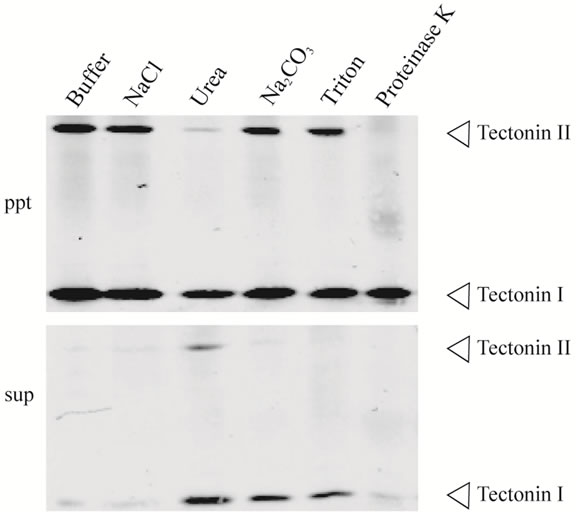
Figure 6. Dissociation of tectonins I and II from the membrane fraction. The precipitate obtained by ultracentrifugation at 200,000 × g (Figure 5, Lane 6), was suspended in buffer containing NaCl, urea, Na2CO3, Triton X-100, or proteinase K. After incubation, each suspension was subjected to ultracentrifugation and separated into supernatant (sup) and precipitate (ppt). The experimental details are described in Materials and Methods section. Each lane corresponds to 1 mg (wet weight) of microplasmodia.
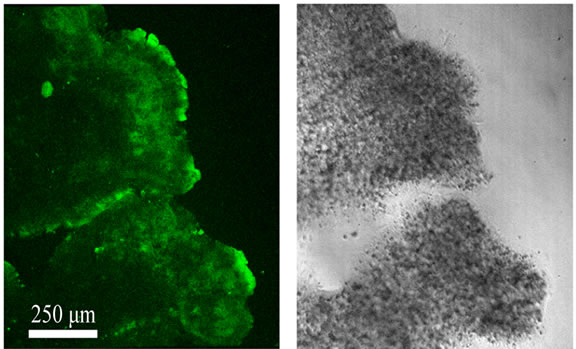
Figure 7. Immunofluorescence micrographs of plasmodia using anti-tectonin I antibody. Fluorescence signal of Alexa Fluor 488 and the corresponding transmitted light image are indicated in the left and right panels, respectively.
orientation on vesicles may be different. Many proteins such as lectin [6-9], which have tectonin domains, act in phagocytosis or cell surface recognition. Similarly, tectonins I and II from P. polycephalum may be involved in predation by phagocytosis. In future studies, we will focus on the relationship between the phagocytic pathway of plasmodia and the function of tectonin II, which is not found in amoebae.
4. ACKNOWLEDGEMENTS
We wish to thank Mr. M. Ogawa (Okayama University of Science) for their technical assistance in this study.
![]()
![]()
REFERENCES
- Huh, C.G., Aldrich, J., Mottahedeh, J., Kwon, H., Johnson, C. and Marsh, R. (1998) Cloning and characterization of Physarum polycephalum tectonins. Homologues of limulus lectin L-6. Journal of Biological Chemistry, 273, 6565-6574. doi:10.1074/jbc.273.11.6565
- Saito, T., Kawabata, S., Hirata, M. and Iwanaga, S. (1995) A novel type of limulus lectin-L6. Purification, primary structure, and antibacterial activity. Journal of Biological Chemistry, 270, 14493-14499.
- Fulop, V. and Jones, D.T. (1999) β propellers: Structural rigidity and functional diversity. Current Opinion in Structural Biology, 9, 715-721. doi:10.1016/S0959-440X(99)00035-4
- Jawad, Z. and Paoli, M. (2002) Novel sequences propel familiar folds. Structure, 10, 447-454. doi:10.1016/S0969-2126(02)00750-5
- Smith, T.F., Gaitatzes, C., Saxena, K. and Neer, E.J. (1999) The WD repeat: A common architecture for diverse functions. Trends in Biochemical Sciences, 24, 181- 185. doi:10.1016/S0968-0004(99)01384-5
- Chen, S.C., Yen, C.H., Yeh, M.S., Huang, C.J. and Liu, T.Y. (2001) Biochemical properties and cDNA cloning of two new lectins from the plasma of Tachypleus tridentatus: Tachypleus plasma lectin 1 and 2. Journal of Biological Chemistry, 276, 9631-9639. doi:10.1074/jbc.M008414200
- Schroder, H.C., Ushijima, H., Krasko, A., Gamulin, V., Thakur, N.L., Diehl-Seifert, B., Muller, I.M. and Muller, W.E.G. (2003) Emergence and disappearance of an immune molecule, an antimicrobial lectin, in basal metazoan: A techylectin-related protein in the sponge Suberites domuncula. Journal of Biological Chemistry, 278, 32810-32817. doi:10.1074/jbc.M304116200
- Low, D.H.P., Ang, Z., Yuan, Q., Frecer, V., Ho, B., Chen, J. and Ding, J.L. (2009) A novel human tectonin protein with multivalent-propeller folds interacts with ficolin and binds bacterial LPS. PLoS ONE, 4, e6260. doi:10.1371/journal.pone.0006260
- Low, D.H.P., Frecer, V., Saux, A.L., Srinivasan, G.A., Ho, B., Chen, J. and Ding, J.L. (2010) Molecular interfaces of the galactose-binding protein tectonin domains in host-pathogen interaction. Journal of Biological Chemistry, 285, 9898-9907. doi:10.1074/jbc.M109.059774
- Ogawa, M., Yoshikawa, Y., Kobayashi, T., Mimuro, H., Fukumatsu, M., Kiga, K., Piao, Z., Ashida, H., Yoshida, M., Kakuta, S., Koyama, T., Goto, Y., Nagatake, T., Nagai, S., Kiyono, H., Kawalec, M., Reichhart, J.M. and Sasakawa, C. (2011) A tecpr1-dependent selective autophagy pathway targets bacterial pathogens. Cell Host Microbe, 9, 376-389. doi:10.1016/j.chom.2011.04.010
- Montfort, W., Villafranc, J.E., Monzingo, A.F., Ernst, S.R., Katzin, B., Rutenber, E., Xuong, N.H., Hamlin, R. and Rpbertus, J.D. (1987) The three-dimensional structure of ricin at 2.8 A. Journal of Biological Chemistry, 262, 5398-5403.
- Dee, J. (1966) Multiple alleles and other factors affecting plasmodium formation in the true slime mold Physarum polycephalum Schw. The Journal of Protozoology, 13, 610-616.
- Dee, J. and Anderson, R.W. (1985) The effect of ploidy on the stability of plasmodial heterokaryons in Physarum polycephalum. Journal of General Microbiology, 131, 1167-1179.
- Daniel, J.W. and Baldwin, H.H. (1964) Methods of culture for plasmodial myxomycetes. In: Prescott, D.M., Ed., Methods in Cell Physiology, Academic Press, London, 9-40.
- Kohama, K., Ishikawa, R. and Ishigami, M. (1994) Large-scale culture of Physarum: A simple method for growing several hundred grams of plasmodia. In: Celis, J.E., Ed., Cell Biology: A Laboratory Hand-Book, Academic Press, London, 452-455.
- Daniel, J.W. and Rush, H.P. (1962) Methods for inducing sporulation of pure cultures of the myxomycete Physarum polycephalum. Journal of Bacteriology, 83, 234-240.
- Harlow, E. and Lane, D. (1988) Storing and purifying antibodies. Cold Spring Habor, New York, 283-318.
- Melera, P.W. and Rusch, H.P. (1973) A characterization of ribonucleic acid in the myxomycete Physarum policephalum. Experimental Cell Research, 82, 197-209. doi:10.1016/0014-4827(73)90262-0
- Hayase, M., Maekawa, A., Yubisui, T. and Minami, Y. (2008) Properities, intracellular localization, and stagespecific expression of membrane-bound β-glucosidase, BglM1, from Physarum polycephalum. The International Journal of Biochemistry & Cell Biology, 40, 2141-2150. doi:10.1016/j.biocel.2008.02.019
NOTES
*Corresponding author.

