Advances in Biological Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17278 , 6 pages DOI:10.4236/abc.2012.21008
Applying the mutation of Bacillus subtilis and the optimization of feather fermentation medium to improve Keratinase activity
![]()
Jilin Business and Technology College, Changchun, China
Email: jlsyzhangxin@sohu.com
Received 7 November 2011; revised 28 December 2011; accepted 8 January 2012
Keywords: Keratinase Activity; Bacillus subtilis; Mutation; Optimization
ABSTRACT
Energetic Bacillus subtilis was preliminarily isolated from feather meal selection medium experiment, which could be used to produce Keratinase. Through using the ultraviolet ray produced by ultraviolet light and the compound mutation of sodium nitrite solution, a mutated strain was produced which can yield Keratinase with a high activity. The activity of Keratinase was 75.9% higher than that before the compound mutation. This was achieved by optimizing the fermentation medium of mutated strain. The optimum fermentation medium had the parameters as feather meal 5.5%, maize silage 0.8%, K+ 0.018 mol/L, Mg2+ 0.065 mol/L, Ca2+ 0.072 mol/L, Fe2+ 0.010 mol/L, and Na+ 0.088 mol/L. Based on the optimized fermentation medium, the highest yielding rate for producing enzyme was 1.117 U/mL, 25.22% higher than that before the optimization. The amino acid in the fermentation medium fluid reached 22.66 mg/mL. This paper presents a simple, and low cost way to produce high quality bio-fermentation feather meal.
1. INTRODUCTION
Poultry feather was often used to feed animals since it was mostly composite of Stearic keratin. The percentage of Stearic keratin in Poultry feather was around 75% - 85%. However, the amino acids in feather Keratin were mostly Hydrophobic amino acids, especially, in which Cystine disulfide bond was difficult to be hydrolyze by Protein hydrolase, so, the activity of feather Keratin was low [1]. If the poultry feather was used to feed animals directly, it will be very difficult for animal to absorb the nutrition. Therefore, it is important to develop an advanced technology to deeply process feather into feedstuff.
When the macromolecule Keratoprotein of feather meal was degraded into small molecular by Bacillus subtilis fermentation which was easier to digeste for animals, new amino acids, bacterial protein, growth factors, immune substance, and beneficial active cell were produced, which not only provided protein sources for animal growth, but also could provide the balanced amino acids content and high nutrition value.
In recent years, some researchers focused their studies on feather meal degradation using mutation strains of Bacillus subtilis [2]. In Reference [3], the author produced mutation strains of bacillus subtilis with high feather degradation ability using ultraviolet.
In this paper, we conducted compound mutation to Bacillus Subtilis using ultraviolet and sodium nitrite. The mutation strains with high Keratinase activity were screened. The results showed that the Keratinase activity improved a lot than before. In the optimization research, we got the optimal fermentation medium composition, using which, the Keratinase activity reached 1.117 U/mL, improved 25.22%, and the amino acids content in the broth could reach 22.66 mg/mL.
2. MATERAILS AND METHODS
2.1. Experimental Materials
The experimental materials were raw Feather meal, maize silage, and medium. The materials of medium used for feather meal screening were feather meal 39 g, K2HPO4 1.4 g, KH2PO4 0.7 g, NaCl 1 g, H2O 1 L, pH value 7.5. The materials of medium used for the whole feather fermentation were feather 60 g, K2HPO4 1.4 g, KH2PO4 0.7 g, NaCl 1 g, H2O 1 L, pH value 7.5.
The materials of medium used for feather meal optimization were feather meal 5.5%, Maize silage powder 0.8%, K+ 0.018 mol/L, Mg2+ 0.065 mol/L, Ca2+ 0.072 mol/L, Fe2+ 0.010 mol/L, Na+ 0.088 mol/L. pH value 7.0 - 8.0.
2.2. Strains Screening Methods
Broiler feather was bought from feather Recycle Companies. Feather meal selection medium was used to selectively culture and isolate by coating diluting. The vigorous colonies were selected as preliminary screening strains.
The preliminary screening strains were respectively put into 0.2 L feather fermentation Mediums. The temperature was adjusted to 36˚C ± 1˚C. The time of shaking culture was set to 72 hours. The shaking speed of the culture was 150 r/min. Degradation degree of feather Keratin was monitored and measured. The strains which have a high degradation degree with feather would be picked up as reserved strains. Figure 1 shows the looking of strain after 72 hours culture. It is difficult to see clear feather shapes, which indicates a good degradation degree. Figure 2 shows a non-degradative feather, which Figure 3 shows a partly degradative feather.
The reserved strains were put into a 500 mL Erlenmeyer flask containing 150 mL feather meal screening medium. The temperature was adjusted to 36˚C ± 1˚C. The time of shaking culture was set as 48 hours. The supernatant was filtered and centrifugated. The condition were centrifuge speed 8000 r/min, temperature 4˚C, time 15 min. Then, the enzyme activity of supernatant was measured. The strains with high enzyme activity were isolated from the supernatant as raw strains for mutation breeding.
The broth was filtered and centrifugated after fermentation, and then 1 mL supernatant was picked up, and was mixed with 2 mL Tris-HCI buffer (50 mM Tris-HCI, pH 8.0), and then mixed with 10 mg feather meal. The mixture then was put into an insulation oscillation machine with 40˚C water in it. After 6 hours reaction, 2 mL 10% TCA was put into the mixture till the end of the reaction. Thirty minutes later, I did the centrifugation for another 15 min, while the temperature was adjusted to 4˚C, while the centrifuge speed was adjusted to 8000 r/min. The 280 nm OD value of the supernatant was mea-

Figure 1. The strains after 72 hours culture.
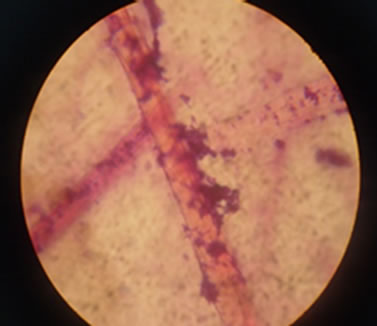
Figure 2. Non-degradative feathers in the microscope.
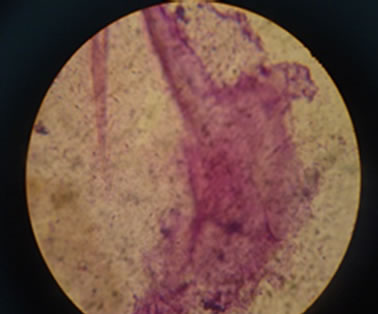
Figure 3. Degradative feathers in the microscope.
sured. In the control experiment, the TCA was put into supernatant firstly, other steps is the same with the steps referring to [4].
The definition of enzyme activity: the enzyme activity was defined as the difference of 280 nm OD values between experimental samples and control experimental samples. 1U enzyme was needed when 1 unit absorbance was added. The formula of enzyme activity was described as Equation (1).
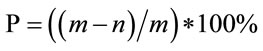 . (1)
. (1)
where P is degradation degree of feather Keratin, m is dry weight of feather meal, n is dry weight of filter residue after fermentation.
2.3. Mutation Breeding Methods
Bacillus subtilis with high enzyme production activity was selected as raw mutation strains. We conducted ultraviolet mutation, sodium nitrite mutation, and compound mutation of ultraviolet and sodium respectively.
After 18 hours culture, slant strains were transferred into the 50 mL beef-protein medium. The culture condition was temperature 36˚C ± 1˚C, time 18 hours, speed 3000 r/min. Then, it was centrifugated for 15 min. Cells were suspendly washed one time. Then we put it into 105 cfu/mL bacterial suspension. After gradient dilution, the 0.1 mL bacterial suspension was respectively coated on the medium plates. After 24 hours culture with a temperature of 36˚C ± 1˚C, I measured the number of cells.
Ultraviolet lamp was preheated for 30 min. 5 mL bacteria suspension was put on a plate with a diameter of 90 mm. Then, we turned on a magnetic stirrer, used ultraviolet lamp to irradiate the plate for 1min, then, opened the cover, and then used ultraviolet lamp to irradiate directly. Bacterial suspension was fixed in a distance of 30 cm from the 15 W ultraviolet lamp. The irradiation time was respectively set as 5 s, 10 s, 15 s, 20 s, 25 s, 30 s, 35 s, 40 s, and 45 s. The mutation bacterial suspension was gradiently diluted under a red light. 0.1 mL bacterial suspension was coated on the medium plate, and was cultured for 24 hours. The temperature was adjusted to 36˚C ± 1˚C.
1 mL Sodium nitrite with a concentration of 0.2 mol/L was put in a large tube, and then I put in 1 mL bacterial suspension, and then put 1 mL acetic acid buffer with a pH value of 4.5. After fast homogenized mixing, the mixture was conducted to a water bathing mutation with a temperature of 27˚C. The mutation time was respectively set as 30 s, 60 s, 90 s, 120 s, 150 s, and 180 s. 2 mL Na2HPO4 with a concentration of 0.07 mol/L was put in the mixture in order to stop the reaction. Then, 5 mL sterile normal saline was put in it for diluting. After gradient dilution under a red light, the 0.1 mL mutation bacterial suspension was coated on the medium plate, and was cultured for 24 hours. The temperature was adjusted to 36˚C ± 1˚C.
Using the optimal mutation dose of ultraviolet and sodium nitrite, we conducted the sodium nitrite mutation to the strains after ultraviolet mutation. After gradient dilution under a red light, the 0.1 mL mixture was coated on the medium plate, and was cultured without light for 24 hours. The temperature was adjusted to 36˚C ± 1˚C.
The inactivation rate of mutation dose was measured. The mutation colony was coated on the Casein screening medium plate using the sterilizing toothpick, and then was cultured without light for 48 hours. The temperature was adjusted to 36˚C ± 1˚C. We measured the diameter of colony and the transparent circled on the Casein screening medium plate, and then calculated the relative diameter (RD) value. The mutation strains with high activity and RD value were selected and reserved as slant strains. The formula of mutation inactivation rate was described as Equation (2):
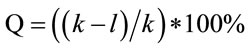 . (2)
. (2)
where, Q is mutation inactivation rate, k is the number of colony before mutation, l is the number of colony after mutation.
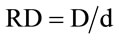 . (3)
. (3)
Equation (3) is the formula of HE value. Where, D is the diameter of transparent circle, d is the diameter of colony on the medium plate.
The 10 mL mutation strains were put into a 500 mL Erlenmeyer flask with 150 mL feather meal medium in it. The temperature was adjusted to 36˚C ± 1˚C. The time of shaking culture was set as 48 hours. The supernatant was filtered and centrifugated. The condition was centrifuge speed 8000 r/min, temperature 4˚C, time 15 min. Then, enzyme activity of supernatant was measured. The strains with a high enzyme activity were isolated from the supernatant as raw strains for mutation breeding.
2.4. Methods of Optimization of Fermentation Medium
We selected sixteen levels from seven factors which could affect the strains growth, replication, and enzyme production of microbe, set the activity of Keratinase as response values, set respectively the addition amount of Fe2+, Na+, K+, Mg2+, Ca2+, Maize silage, and feather meal as dependent variables, optimized the medium components. We designed the experiment using U*16(1610) use-table. The liquid volume in flask was 150 mL/500 mL, and the inoculation amount was 5%, and the shaking speed of the culture was 150 r/min, and the temperature was 36˚C ± 1˚C, the time period was 48 hours. Then, we measured the enzyme activity of the supernatant.
3. RESULTS AND DISCUSSIONS
3.1. Experimental Results of Strain Screening
Eight strains which can degrade feather were isolated, and were respectively named as E, F, G, H, J, K, M, and N. We measured the feather Keratin degradation rate among the whole feather fermentation medium. Table 1 shows the results. Where, the strains of E, F, H, and M are better for degrading feather Keratin, which will be used for the secondary screening. The “+” represents the degradation result is bad, “++” represents the result is normal, “+++” represents the result is good, “++++” represents the result is the best. Table 2 shows that F strain can produce Keratinase with a highest activity. Therefore, the F strain was selected as a raw mutation strain. We confirmed that the F strain was Bacillus Subtilis after preliminary strain identification.
3.2. Experimental Results of Mutation Condition Optimization
Table 3 shows F strain inactivating efficacy in the different ultraviolet dose. Figure 4 shows the variation curve of inactivation rate with different ultraviolet irradiation time. Table 4 shows measurement results of HE value and enzyme activity after F strain ultraviolet mutation. According to the experiment, we presented the F
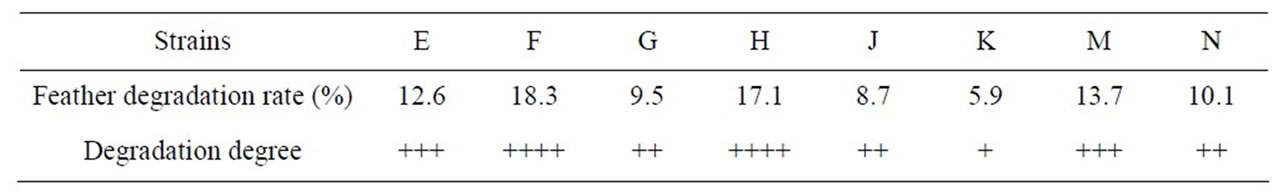
Table 1. Feather degradation degree after 72 hours strains culture in feather fermentation medium.
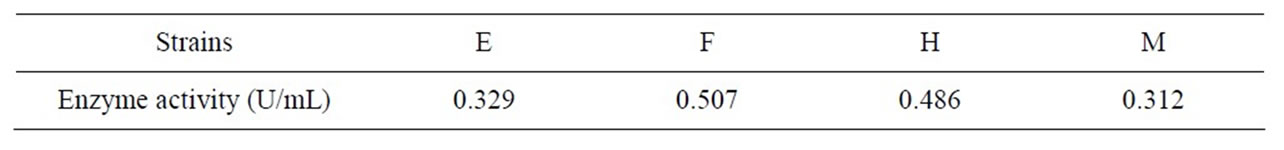
Table 2. Measurement results of strains Keratinase activity after shaking secondary screening.
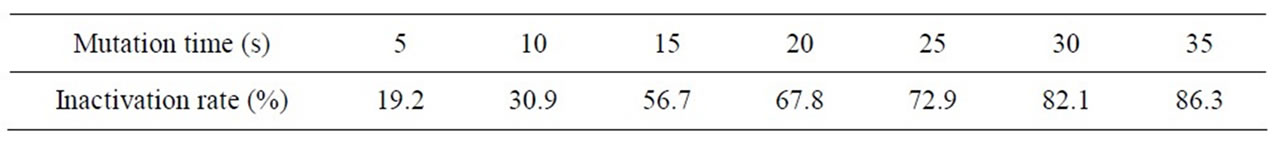
Table 3. F Strain inactivating efficacy in the different ultraviolet dose.
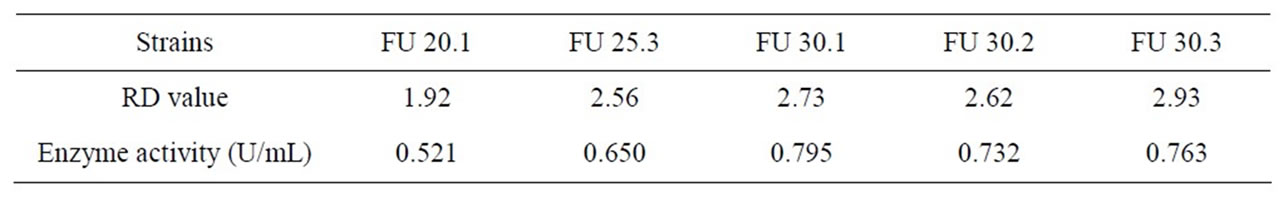
Table 4. Measurement results of RD value and enzyme activity after F strain ultraviolet mutation.
strain optimal ultraviolet mutation condition is ultraviolet lamp 15 W, mutation distance 30cm, mutation time 25 s - 30 s, the Inactivation rate is 72.9% - 82.1%. The strain positive variation is obvious. The RD value is 2.93, the highest Keratinase activity is 0.795 U/mL which improves 56.8% than that before mutation processing.
Table 5 shows F strain inactivating efficacy in the different sodium nitrite dose. Figure 5 shows the variation curve of inactivation rate with different sodium nitrite dose. Table 6 shows measurement results of RD value and enzyme activity after F strain sodium nitrite mutation. According to the experiment results, we presented the F strain optimal sodium nitrite mutation condition is sodium nitrite solution 0.2 mol/L, mutation time 120 s - 150 s, the Inactivation rate is 69.2% - 81.5%. The strain positive variation is obvious. The HE value is 2.81, the highest Keratinase activity is 0.689 U/mL which improves 35.9% than that before mutation processing.
Table 7 shows that among the compound mutation strains, the FUN30.2 has the highest Keratin activity. The optimal mutation condition is ultraviolet lamp 15 W, mutation distance 30 cm, mutation time 30 s, 0.2 mol/L sodium nitrite solution, time 120 s. The highest Keratinase activity is 0.892 U/mL which improves 75.93% than that before mutation processing. FUN 30.2 was confirmed as optimal strains.
3.3. Experimental Results of Medium Optimization
The results showed the optimal compound ratio of feather
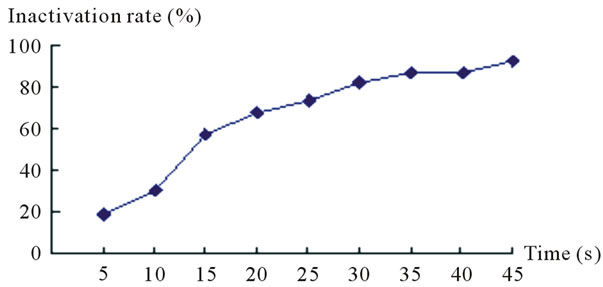
Figure 4. Diagram of inactivating efficacy in the different ultraviolet dose.
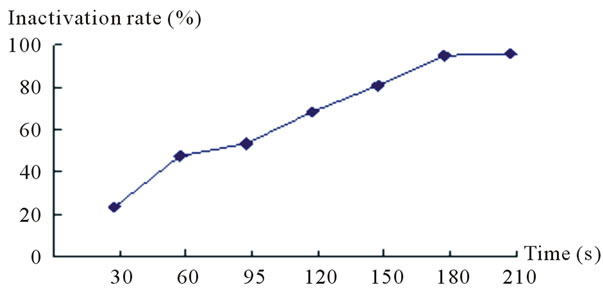
Figure 5. Diagram of inactivating efficacy in the different sodium nitrite dose.
meal medium was feather meal 5.5%, Maize silage 0.8%, K+ 0.018 mol/L, Mg2+ 0.065 mol/L, Ca2+ 0.072 mol/L, Fe2+ 0.010 mol/L, Na+ 0.088 mol/L. After medium optimization, the strain producing enzyme activity was 1.117 U/mL which improves 25.22%. Comparing to other References, we provided a simply method to reuse feather as
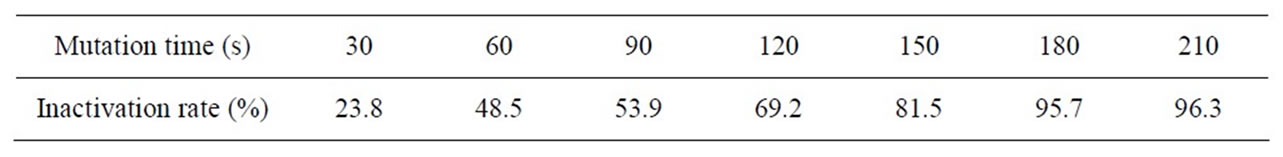
Table 5. F strain inactivating efficacy in the different sodium nitrite dose.
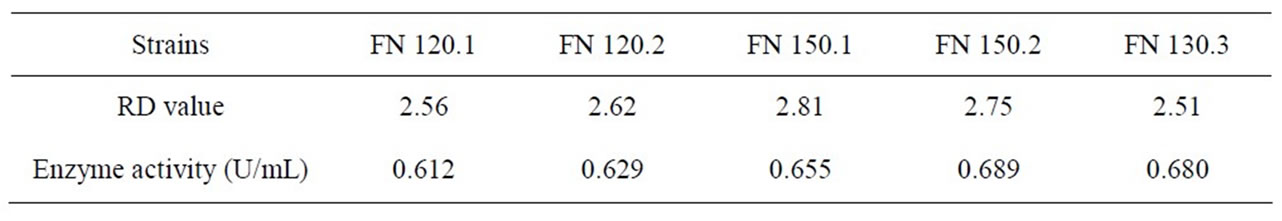
Table 6. Measurement results of RD value and enzyme activity after F strain sodium nitrite mutation.
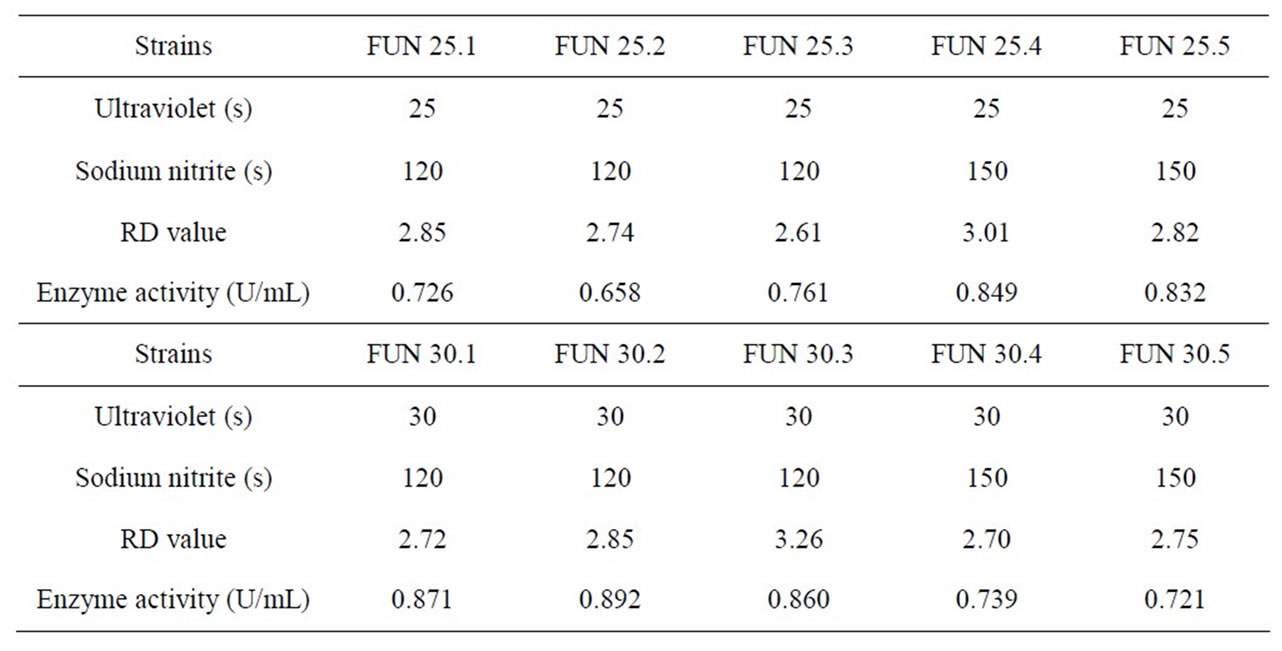
Table 7. Measurement results of RD value and enzyme activity after F strain compound mutation of ultraviolet and sodium nitrite.
possible animal feed through improvement of keratinase activity by using optimal mutation dose.
3.4. Testing Results of Productions
The sensory index of the biodegradation feather meal is solid, brown-yellow, powder, little fragrance. Table 8 shows the physicochemical and microbial index of the biodegradation feather meal.
4. CONCLUSIONS
This paper presented a compound mutation method with ultraviolet and sodium nitrite processing. Using this method, the mutation strain of Bacillus Subtilis FUN 30.2 which can produce the high activity Keratinase was isolated. The mutation condition was ultraviolet lamp 15 W, mutation distance 30 cm, mutation time 30 s, sodium nitrite solution 0.2 mol/L, and time 120 s. The Keratinase activity of the mutation strain was 0.892 U/mL which improved 75.93% than that before mutation processing. Through the optimization experiments, this paper provided the optimal component ratio of feather medium which was feather meal 5.5%, Maize silage 0.8%, K+ 0.018
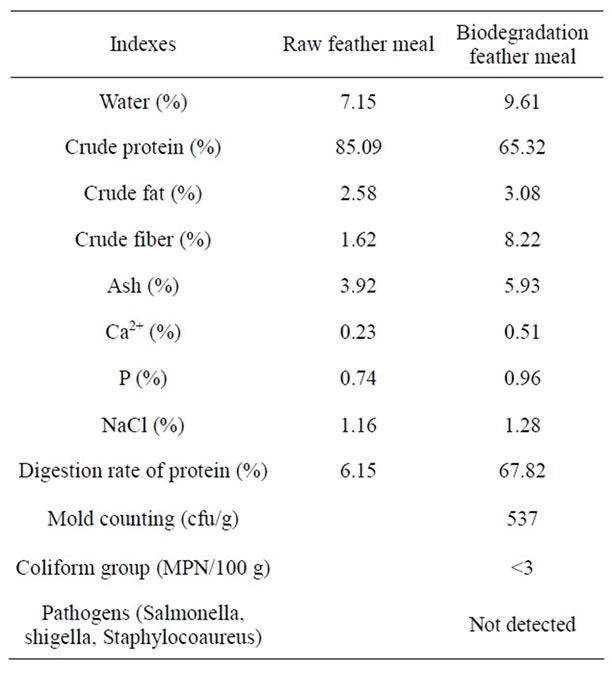
Table 8. Physicochemical and microbial index of the biodegradation feather meal.
mol/L, Mg2+ 0.065 mol/L, Ca2+ 0.072 mol/L, Fe2+ 0.010 mol/L, and Na+ 0.088 mol/L. After medium optimization, the strain producing enzyme activity improved 25.22%.
By fermenting processing, feather Keratin was transformed into amino acids, short Peptide, and bacterial protein. The amino acids content in the broth reached 22.66 mg/mL. This paper provided a simply method to reuse feather as possible animal feed through improvement of keratinase activity by using mutated Bacillus subtilis.
REFERENCES
- Cai, C.G. and Zheng, X.D. (2006) Research advance on Keratinase source, characteristics, and biology engineering. Food and Fermentation Industries, 32, 111-113.
- Wang, W.J., Cheng, H.Y. and Yang, Y.Q. (2010) Research advance on degradation of keratin in feathers. Feed Industries, 31, 39-42.
- You, Y.W., You, S.B., and Yue, S.S. (2006) Induced mutation and screening of protease deficient strain of Bacillus subtilis YYW-1. Shandong Science, 19, 12-14.
- Zhang, H.J., Liu, D.C. and Yang, G.Y. (2004) Exploring the way of determining the vitality of the different proteases by ultraviolet spectrophotometry. Cereal & Feed Industry, 9, 44-45.

