Optics and Photonics Journal
Vol.3 No.1(2013), Article ID:28936,6 pages DOI:10.4236/opj.2013.31008
Investigation of Silver Nanostructures and Their Influence on the Fluorescence Spectrum of Erbium-Doped Glasses*
Department of Physics, Alabama A&M University, Normal, USA
Email: #rami.bommareddi@aamu.edu
Received November 26, 2012; revised December 27, 2012; accepted January 4, 2013
Keywords: Silver Nanoparticles; Field Enhanced Er3+ Luminescence; Ag; Er3+ Co-Doped Oxide Glass; Sodium Borate Glass
ABSTRACT
Sodium borate glasses embedded with silver were made by the melt quenching technique. Glass transition temperature was recorded by thermal analysis of the sample. As made glasses revealed emission in the visible region under nitrogen laser and excimer laser excitations. Heat treatment was used to induce silver metallic particles. Absorption spectra revealed a peak at 417 nm due to surface Plasmon resonance. Particle size was estimated to be 2.6 ± 0.2 nm. Erbium and silver co-doped multielement oxide glasses were made by the melt quenching technique followed by heat treatment to induce nanoparticles. In heat treated samples, Er3+ luminescence increased 4× due to enhanced field in the vicinity of silver particles. Under excimer laser excitation, Er3+ and 2% Ag co-doped glass revealed Er3+ transitions due to enhanced field at the rare-earth ions. Under 795 nm laser excitation Er3+ green upconversion signals are found to be 4× stronger in 2% Ag co-doped, heat treated sample, than the others.
1. Introduction
Glasses are easy to make and at the same time they can be tailored to specific applications. Rare-earth ion doped glasses have wide ranging applications because of their use as luminescent devices and lasers [1]. The vibrational frequencies of glasses [2] are high when compared to those of halide crystals [3]. As a result quantum yield is low in glasses for some of the fluorescing levels because, the nonradiative relaxation rate is high. To overcome this problem there was some interest to make oxyfluoride glasses which facilitate the crystallization of fluorides by heat treatment [4]. Alternately the rare-earth ion luminescence also increases if the electric field intensity increases at the location of the rare-earth ion [5] or if there is efficient energy transfer to the luminescent centers [6]. For this purpose there have been some efforts to make metal and rare-earth ion co-doped glasses [7,8]. Metals like silver, gold and copper grow as nanoparticles upon heat treatment due to reduction [9]. Surface Plasmon resonance is induced in these metal particles at optical frequencies [9] which in turn were known to enhance rare-earth luminescence [10]. Though there are several efforts in this direction the mechanisms responsible for luminescence enhancement are not fully understood.
Here we have synthesized silver doped sodium borate glasses and investigated surface Plasmon resonance studies in them. We also made metal and rare-earth ion codoped glasses and investigated their luminescence characteristics. Our results are summarized here. This work is carried out because these investigations help in the design of highly efficient luminescent materials.
2. Experimental
Sodium borate glass was made with the following composition: Na2CO3 (33 mol%), B2O3 (65 mol%) and AgO (2 mol%). Appropriate quantities of the chemicals were mixed thoroughly and then melted in a box furnace using alumina crucible, in ambient air at 1400˚C for 50 minutes. At high temperatures sodium carbonate decomposes into sodium oxide. The resulting melt was poured into an alumina mold and allowed the melt to cool to room temperature naturally. The resulting glass was polished on all sides for the spectral recordings. Using similar procedure we made a few other glasses whose composition and melting temperatures are shown in Table 1. All the samples were annealed at 200˚C for an hour. Thermal analysis of the glass was performed using NETZSCH model DSC 404 C Pegasus® differential scanning calorimeter (DSC). Sample absorption spectrum was recorded using a Cary 3E spectrophotometer. Fluorescence was generTable 1. Sample composition of different glasses.
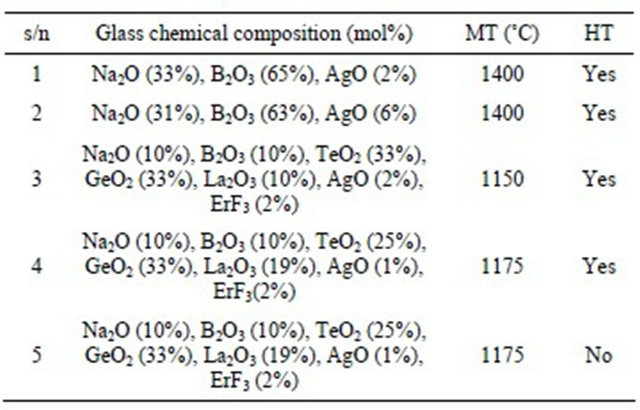
s/n: sample number; MT: melting temperature; HT: heat treated.
ated by exciting the sample with Argon ion, Nitrogen, Excimer or Ti: Sapphire lasers. The emitted light was collected by a fiber whose other end was attached to the entrance slit of a medium resolution monochromator. The photomultiplier tube (PMT) output was acquired by a computer for further processing. For lifetime measurement the PMT output was acquired by a Stanford research systems multichannel scaler (SR430). Lifetimes were derived by fitting the decay signals to single exponentials. An optical microscope was used to image larger particles.
3. Results and Discussion
The glass has to be heat treated to induce metallic particles. So, one has to know the approximate temperature range for heat treatment. Glass transition temperature is defined for amorphous materials. Above this temperature the material is in the rubber-like state and dopant ions migrate to form nanocrystals. For this purpose glass transition temperature was measured using a differential scanning calorimeter under nitrogen atmosphere at a constant heating rate 10˚C/min (Figure 1). It is clear from Figure 1 that the glass transition temperature is 467˚C. Absorption spectrum of the as made glass did not reveal any distinct peak in the visible region, but the absorption increased gradually at shorter wavelengths. All these glass samples did not reveal emission under any Ar+ laser excitation. However, when the samples were excited with a 337.1 nm N2 laser, it revealed three broad peaks in the visible region centered at 450, 500 and 560 nm. These peaks overlapped at higher silver concentrations (Figure 2). The measured lifetimes of 450, 500 and 560 nm signals are 39.2, 57.3 and 34 µs respectively. One such decay curve is shown in Figure 3. The decay time was obtained by fitting a single exponential function to the decay signal (fitting is not shown). The long lifetimes of these peaks indicate that these peaks are not due to surface Plasmon (SP) because the emission wavelengths are
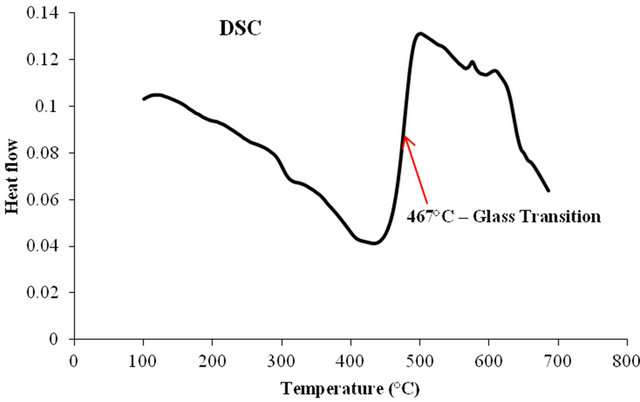
Figure 1. DSC of Ag doped sodium borate glass showing the glass transition temperature at 467˚C.
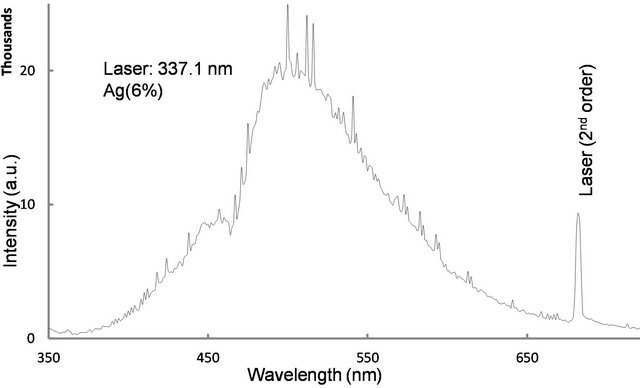
Figure 2. Fluorescence spectrum of silver doped Sodium borate glass observed under 337.1 nm N2 laser excitation. The peaks centered at 450, 500, and 560 nm overlapped.
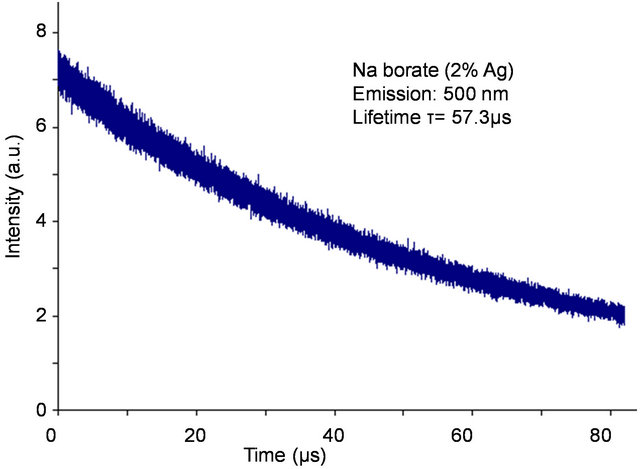
Figure 3. Temporal evolution of 450 nm emission observed under 337.1 nm laser excitation.
longer and the measured lifetimes are much longer than those expected for Plasmon relaxation times. The emission is due to molecular aggregates of silver [10,11]. Under 308 nm laser (excimer) excitation, sodium borate doped with Ag (2%) exhibited emission at 355, 510 and 700 nm (Figure 4). However in a Ag (6%) doped sodium

Figure 4. Emission observed from sodium borate glass embedded with Ag (2 mol%) under 308 nm excimer laser excitation.
borate the peaks are shifted to 355, 480 and 840 nm and the 480 nm peak intensity is much higher than that of 840 nm (not shown). The measured lifetimes of the emission peaks at 355, 510 and 700 nm are respectively 48, 180 and 95 µs. The difference in lifetimes suggest that the fluorescing levels are different from those excited by the nitrogen laser.
One of our objectives is to induce metallic nanoparticles in the glass. So the glass was subjected to several heat treatments successively and we monitored its absorption spectrum after each treatment (Figure 5). Curves (a) and (b) were recorded before and after heat treatment at 470˚C for 8 h. Heat treatment increased the overall absorption in the visible region. Upon heat treatment at 500˚C for 17 h, the sample revealed a small absorption peak at 417 nm (curve (c)). The absorption at 417 nm is due to surface plasmon resonance (SPR) [13,14]. After heat treating the same sample at 520˚C for 6 h, its absorption peak intensity increased (curve (d)). This suggests that the particle concentration has increased. On the other hand, when the same sample was heat treated for 12 h at 520˚C the absorption peak wavelength shifted to 380 nm, suggesting that the particle size has decreased. When the spectrum of the as made glass was subtracted from that of the heat treated glass it revealed a prominent peak at 417 nm (Figure 6). This peak confirms the existence of Ag nanoparticles in the glass. At plasma resonance local field enhances by a large value, which in turn contributes to fluorescence enhancement of the dopant ions. From a measurement of its full-width at half maximum (FWHM), 50 nm, the metallic particle size was estimated [5,13] as 2.6 ± 0.2 nm, by using 2R = vf/Δω1/2, where the Fermi velocity, vf = 1.39 × 106 m/s. Figure 6 also reveals couple of small peaks in the 500 - 600 nm region which fall in the region of silver exciton spectrum [15]. An optical microscope image of the sample revealed the presence of micron size Ag particles (Figure 7)
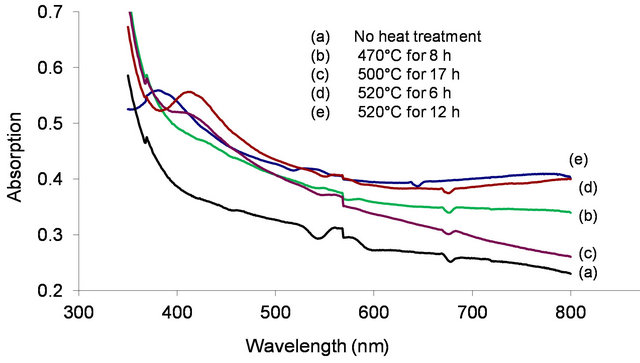
Figure 5. Absorption spectrum of Ag doped sodium borate glass observed before and after heat treatment at different temperatures and time durations.
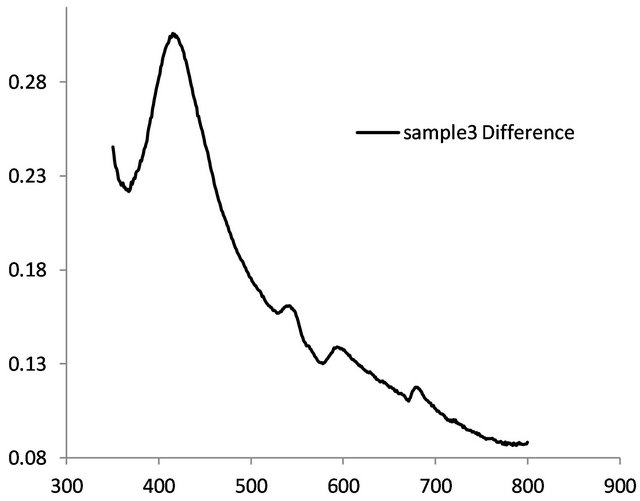
Figure 6. Absorption spectrum of surface plasmon resonance.
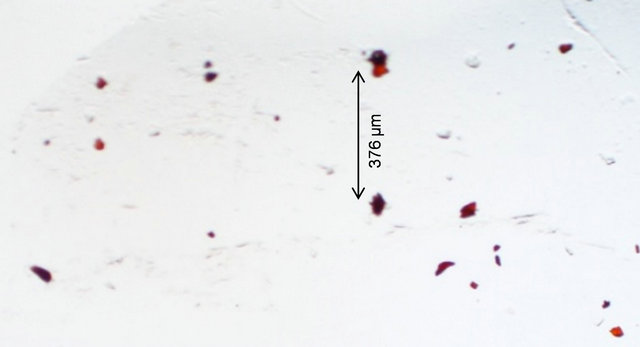
Figure 7. Optical microscope image of a heat treated glass reveals micron size particles.
in it, in addition to nanoparticles.
We were interested in studying the effect of silver particles, if any, on the luminescence of rare-earth ions. Because the vibrational frequency of borates is very large [2] rare-earths do not exhibit high emission intensities in them. So we modified the glass composition as shown in Table 1 for samples 3-5. The glasses contain 2 mol% Er3+ as co-dopant in addition to silver. For samples 3 to 5 shown in Table 1, the Er3+ concentration is 2 mol% but the silver concentration is different, 1 mol% or 2 mol%. Absorption spectrum revealed all the Er3+ peaks as expected (Figure 8). The ground state of Er3+ is 4I15/2 and the excited states are identified on the same figure. A partial energy level diagram is constructed using the absorption spectrum (Figure 9). The 488 nm laser resonantly excites the 4F7/2 level of Er3+. The excited ions relax nonradiatively to 4S3/2 level causing the latter to emit green fluorescence in the range 500 - 600 nm (4S3/2, 2H11/2à4I15/2). Figure 10 compares the emission spectra of two samples. Both the samples have the same Er3+ concentration (2 mol%); but different Ag concentrations (1 mol% and 2 mol%). Optical microscope image revealed that the 2% Ag-doped sample has higher concentration of Ag metallic particles. The Er3+ fluorescence intensity in 2% Ag co-doped sample is 4× higher than that of 1 mol% Ag doped sample. When we recorded the emission on the short wavelength side of the laser another interesting effect occurred. Er3+ and Ag (2%) codoped sample revealed emission at 415 nm that was absent or too weak in 1% Ag doped sample (Figure 11). This indicates that Ag particles are playing a role on Er3+ emission intensity. To further understand the effect of Ag particles we measured the lifetime of Er3+ fluorescence in all the glasses. The lifetime of 4S3/2 level is 10 µs in all the glasses. This implies that we do not have any evidence that supports energy transfer from Ag aggregates (molecular form) to Er3+. This also suggests that the enhancement in Er3+ luminescence is due to field enhancement caused by nearby Ag metallic particles [7]. Probably 488 nm laser weakly excites SPR in Ag particles, which in turn enhance field at the nearby Er3+ ion sites. Under 308 nm excitation, sample 5 (not heat treated) revealed three broad emission peaks centered at 355, 510 and 700 nm, whose positions and shapes are same as that of 2% Ag-doped (sample 1) sodium borate glass (see Figure 4). On the other hand, the heat treated and codoped glass (sample 3) revealed Er3+ peaks at 390, 480, 510, 540, 790 and 820 nm (Figure 12). Obviously this indicates that Ag particles influenced emission intensities of Er3+ transitions.
Accordingly, 2% Ag doped sample revealed abundant metallic particles, after heat treatment. So, in this sample a relatively large number of Er3+ ions are in the vicinity of Ag metallic particles. Surprisingly, in a 2 mol% Ag sample an intense peak appeared at 420 nm (not shown) and no such peak occurred in 1 mol% Ag sample. This sample was heat treated at 370˚C for an hour. Optical microscope images revealed crystallites, whose sizes extend up to several microns. When the samples were excited with 795 nm laser green emission occurred from 4S3/2, 2H11/2 levels (4S3/2, 2H11/2à4I15/2), as shown in Figure 13. Upconversion occurs due to stepwise two-photon excitation. One such scheme is shown in Figure 9. All
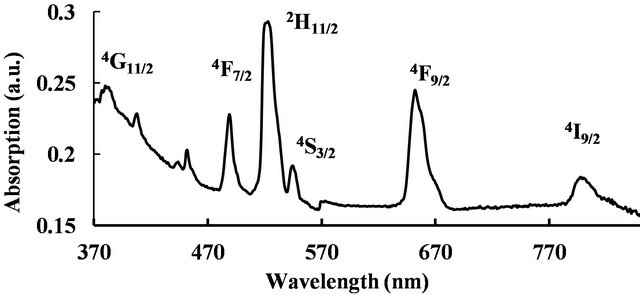
Figure 8. Absorption spectrum of Er3+ doped glass.
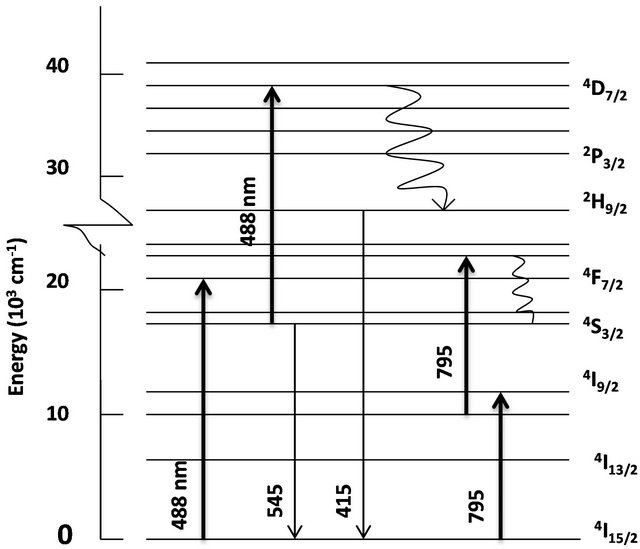
Figure 9. Partial energy level diagram of Er3+-doped glass. Upward and downward arrows represent absorption and emission transitions. Wavy arrows represent nonradiative transitions.
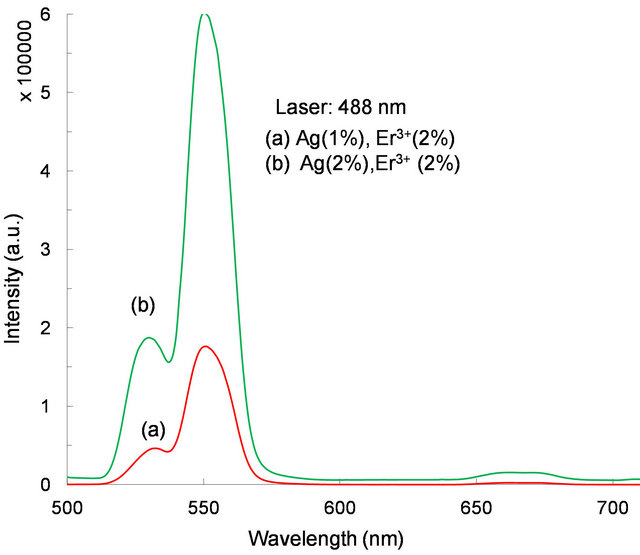
Figure 10. Fluorescence spectrum (4S3/2, 2H11/2à4I15/2) observed under 488 nm laser excitation from samples 3 and 4.
the samples contain 2% Er3+ but different Ag concentrations. These observations suggest that the increased emission may be due to enhanced field in the vicinity of Ag nanoparticles.
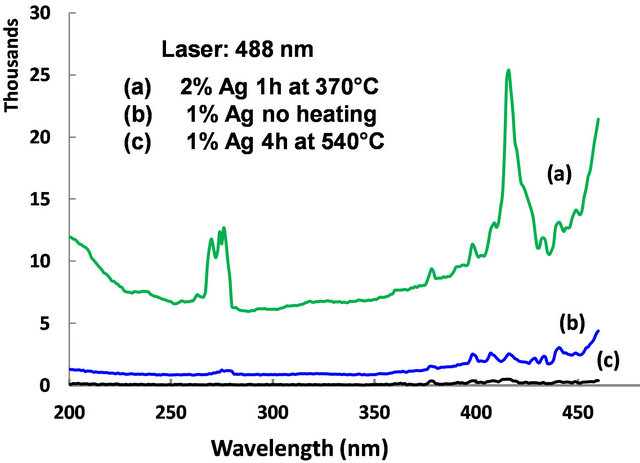
Figure 11. Emission spectrum observed from samples 3-5 under 488 nm laser excitation.
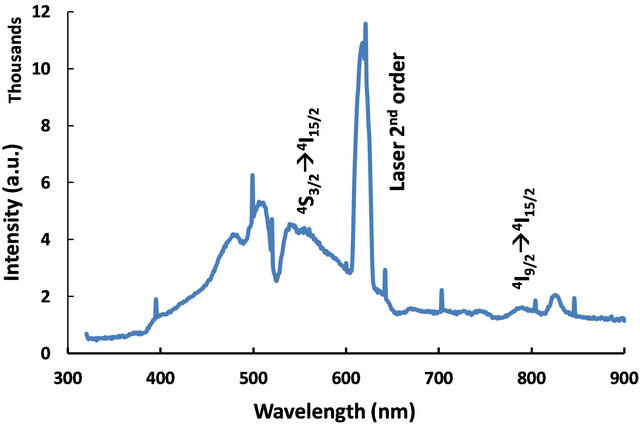
Figure 12. fluorescence spectrum observed from sample 3 under 308 nm laser excitation.
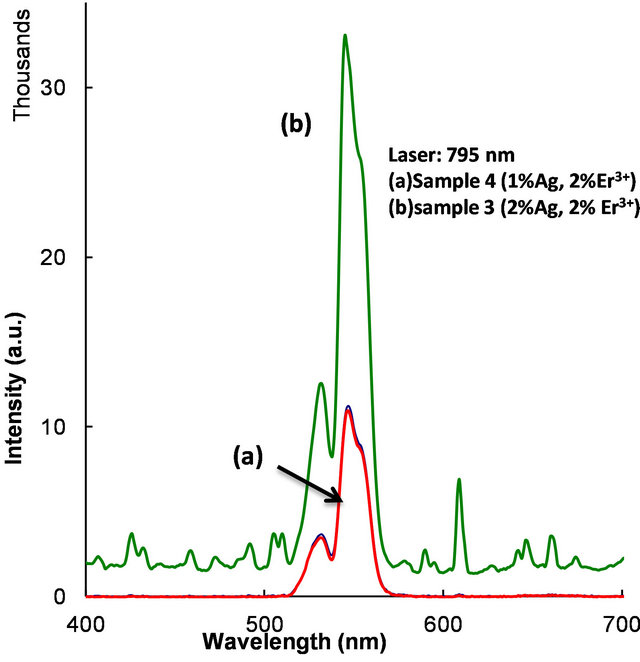
Figure 13. Energy upconversion spectrum (4S3/2, 2H11/2à4I15/2) observed under 795 nm laser excitation.
4. Conclusion
Sodium borate glasses embedded with silver were made by the melt quenching technique. Glass transition temperature was found to be 467˚C by thermal analysis of the sample. As made glasses revealed emission in the visible region under nitrogen laser and excimer laser excitations, due to the formation of Ag aggregates (molecular form). Heat treatment was used to induce silver metallic particles. Absorption spectra revealed a peak at 417 nm due to surface Plasmon resonance. Particle size was estimated to be 2.6 nm. Erbium and silver co-doped multielement oxide glasses were made by the melt quenching technique followed by heat treatment to induce Ag nanoparticles. In heat treated samples Er3+ luminescence increased 4× due to field enhancement in the vicinity of silver particles.
REFERENCES
- B. R. Reddy, “Stepwise Two-Photon Excitation Studies in Fibers and Crystals for Energy Upconversion and Infrared Quantum Counter Devices,” Proceedings of SPIE, Vol. 2000, 2000, pp. 87-114.
- C. B. Layne, W. H. Lowdermilk and M. J. Weber, “Multiphonon Relaxation of Rare-Earth Ions in Oxide Glasses,” Physical Review B, Vol. 16, No. 1, 1977, pp. 10-20. doi:10.1103/PhysRevB.16.10
- L. A. Riseberg and H. W. Moose, “Multiphonon Orbit Relaxation of Excited States of Rare-Earth Ions in Crystals,” Physical Review, Vol. 174, No. 2, 1968, pp. 429- 438. doi:10.1103/PhysRev.174.429
- M. Dejneka, “Rare-Earth Luminescence in Novel Oxyfluoride Glasses and Glass Ceramics,” Proceedings of SPIE, Vol. 3280, 1998, pp. 132-139. doi:10.1117/12.305402
- T. Hayakawa, S. T. Selvan and M. Nogami, “Field Enhancement Effect of Small Ag Particles on the Fluorescence from Eu3+-Doped SiO2 Glass,” Applied Physics Letters, Vol. 74, No. 11, 1999, pp. 1513-1515. doi:10.1063/1.123600
- C. Strohhofer and A. Polman, “Silver as a Sensitizer for Erbium,” Applied Physics Letters, Vol. 81, No. 8, 2002, pp. 1414-1416. doi:10.1063/1.1499509
- L. P. Naranjo, C. B. D. Araujo, O. L. Malta, P. A. Santa Cruz and L. R. P. Kassab, “Enhancement of Pr3+ Luminescence in PbO-GeO2 Glasses Containing Silver Nanoparticles,” Applied Physics Letters, Vol. 87, No. 24, 2005, pp. 241914-241916. doi:10.1063/1.2143135
- L. R. P. Kassab, C. B. D. Araujo, R. A. Kobayashi, R. D. Pinto and D. M. Silva, “Influence of Silver Nanoparticles in the Luminescence Efficiency of Pr3+-Doped Tellurite Glasses,” Journal of Applied Physics, Vol. 102, No. 10, 2007, pp. 103515. doi:10.1063/1.2817980
- B. J. Messinger, K. U. von Raben, R. K. Chang and P. W. Barber, “Local Fields at the Surface of Noble-Metal Microsphere,” Physical Review B, Vol. 24, No. 2, 1981, pp. 649-657. doi:10.1103/PhysRevB.24.649
- V. O. Obadina and B. R. Reddy, “Effect of Silver Nanoparticles on the Spectral Properties of Rare-Earth Ions in a Sodium Borate Glass,” Proceedings of SPIE, Vol. 8271, 2012, Article ID: 827104. doi:10.1117/12.905458
- C. Sonnichsen, T. Franzl, T. Wilk, G. von Plessen and J. Feldmann, “Plasmon Resonances in Large Noble-Metal Clusters,” New Journal of Physics, Vol. 4, 2002, pp. 93.1-93.8.
- H. Guo, X. Wang, J. Chen, and F. Li, “Ultrviolet Light Induced White Light Emission in Ag and Eu3+ Co-Doped Oxyfluoride Glasses,” Optics Express, Vol. 18, No. 18, 2010, pp. 18900-18905. doi:10.1364/OE.18.018900
- S. Lee, et al., “Spectral Change in Silver-Doped SodiumBorate Glass by Using Femtosecond Laser Irradiation,” Journal of Korean Physical Society, Vol. 52, 2008, pp. 1665-1668. doi:10.3938/jkps.52.1665
- J. A. Jimenez, S. Lysenko, G. Zhang and H. Liu, “Optical Properties of Silver-Doped Aluminophosphate Glasses,” Journal of Material Science, Vol. 42, No. 5, 2007, pp. 1856-1863. doi:10.1007/s10853-006-0898-6
- P. Gangopadyaya, R. Kesavamoorthy, S. Bera, P. Magudapathy, K. G. M. Nair, B. K. Panigrahi and S. V. Narasimhan, “Optical Absorption and Photoluminescence Spectroscopy of the Growth of Silver Nanoparticles,” Physical Review Letters, Vol. 94, No. 4, 2005, Article ID: 047403. doi:10.1103/PhysRevLett.94.047403
NOTES
*This research was supported by NSF grant HRD 0927644.
#Corresponding author.

