Journal of Surface Engineered Materials and Advanced Technology
Vol.2 No.1(2012), Article ID:16998,3 pages DOI:10.4236/jsemat.2012.21008
Estimate of the Crystallization Kinetics in Stoichiometry Compositions Films of Ge:Sb:Te
![]()
1Ciencias Ambientales del Instituto Nacional de Investigaciones Nucleares, La Marquesa, México; 2CICATA-IPN Unidad Querétaro, Querétaro, México; 3Centro de Investigación en Materiales Avanzados, Chihuahua, México; 4CINVESTAV-Qro., Querétaro, México.
Email: *carlos.rivera@inin.gob.mx
Received October 6th, 2011; revised November 19th, 2011; accepted November 27th, 2011
Keywords: Crystallization; Ge:Sb:Te; JMAK Model
ABSTRACT
The aim of this work is to compare the isothermal crystallization kinetic in the films along GeTe-Sb2Te3 line with composition Ge2Sb2Te5, Ge1Sb2Te4 Ge1Sb4Te7 and Ge4Sb1Te5 using mainly Johnson-Mehl-Avrami-Kolmogorov (JMAK) model. Results obtained have shown different crystallization mechanism in the investigated films. In Ge2Sb2Te5 and Ge1Sb2Te4 films the analysis of the kinetic results (Avrani coefficient) showed that at the beginning of crystallization a metastable phase appeared with the Ge1Sb4Te7 composition, this is followed by the nucleation and growth of the stable fcc phase up to full crystallization. In contrast Ge4Sb1Te5 and Ge1Sb4Te7 films show diffusion control growing from small dimension grains with decreasing nucleation rate.
1. Introduction
Phase-change memory technology is based on the high speed reversible amorphous-to-crystalline transformation of a thin film material. The limiting process in rewritable media is the slow crystallization process. For that reason, in recent years many experimental and theoretical studies to investigate the amorphous-to-crystalline phase transformation have appeared in the literature.
Studies of crystallization kinetics of phase change films, are often analyzed using the Johnson-Mehl-Avrami-Kolmogorov (JMAK) model for isothermal annealing (see the following reference [1-5]), which allow to determine the activation energy for the crystallization process. According to the JMAK model the transformed volume fraction x can be determined by the following expression.
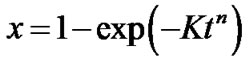 (1)
(1)
where K = γexp(–E/kT) and γ, E, t and n are the frequency factor, effective activation energy, time of annealing and Avrami exponent, respectively. In materials with random nucleation and isotropic growth the plot 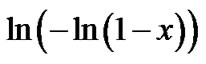 vs
vs 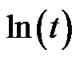 should be a straight line with a slope corresponding to the Avrami exponent, which provides information about the mechanisms of crystallization.
should be a straight line with a slope corresponding to the Avrami exponent, which provides information about the mechanisms of crystallization.
The kinetic parameters in the literature for Ge2Sb2Te5, Ge1Sb2Te4, Ge1Sb4Te7 and Ge4Sb1Te5 (the most common material used in phase change technology) show a large discrepancy. Values from 1.2 to 4.4 for the Avrami exponent and from 0.81 to 4.3 eV for the effective activation energy have been reported [1-7].
The aim of this work is to compare the isothermal crystallization kinetic in the films along GeTe-Sb2Te3 line.
2. Methodology
The amorphous Ge2Sb2Te5, Ge1Sb2Te4, Ge1Sb4Te7 and Ge4Sb1Te5 films, with thickness about of 200 nm, were produced using thermal evaporation from bulk alloys. The composition of films was evaluated by energy dispersive spectroscopy (EDS) and it did not differ more than 2% from the composition of the bulk materials.
In situ optical reflection (using a laser diode emitting at 650 nm) and X-ray measurements were carried out using a resistance heater. The temperature was controlled with a device which was programmed to heat the sample to a predetermined temperature for isothermal measurements. X-ray diffraction measurements were carried out using a Rigaku X-ray diffractometer with a Cu tube.
In the reflectance measurements the volume fraction of crystalline phase was calculated using the generally employed assumption that the signals are linearly related to the transformed crystalline volume fractions x using the next expression.
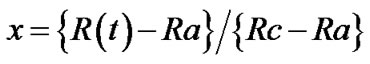 (2)
(2)
where Ra and Rc are the reflectance of amorphous and crystalline phase, respectively.
3. Results and Discussion
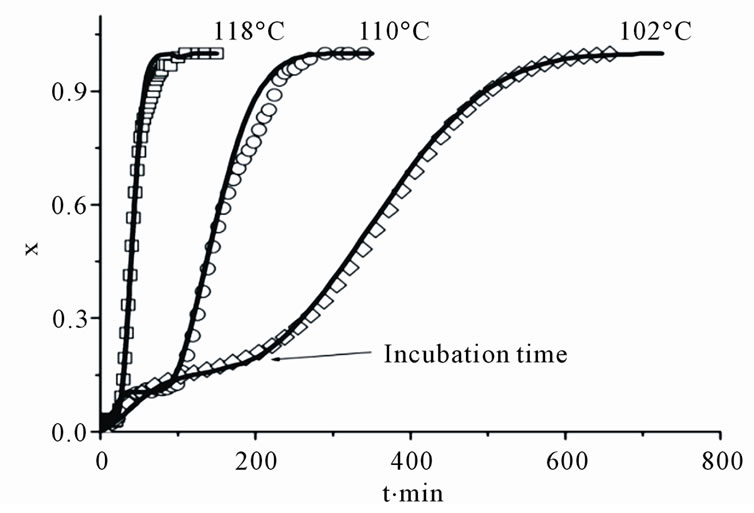
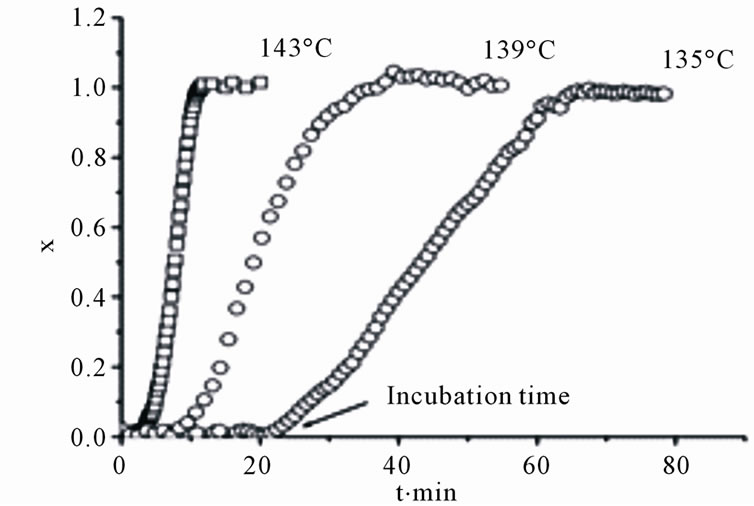
The next Figure 1 and Figure 2, show the evolution in the volume fraction of the transformed crystalline phase obtained from reflectance measurements during isothermal annealing at the indicated temperatures in films with the following compositions: Ge2Sb2Te5, Figure 1(a), and Ge4Sb1Te5, Figure 2(a). All films show long incubation time for crystallization, or time required to reach a critical nuclei size which lead to an abrupt increasing in the crystalline volume fraction. However there are two well defined crystallizations behaviors; in films with the
 (a)
(a)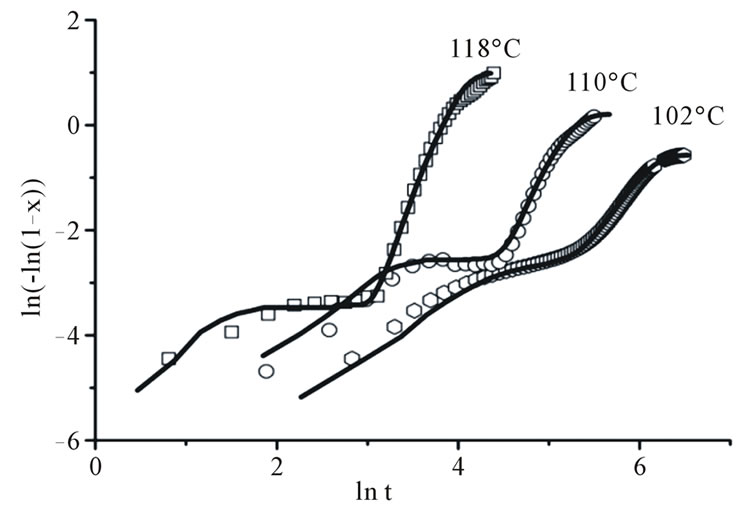 (b)
(b)
Figure 1. The dependencies of volume fraction (a) and an Avrami plot (b) for a Ge2Sb2Te5 film obtained from reflection measurements (points), results of simulations using the model with metastable phase formation are the continuous lines.
 (a)
(a)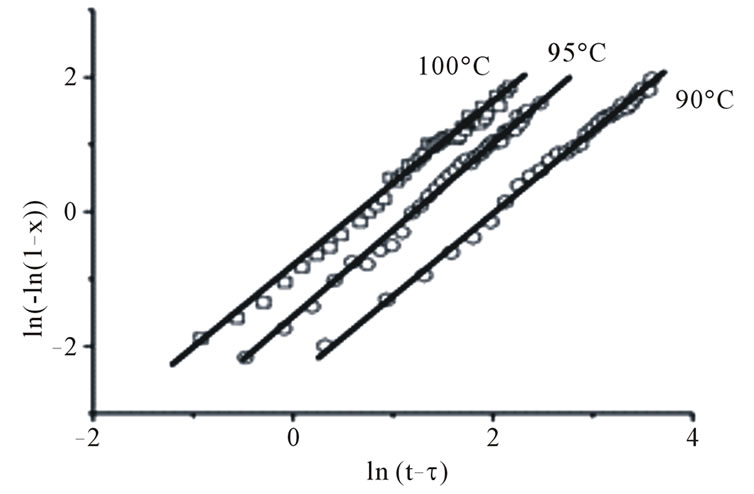 (b)
(b)
Figure 2. The dependencies of volume fraction (a) and Avrami plot (b) for a Ge4Sb1Te5 film obtained from reflection measurements (points), the results of fitting using JMAK models are the continuous lines.
Ge2Sb2Te5 and Ge1Sb2Te4 (not shown) compositions, the results show a relative large amounts of crystallized phase (about 20%) during the incubation time, whereas in those with the other two compositions (Ge1Sb4Te7 and Ge4Sb1Te5) the crystallized material during this time is much smaller (less than 2%).
X-ray measurements indicate that during incubation time, a metastable phase with the fcc Ge1Sb4Te7 composition is formed in the Ge2Sb2Te5 and Ge1Sb2Te4 films. This metastable phase has a lower crystallization temperature and it probably appears in the two ternary alloys due to local fluctuations in the composition of the amorphous films [8,9]. Thus, the process of isothermal crystallization in Ge2Sb2Te5 and Ge1Sb2Te4 takes place in two stages: in the first stage, nuclei of a metastable phase with composition Ge1Sb4Te7 precede the formation of the stable fcc phases; in the second stage, the nuclei transform into the equilibrium fcc stoichiometric structures with a composition correspondent to the parent amorphous material.
In materials where phase transformation starts with the formation of metastable phases, the isothermal crystallization process cannot be simply described by the JMAK theory and the Avrami plots are not linear which implies that Avrami exponent n does not remain constant during the crystallization process (Figure 1(b)). The kinetics of two-stage crystallization can be described by a modified JMAK model which assumes that the metastable phase grows up to a certain fraction and then stops growing. The second stage consists of simultaneous nucleation and growth of the stable phase into the metastable (until the grain limit is reached) and within the amorphous phases [8,9].
In films with compositions Ge4Sb1Te5 and Ge1Sb4Te7, the X-ray diffraction data shows crystallization into the same and final phase during the whole annealing time. In this case it is possible to neglect the amount of crystallized material during the incubation time τ and define τ as the beginning of the transformation [1], the JMAK equation is now expressed:
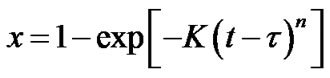 (3)
(3)
According to Equation (3) the plot 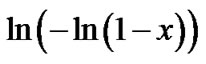 vs
vs 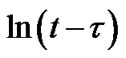 must be a straight line with the slope n (Figure 2(b)). This means that crystallization process in Ge4Sb1Te5 and Ge1Sb4Te7 material display random nucleation and isotropic growth.
must be a straight line with the slope n (Figure 2(b)). This means that crystallization process in Ge4Sb1Te5 and Ge1Sb4Te7 material display random nucleation and isotropic growth.
The values of Avrami exponent n (n = 1.8 for Ge4Sb1Te5 and n = 1.94 for Ge1Sb4Te7) and effective activation energy Ea (Ea = 3.46 eV for Ge4Sb1Te5 and Ea = 1.7 eV for Ge1Sb4Te7) have been calculated from reflection measurements. According to J. W. Christian [10], the values of n in the range 1.5 < n < 2.5 correspond to a crystallization process dominated by all shapes growing from small dimensions with decreasing nucleation rate.
Relative low effective activation energy obtained for Ge1Sb4Te7, low crystallization temperature, and the possibility to growth from small dimension allow suggesting that the appearance of nanocrystalline nuclei with the Ge1Sb4Te7 phase in the process of crystallization of the Ge2Sb2Te5 and Ge1Sb2Te4 materials could be related to local fluctuations in composition of the amorphous matrix.
4. Conclusion
In this work the crystallization mechanism in phasechange materials along GeTe-Sb2Te3 line is compared. The traditional JMAK model did not explain the isothermal crystallization kinetics results in all samples. In Ge2Sb2Te5 and Ge1Sb2Te4 films crystallization starts with formation of a metastable phase with Ge1Sb4Te7 composition. In contrast in Ge1Sb4Te7 and Ge4Sb1Te5 crystallization kinetics can be described by a JMAK model which take into account incubation times
REFERENCES
- V. Weidenhof, I. Friedrich, S. Ziegler and M. Wuttig, “Laser Induced Crystallization of Amorphous Ge2SbTe5 Films,” Journal of Applied Physics, Vol. 89, No. 6, 2001, pp. 3168-3176. doi:10.1063/1.1351868
- T. H. Jeong, M. R. Kim and H. Seo, “Crystallization Behavior of Sputter-Deposited Amorphous Ge2Sb2Te5 Thin Films,” Journal of Applied Physics, Vol. 86, No. 2, 1999, pp. 774-778. doi:10.1063/1.370803
- J. Gonzalez-Hernandez, B. S. Chao, D. Strand, S. R. Ovshinsky, D. Pawlik and P. Gasiorowski, “The Relationship between Crystal Structure and Performance as 58 Optical Recording Media in Te-Ge-Sb Thin Films,” Applied Physics communications, Vol. 11, No. 4, 1992, pp. 557-581.
- J. Tominaga, T. Nakano and N. Atoda, “Double Optical Phase Transition of GeSbTe Thin Films Sandwiched between Two SiN Layers,” Japanese Journal of Applied Physics, Vol. 37, 1998, pp. 1852-1854. doi:10.1143/JJAP.37.1852
- G. Ruitenberg, A. K. Petford-Long and R. C. Doole, “Determination of the Isothermal Nucleation and Growth Parameters for the Crystallization of Thin Ge2Sb2Te5 Films,” Journal of Applied Physics, Vol. 92, No. 6, 2002, 92, p. 3166. doi:10.1063/1.1503166
- N. Ohshima, “Crystallization of Germanium-AntimonyTellurium Amorphous Thin Film Sandwiched between Various Dielectric Protective Films,” Journal of Applied Physics, Vol. 79, No. 11, 1996, pp. 8357-8363. doi:10.1063/1.362548
- Z. L. Mao, H. Chen and A. L. Jung, “The Structure and Crystallization Characteristics of Phase Change Optical Disk Material Ge1Sb2Te4,” Journal of Applied Physics, Vol. 78, No. 4, 1995, pp. 2338-2342. doi:10.1063/1.360152
- B. Laine, C. Rivera-Rodriguez, E. Morales-Sanchez, E. Prokhorov, G. Trapaga and J. Gonzalez-Hernandez, “Isothermal Phase Transformation Kinetics in Stoichiometric GeSbTe Thin Films,” Journal of Non-Crystalline Solids, Vol. 345-346, 2004, pp. 173-177. doi:10.1016/j.jnoncrysol.2004.08.017
- D. Claudio, J. Gonzalez-Hernandez, O. Licea, B. Laine, E. Prokhorov and G. Trapaga, “An Analytical Model to Represent Crystallization Kinetics in Materials with Metastable Phase Formation,” Journal of Non-Crystalline Solids, Vol. 352, 2006, pp. 51-55.
- J. W. Christian, “The Theory of Transformation in Metals and Alloys,” Pergamon Press, Oxford, 1975.
NOTES
*Corresponding author.

