International Journal of Organic Chemistry
Vol.05 No.04(2015), Article ID:62158,9 pages
10.4236/ijoc.2015.54028
Ligand Exchange Reaction of Ferrocene with Heterocycles
Yutaka Okada, Hiroshi Huruya, Yasuhiro Imori
Department of Applied Chemistry, Ritsumeikan University, Kusatsu-Shi, Japan

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 October 2015; accepted 21 December 2015; published 24 December 2015
ABSTRACT
The ligand exchange reaction with heterocycles containing nitrogen, oxygen or sulfur atoms was carried out. For the reaction with heterocycles, the order of the reactivity was S-heterocycles > N-heterocycles > O-heterocycles. Furthermore, when the results for the heterocycles were compared to those for the corresponding hydrocarbons, the hydrocarbons had a higher reactivity. These results mean that the reactivity would be mainly governed by the electron density of these arenes.
Keywords:
Ferrocene, Ligand Exchange Reaction, Heterocycle

1. Introduction
The ligand exchange reaction is one of the typical reactions of ferrocenes. This is the reaction which was siscovered by Nesmeyanov et al. (Scheme 1) [1] . As for this reaction, the Cp ring of the ferrocene is exchanged for the aromatic ring, like benzene, in the presence of AlCl3 and the product is able to be isolated as a stable PF6 salt. Recently, this reaction is used for the preparation of several functional materials containing ferrocene moiety [2] [3] .
This reaction is initiated by pulling the Cp ring by AlCl3 in the vertical direction, so that the ferrocene decomposes into two fragments. The resulting fragment containing an iron atom is coordinated by an aromatic ring (Scheme 2) [4] [5] .
The authors have reported the substituent effects for the reaction between an alkylferrocene and alkylbenzenes [6] - [8] . In this study, the reaction with heterocycles containing nitrogen, oxygen or sulfur atoms was carried out. These results were compared to the reaction with carbocycles.
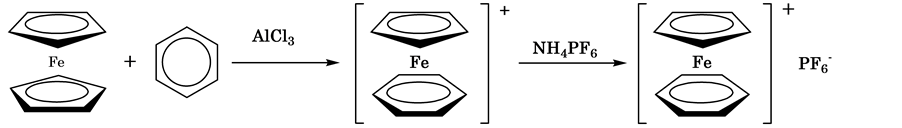
Scheme 1. The ligand exchange reaction.
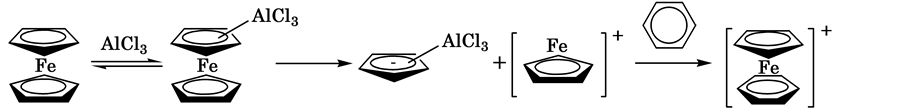
Scheme 2. The resulting fragment.
2. Experimental
2.1. Measurements of Ligand Exchange Reaction
The reaction was carried out in a small vessel, which contained the substrates and the solvent. The vessel was heated to a constant temperature in an oil bath. The reaction mixture was analyzed by HPLC at a specific time [7] [8] .
2.2. Measurement Apparatus
The 1H-NMR spectra were measured in chloroform-d at room temperature using a JEOL A-400 spectrometer. The NMR data are shown in Tables 1-4.
The mass spectra were obtained using a Shimadzu LCMS-QP8000. The ionization mode was used for the Atmospheric Pressure Chemical Ionization method.
3. Results and Discussion
3.1. The Ligand Exchange Reaction with Five-Membered Heterocycles
The yields of the reaction are shown in Table 5. For pyrrole, there are no products under these conditions. For thiophene, a small amount of the ligand exchange product was produced.
Pyrrole and thiophene are analogues containing nitrogen and sulfur, respectively. The difference in the reactivity would be due to the difference in the π-electron density of the aromatic ring. Namely, the electronegativity of nitrogen is 2.6, and that of sulfur is 3.0. Therefore, concerning the deviation of π-electron on the heterocycle, the sulfur analogue is larger than the nitrogen one, so the former would not act as an η5-ligand,
The yield of the ligand exchange product for thiophene was 3.1% - 4.8%, which was much lower than that of the hydrocarbon mentioned in previous papers [7] [8] . These results are supported by the fact that the product from thiophene shows lower chemical shifts of the Cp ring than that of the other products (Table 6).
3.2. The Ligand Exchange Reaction with Fused Five-Membered Heterocycles
As shown in Table 7, the product from benzothiophene was detected, whereas no product was detected from indole and benzofuran. The carbon analogue, indene, produced the exchange product. Furthermore, the NMR data (Table 6) showed that the CpFe moiety coordinates to the benzene ring, not but the five-membered ring.
As mentioned above, the electronegativity of nitrogen is higher than that of sulfur. The electronegativity of oxygen is 3.4 which is higher than that of nitrogen. Therefore, these hetero atoms would produce a decrease in the π-electron density and deviation on the benzene ring. These would decrease the ability to coordinate as the η5-ligand. The sulfur analogue, benzothiophene, gave the exchange product as well as the carbon analogue, indene, because of the lower electronegativity of the sulfur atom.
However, the yield of the ligand exchange product for benzothiophene was much lower than that of the tricyclic compounds as will be mentioned later.
Table 1. 1H-NMR chemical shifts (p.p.m.) of substrates for the ligand exchange reactions on acetone-d6.
Table 2. 1H-NMR chemical shifts (p.p.m.) of arene complexes of ferrocene on acetone-d6.
Table 3. 13C-NMR chemical shifts (p.p.m.) of substrates for the ligand exchange reactions on acetone-d6.
Table 4. 13C-NMR chemical shifts (p.p.m.) of arene complexes of ferrocene on acetone-d6.
Table 5. Yields of ligand exchange reactions of ferrocene with five-membered heterocycles.
Table 6. The difference of chemical shifts (p.p.m.) between the ligand exchange products and the substrates.
1)Δδ = δ(ligand exchange product) − δ(substrate).
Table 7. Yields of ligand exchange reactions of ferrocene with fused five-membered heterocycles.
3.3. The Ligand Exchange Reaction with Fused Six-Membered Heterocycles
As shown in Table 8, there are no products from the reaction between ferrocene and fused six-membered heterocycles, quinoline and isoquinoline. For the carbon analogue, naphthalene, the expected product was produced.
These results would be due to the electron withdrawing effect by the hetero atom similar to the case of five- membered heterocycles.
3.4. The Ligand Exchange Reaction with Fused Tricyclic Heterocycles
The ligand exchange reaction between ferrocene and the fused tricyclic heterocycles was carried out. As the results, the expected products were formed for all the tested heterocycles; i.e. carbazol, dibenzofuran and dibenzothiophene (Table 9). The NMR data showed that these products have the CpFe moiety on the end ring. This structure would be more stable than that bearing the CpFe moiety at the central ring due to the aromaticity of the rings.
The yields for these reactions were much higher than those for above-mentioned heterocycles. This would be due to the electron withdrawing effect by the hetero atom is divided into the two end rings, so that the effect is weakened.
Furthermore, the end ring protons bearing no CpFe moiety are shifted to a lower field as the NMR data show in Table 6. These shifts show that electrons are supplied from the end ring to produce the bond between the CpFe and the aromatic ring. On the contrary, this electron transfer caused the lower field shifts of the ring protons bearing the CpFe moiety. This effect, which does not appear for monocyclic and bicyclic heterocycles, would increase the reactivity of these tricyclic heterocycles.
As shown in Table 9, the yields for the carbazol, dibenzofuran and dibenzothiophene were almost equal, so the reactivities of these heterocycles were difficult to compare to each other. Therefore, these three heterocycles were put in a vessel at the same time, and the reaction was completed. The results are shown in Table 10.
From Table 10, for the mixture of carbazol and dibenzofuran, only the product with carbazol was formed (Entry 1). For the mixture of carbazol and dibenzothiophene, the ratio of products was 95:5, and for the mixture of dibenzofuran and dibenzothiophene, the ratio was 36:64 (Entries 2 and 3). These ratios were determined from the relative intensity of the LC-MS’s molecular weight related ion. Based on these results, the order of the reactivities of these heterocycles is determined to be carbazol > dibenzothiophene > dibenzofuran.
This order was supported by the NMR data. In general, electrons are supplied from the Cp ring to the Fe atom and the coordinated aromatic ring in these ligand exchange products, so that the chemical shifts of the Cp protons in the products are at a lower field than those in ferrocene. Table 1 shows such lower field shifts of the Cp protons. Moreover, the shift amount was the lowest for carbazole, and the highest for dibenzofuran. The fact that the shift amount was low means that the supplied amount of electron is low. This result shows that the intensity
Table 8. Yields of ligand exchange reactions of ferrocene with fused six-membered heterocycles.
Table 9. Yields of ligand exchange reactions of ferrocene with fused tricyclic heterocycles.
Table 10. Ratio of ligand exchange products of ferrocene with fused tricyclic heterocycles.
1)N = carbazol, O = dibenzofuran, S = dibenzothiophene.
of the Cp-Fe bond in the ligand exchange products does not change from that of ferrocene. Therefore, the product would be thermodynamically stable. As mentioned in the Introduction, the ligand exchange is a two-step reaction. The first step is pulling the Cp ring by AlCl3, and the second step is the coordination of an aromatic ring. Therefore, the reactivity would depend on the second step. The transition state of the second step should be similar to the ligand exchange product, therefore, the order of stability of the transition state is equal to that of the product.
4. Conclusion
For the ligand exchange reaction with heterocycles, the order of the reactivity was S-heterocycles > N-hetero- cycles > O-heterocycles. Furthermore, the reactivity of these heterocycles was lower than those for the corresponding hydrocarbons. The reactivity would be affected by the electron density of these arenes.
Cite this paper
YutakaOkada,HiroshiHuruya,YasuhiroImori, (2015) Ligand Exchange Reaction of Ferrocene with Heterocycles. International Journal of Organic Chemistry,05,282-290. doi: 10.4236/ijoc.2015.54028
References
- 1. Nesmeyanov, A.N., Vol’kenau, N.A. and Isaeva, L.S. (1967) Mobility of the Halogen in the Cyclopentadienyl Ring of Chlorocyclopentadienylphenyliron. Doklady Chemistry, 176, 106-109.
- 2. Li, F.-C., Tsai, S.-C., Yeh, C.-Y., Yeh, J.-Y., Chou, Y.-S., Ho, J.-R. and Tsiang, R.C.-C. (2014) Organic Thin-Film- Transistor Au/Poly(3-Hexylthiophene)/(Bilayer Die-lectrics)/Si Having Carbon Nanotubes Chemically Bonded to Poly(3-Hexylthiophene) in the Active Layer. Journal of Nano-science and Nanotechnology, 14, 5019-5027.
http://dx.doi.org/10.1166/jnn.2014.9259 - 3. Cha, I., Yagi, Y., Kawahara, T., Hashimoto, K., Fujiki, K., Tamesue, S., Yamauchi, T. and Tsubokawa, N. (2014) Grafting of Polymers onto Graphene Oxide by Trapping of Polymer Radicals and Ligand-Exchange Reaction of Polymers Bearing Ferrocene Moieties. Colloids and Surfaces, A441, 474-480.
http://dx.doi.org/10.1016/j.colsurfa.2013.10.002 - 4. Astruc, D. and Dabard, R. (1976) On the Mechanism of Ligand Exchange and Complexation by Aluminum Chloride of Ferrocene and Its Alkylated and Acylated Derivatives. Journal of Organometallic Chemistry, 111, 339-347.
http://dx.doi.org/10.1016/S0022-328X(00)98142-7 - 5. Astruc, D. and Dabard, R. (1976) Ligand Exchange Reactions between Arenes and Ferrocene Derivatives Bearing at the α Position One or Two Substituents Complexed by Aluminum Trichloride. Tetrahedron, 32, 245-249.
http://dx.doi.org/10.1016/0040-4020(76)87009-3 - 6. Astruc, D. (1983) Organo-Iron Complexes of Aromatic Compounds. Applications in Synthesis. Tetrahedron, 39, 4027-4095.
http://dx.doi.org/10.1016/S0040-4020(01)88627-0 - 7. Hayashi, T., Okada, Y. and Shimizu, S. (1996) Substituent Effects for the Disproportionation, Synproportionation and Ligand Exchange Reaction of t-Butylferrocenes. Transition Metal Chemistry, 21, 418-422.
http://dx.doi.org/10.1007/BF00140783 - 8. Okada, Y., Yoshigami, Y. and Hayashi, T. (2003) Steric Effects on the Ligand Exchange Reactions of Alkylferrocenes. Transition Metal Chemistry, 28, 794-799.
http://dx.doi.org/10.1023/A:1026006314010


