International Journal of Organic Chemistry
Vol.03 No.02(2013), Article ID:33048,7 pages
10.4236/ijoc.2013.32018
Al-Pillared Ghassoulite Clay as a New Green Catalyst for the Synthesis of Benzothiazoles and Benzimidazoles: Effect of Amine/CEC Ratio
Rachid Azzallou1,2, Rachid Mamouni2*, Kimberly Stieglitz3, Nabil Saffaj4, Mohammadine El Haddad5, Said Lazar1
1Laboratoire de Biochimie, Environnement & Agroalimentaire, URAC 36, Université Hassan II-Mohammedia,
Mohammedia, Morocco
2Equipe de Chimie Bio-Organique Appliquée, Faculté des Sciences, Université Ibn Zohr, Agadir, Morocco
3Department of Science, Technology, Engineering and Mathematics (STEM), Biotechnology Division Roxbury Community College, Boston, USA
4Laboratoire de Chimie, Faculté Polydisciplinaire, Ouarzazate, Morocco
5Equipe de Chimie Analytique & Environnement, Faculté Polydisciplinaire, Université Cadi Ayyad, Safi, Morocco
Email: *mamounirachid@yahoo.fr
Copyright © 2013 Rachid Azzallou et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received March 22, 2013; revised May 4, 2013; accepted May 21, 2013
ABSTRACT
Catalysts based on pillared clays were synthesized, by preadsorption of amines between the clay layers prior to pillaring with aluminium precursora, from Moroccan Ghassoulite clay. A comparison of catalytic activity, in the synthesis of benzothiazole and benzimidazole derivatives, of these catalysts is discussed. It is shown that Al-pillared (x CEC) catalyst can be quantitatively recovered and be reused effectively for many times.
Keywords:
Cation Exchange Capacity (x CEC); Benzimidazole; Benzothiazole; Catalyst; Pillared
1. Introduction
Clay minerals are attractive materials for catalysis and adsorption due to low toxicity, high availability and economics. Various methods have been proposed for modifying their properties to obtain materials for specific applications [1]. Treating cationic layered minerals with acid change surface acidity influence textural properties and chemical composition [2]. Intercalation of cationic layered minerals with metal oxide pillars (e.g. Al2O3, TiO2, ZrO2) is another important method used for modi- fication of these clays [3]. These materials, which are called pillared interlayered clays (PILCs), are character- ized by a relatively high surface area (150 -
The Al13-PILC material used in the present study was prepared with a purified Moroccan clay mineral from Moulouya known as a Ghassoulite or Rhassoulite [8].
The benzimidazole and benzothiazole skeletons may be found in numerous pharmaceutical agents with a di- verse spectrum of biological properties [9-12]. Al- though a wide range of methods are available for synthe- sizing benzimidazoles [12-21], and benzothiazoles [22-26], a real need exists for new and simple procedures that support many kinds of structural diversity and vari- ous substitution patterns in the target library. Recently, some microwave-assisted methods were reported for the synthesis of 2-substituted benzothiazoles [27]. Strong oxidant [28] or, more interestingly, catalytic aerobic oxidation involving oxygen as terminal oxidant [29,30] has received considerable attention in the building block construction of benzimidazoles [31-34].
In continuation of our attempts to explore the catalytic activity of some new inorganic supports like Animal Bone Meal (ABM) and Al-pillared clay for useful or- ganic transformations [34-38], we herein report a study of the influence of number and size of the pillars in the interlayer region, which is in turn influenced by the cation exchange capacity (x CEC) of the original clay mineral, in the synthesis of benzimidazole and benzothi- azole derivatives (Scheme 1).
The modified clay support has paved the way for bet- ter dispersion of the Alumina which results in higher catalytic activity.
2. Experimental
2.1. Aluminum Oxide-Pillared Clay Synthesized with Preadsorption of Diethylamine
The starting material is the Na-exchanged ghassoulite. It is added to the vigorously stirred solution of an organic amine compound (diethylamine) dissolved in water. The amounts of amine were 1/4, 1/2 and 3/4 of the cation exchange capacity (CEC) of the clay. The pH of this mixture was adjusted to 8 and the mixture was stirred over night at room temperature until the exchange was complete. Adding the pillaring solution, previously pre- pared, performs a further exchange reaction, drop wise to the vigorously stirred solution of the partially intercalated Na-Gh clay. This mixture is stirred at room temperature over a night. After centrifugation, the solid phase was washed until no chloride was present in the wash water. The solid obtained was then dried at 100˚C and calcined at a heating rate of 2˚C/min to a designated temperature T = 450˚C and kept at this temperature for 4 h. During this calcination process the aluminum precursors are con- verted into rigid aluminum oxide pillars, and the ammo- nium ions are removed. The resulting materials are de- nominated x CEC and the x values are 1/4, 1/2 and 3/4 as shown in Figure 1 and analyzed and interpreted as in previous studies (Figure 1) [8].
2.2. Characterization
The clay’s cation exchange capacity (CEC) was meas- ured using the method generated by Glaeser [39]. Four samples of
By extrapolating the plot of n(OH)titrated = f(n(OH) added) to the intercept with the x-axis, we determined the
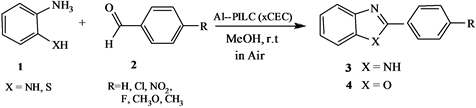
Scheme 1. Synthesis of 2-arylbenzimidazoles and benzothi- azoles catalyzed by Al-pillared (x CEC).
Figure 1.X-ray diffraction patterns of x CEC .
CEC value. The experiment revealed that the negative charge was reduced from 79 for the raw mineral to 49 for the Na-Gh, expressed as milliequivalents of the anionic sites per
The X-ray diffraction data was used to determine the basal spacing of the pillared products. Samples for X-ray diffraction (XRD) measurements were recorded after heating for 2 h in vacuum (0.1 Pa) at 100˚C. Nitrogen adsorption isotherms were carried out at a liquid-nitrogen temperature with an ASAP
2.3. General Procedure for the Synthesis of Bis-Enzimidazoles or Benzothiazoles
The catalyst Al-PILC (x CEC) (100 mg) was added to a mixture of aldehyde 2 (1.0 mmol) and o-phenylenedia- mine
gress of the reaction was monitored by TLC (n-hexane/ EtOAc 2:1). After complete conversion, the reaction mixture was filtered, the catalyst was washed, and the solvent was removed under reduced pressure. The crude material was purified by column chromatography or recrystallization to afford the pure product 3 or 4.
3. Results and Discussion
Al-pillared clays were prepared by a reaction of Na- ghassoulite (Na-Gh) with base-hydrolyzed solutions of Al3+ salts and a subsequent thermal conversion of the intercalated polycations. These pillared clays have rela- tively low micropore volumes [37,38]. By preadsorption of amines between the clay layers prior to pillaring with aluminium precursor, it was possible to increase the mi croporosity of the obtained material. This improvement can be explained by the decrease of the Al-pillars density
Figure 2.Relative pressure.
Table 1. Textural parameters of samples obtained from the nitrogen adsorption.
aPores between 1.7 and 300 nm diameter.
between the clay sheets. This explanation was proved by the decrease of the aluminium amounts in the treated samples determined by chemical analyses. The amount of amine was successively 1/4, 1/2 and 3/4 of the cation exchange capacity of the clay mineral.
As a result of preadsorption of amines, the pillar density decreases because a part of the interlayer space is occu- pied by the amine and during the calcination; the pillaring precursors are converted into rigid aluminum oxide pillars and the organic compounds are removed reflecting an apparent improvement in the orientation of the clay layers. The surface area of this product increase to reach 624 m2∙g−1 and the micropore volume becomes three times higher than that prepared without preadsorption of diethylamine.
Recently we have reported that Al-PILC used in methanol at room temperature or microwave can promote the synthesis of benzimidazole with good yields [37,38]. In this work we report the effect of a family of Al-pil- lared Moroccan clay, differing only in cation exchange capacity (CEC), in the synthesis of benzimidazole or benzothiazoles derivatives.
The reaction was first optimized using benzaldehyde
In air and in the presence of Al-pillared (x CEC), reac- tions between
High yields are obtained in a reasonably short time in the presence of the catalyst Al-pillared (1/4 CEC) than Al-pillared (1/2 CEC, 3/4 CEC) (Figure 3, Table 4).
To explain this phenomenon, we must clarify the in- fluence of preadsorption of amine between the clay lay- ers prior to pillaring with aluminum precursor.
Table 2. Chemical composition of the samples studied (metal oxides in wt%).
Table 3. Optimisation the synthesis of
aYields in pure isolated products. bNR: no reaction.
The preadsorption of amine increase the porosity of pillared clay (Table 1). This improvement can be explanation was proved by the decrease of the aluminum amounts in the treated sample (Table 2) from 1/4 CEC to 3/4 CEC.
The reduction of yields from catalyst 1/2 CEC to 1/4 CEC (Tables 3 and 4) can be explained by complete satu-
Figure 3.Comparison of the catalytic activity of 1/4 CEC, 1/2 CEC and 3/4 CEC in the synthesis of benzimidazole 3a.

Scheme 2. Proposition of mechanism synthetic of benzimidazoles and benzimidazoles via Al-pillared (x CEC).
Table 4. Al-pillared (x CEC) catalyzed formation of benzimidazoles and benzothiazoles.
aYields in pure products isolated by distillation under vacuum identified by 1H-NMR and IR spectroscopy.
ration of interlayer volume with amine molecules which hinders the exchange with the Al13 oligomers. Since these oligomers will be responsible for the formation of pillars, which produce the permanent and final porosity of materials after calcination. The saturation of the inter- layer volume with amine will have negative effect poros- ity (Vp) (Table 1). The Al-pillared (1/4 CEC) sample pre- pared in this study has narrow enough pores to adsorb the molecules and to produce a good catalysis efficiency.
In the presence of pillared clay (1/4 CEC), various sol- vents were tested. Thus, after 20 min the yields of prod- uct
In the absence of solvent, only a 10% yield of
The Al-pillared (1/4 CEC) was quantitatively recovered by simple filtration and regenerated by calcination at 200˚C over 2 h and was reused in future reactions. Inves- tigations were performed on benzaldehyde
Whatever the assay, completion of the reaction was always achieved in 25 min but a decrease in yield was observed at the fourth round. The catalyst was fully re- covered after the first reaction, recovery remained stable till the fifth reaction but the amount of promoter gradually decreased after each assay.
4. Conclusions
To summarize, we have reported a study of the influence of number and size of the pillars in the interlayer region, which is in turn influenced by the cation exchange ca- pacity (x CEC) of the original clay mineral, in the synthesis of benzimidazole and benzothiazole derivatives. High yields are obtained in a reasonably short time in the presence of the catalyst Al-pillared (1/4 CEC) than Al-
Table 5. Studies on the reuse of Al-pillared (1/4 CEC).
pillared (1/2 CEC, 3/4 CEC).
The Al-pillared (1/4 CEC) sample in this study was prepared with pores narrow enough to adsorb the mole- cules and to have an enhanced catalysis efficiency com- pared to other Al-pillared samples. The modified clay support has paved the way for better dispersion of the Alumina which results in higher catalytic activity.
References
- 1. C. H. Zhou, “Emerging Trends and Challenges in Synthetic Clay-Based Materials and Layered Double Hydroxides,” Applied Clay Science, Vol. 48, No. 1-2, 2010, pp. 1-4. doi:10.1016/j.clay.2009.12.018
- 2. P. Komadel and J. Madejova, “Acid Activation Clay Minerals, Handbook of Clay Science,” Elsevier, Amsterdam, 2006, pp. 263-288. doi:10.1016/S1572-4352(05)01008-1
- 3. F. Belkhadem, A. Maldonado, B. Siebenhaar, J.-M. Clacens, J. M. Perez-Zurita, A. Bengueddach and F. Figueras, “Microalorimetric Measurement of the Acid Properties of Pillared Clays Prepared by Competitive Cation Exchange,” Applied Clay Science, Vol. 39, No. 1-2, 2008, pp. 28-37. doi:10.1016/j.clay.2007.04.005
- 4. R. T. Yang, N. Tharappiwattananon and R. Q. Long, “Ion-Exchanged Pillared Clays for Selective Catalytic Reduction of NO by Ethylene in the Presence of Oxygen,” Applied Catalysis B: Environmental, Vol. 19, No. 3-4, 1998, p. 289.
- 5. C. B. Molina, J. A. Casas, J. A. Zazo and J. J. Rodriguez, “A Comparison of Al-Fe and Zr-Fe Pillared of Clays for Catalytic Wet Peroxide Oxidation,” Chemical Engineering Journal, Vol. 118, No. 1-2, 2006, pp. 29-35. doi:10.1016/j.cej.2006.01.007
- 6. T. J. Pinnavaia, M. Tzou, S. D. Landau and R. H. Raythatha, “On the Pillared and Delamination of Smectite Clay Catalysis by Polyoxo Cations of Aluminium,” Journal of Molecular Catalysis, Vol. 27, No. 1-2, 1984, pp. 195-212. doi:10.1016/0304-5102(84)85080-4
- 7. G. J. J. Bartley and R. Burch, “Zr-Containing Pillared Interlayer Clays. Part III. Influence of Method of Preparation on the Thermal and Hydrothermal Stability,” Applied Catalysis, Vol. 19, No. 1, 1985, pp. 175-185. doi:10.1016/S0166-9834(00)82679-2
- 8. A. Elmchaouri and R. Mahboub, “Effects of Preadsorption of Organic Amine on Al-PILCs Structures,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 259, No. 1-3, 2005, pp. 135-141. doi:10.1016/j.colsurfa.2005.02.014
- 9. C. Chen and Y. Chen, “Liquid-Phase Synthesis of 2-Substituted Benzimidazoles, Benzoxazoles and Benzothiazoles,” Tetrahedron Letters, Vol. 45, No. 1, 2004, pp. 113-115. doi:10.1016/j.tetlet.2003.10.095
- 10. N. Siddiqui, A. Rana, S. A. Khan, M. A. Bhat and S. E. Haque, “Synthesis of Benzothiazole Semicarbazones as Novel Anticonvulsants—The Role of Hydrophobic Domain,” Bioorganic & Medicinal Chemistry Letters, Vol. 17, No. 15, 2007, pp. 4178-4182. doi:10.1016/j.bmcl.2007.05.048
- 11. C. J. Lion, C. S. Matthews, G. Wells, T. D. Bradshaw, M. F. G. Stevens and A. D. Westwell, “Antitumour Properties of Fluorinated Benzothiazole-Substituted Hydroxycyclohexa-2,5-Dienones (‘Quinols’),” Bioorganic & Medicinal Chemistry Letters, Vol. 16, No. 19, 2006, pp. 5005-5008. doi:10.1016/j.bmcl.2006.07.072
- 12. S. T. Huang, I. J. Hsei and C. Chen, “Synthesis and Anticancer Evaluation of Bis(benzimidazoles), Bis(benzoxazoles), and Benzothiazoles,” Bioorganic & Medicinal Chemistry Letters, Vol. 14, No. 17, 2006, pp. 6106-6019. doi:10.1016/j.bmc.2006.05.007
- 13. J.-L. Girardet and L. B. Townsend, “Synthesis of β-lLyxofuranosyl Benzimidazoles by an Unexpected Intramolecular Displacement Reaction,” The Journal of Organic Chemistry, Vol. 64, No. 11, 1999, pp. 4169-4172. doi:10.1021/jo981733t
- 14. C.-M. Yeh, C.-L. Tung and C.-M. Sun, “Combinatorial Liquid-Phase Synthesis of Structurally Diverse Benzimidazole Libraries,” Journal of Combinatorial Chemistry, Vol. 2, No. 4, 2000, pp. 341-348. doi:10.1021/cc0000085
- 15. J. J. Chen, Y. Wei, J. C. Drach and L. B. Townsend, “Synthesis and Antiviral Evaluation of Trisubstituted Indole N-Nucleosides as Analogues of 2,5,6-Trichloro-1(beta-D-ribofuranosyl)benzimidazole (TCRB),” Journal of Medicinal Chemistry , Vol. 43, No. 12, 2000, pp. 24492456. doi:10.1021/jm990320x
- 16. D. Tumelty, K. Cao and C. P. Holmes, “Traceless SolidPhase Synthesis of Substituted Benzimidazoles via a Base-Cleavable Linker,” Organic Letters, Vol. 3, No. 1, 2001, pp. 83-86. doi:10.1021/ol006801o
- 17. J. Mann, A. Baron, Y. Opoku-Boahen, E. Johansson, G. Parkinson, L. R. Kelland and S. Neidle, “A New Class of Symmetric Bisbenzimidazole-Based DNA Minor Groove-Binding Agents Showing Antitumor Activity,” Journal of Medicinal Chemistry, Vol. 44, No. 2, 2001, pp. 138-144. doi:10.1021/jm000297b
- 18. B. Raju, N. Nguyen and G. W. Holland, “Solution-Phase Parallel Synthesis of Substituted Benzimidazoles,” Journal of Combinatorial Chemistry, Vol. 4, No. 4, 2002, pp. 320-328. doi:10.1021/cc000098d
- 19. H. Akamatsu, K. Fukase and S. Kusumoto, “New Efficient Route for Solid-Phase Synthesis of Benzimidazole Derivatives,” Journal of Combinatorial Chemistry, Vol. 4, No. 5, 2002, pp. 475-483. doi:10.1021/cc020006f
- 20. C. E. Hoesl, A. Nefzi and R. A. Houghten, “Parallel Solid-Phase Synthesis of 2-Imino-4-oxo-1,3,5-triazino [1,2-a]benzimidazoles via Tandem Aza-Wittig/Heterocumulene-Mediated Annulation Reaction,” Journal of Combinatorial Chemistry, Vol. 5, No. 2, 2003, pp. 155-160. doi:10.1021/cc020077e
- 21. D. Vourloumis, M. Takahashi, K. B. Simonsen, B. K. Ayida, S. Barluenga, G. C. Winters and T. Hermann, “Solid-Phase Synthesis of Benzimidazole Libraries Biased for RNA Targets,” Tetrahedron Letters, Vol. 44, No. 14, 2003, pp. 2807-2811. doi:10.1016/S0040-4039(03)00453-2
- 22. M.-S. Chua, D.-F. Shi, S. Wrigley, T. D. Bradshaw, I. Hutchinson, P. N. Shaw, D. A. Barrett, L. A. Stanley and M. F. G. Stevens, “Antitumor Benzothiazoles. 7.1 Synthesis of 2-(4-Acylaminophenyl)benzothiazoles and Investigations into the Role of Acetylation in the Antitumor Activities of the Parent Amines,” Journal of Medicinal Chemistry, Vol. 42, No. 3, 1999, pp. 381-392. doi:10.1021/jm981076x
- 23. E. Kashiyama, I. Hutchinson, M.-S. Chua, S. F. Stinson, L. R. Phillips, G. Kaur, E. A. Sausville, T. D. Bradshaw, A. D. Westwell and M. F. G. Stevens, “Antitumor Benzothiazoles. 8.1 Synthesis, Metabolic Formation, and Biological Properties of the Cand N-Oxidation Products of Antitumor 2-(4-aminophenyl)benzothiazoles,” Journal of Medicinal Chemistry, Vol. 42, No. 20, 1999, pp. 41724184. doi:10.1021/jm990104o
- 24. I. Hutchinson, M.-S. Chua, H. L. Browne, V. Trapani, T. D. Bradshaw, A. D. Westwell and M. F. G. Stevens, “Antitumor Benzothiazoles. 14.1 Synthesis and in Vitro Biological Properties of Fluorinated 2-(4-Aminophenyl)benzothiazoles,” Journal of Medicinal Chemistry, Vol. 44, No. 9, 2001, pp. 1446-1455. doi:10.1021/jm001104n
- 25. W. Leng, Y. Zhou and Q. Xu Liu, “Synthesis and Characterization of Nonlinear Optical Side-Chain Polyimides Containing the Benzothiazole Chromophores,” Macromolecules, Vol. 34, No. 14, 2001, pp. 4774-4779. doi:10.1021/ma0100347
- 26. I. Hutchinson, S. A. Jennings, B. R. Vishnuvajjala, A. D. Westwell and M. F. G. Stevens, “Antitumor Benzothiazoles. 16.1 Synthesis and Pharmaceutical Properties of Antitumor 2-(4-Minophenyl)benzothiazole Amino Acid Prodrugs,” Journal of Medicinal Chemistry, Vol. 45, No. 3, 2002, pp. 744-747. doi:10.1021/jm011025r
- 27. F. M. Moghaddam, G. R. Bardajee, H. Ismaili and S. M. “Dokht Taimoory,” Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry, Vol. 36, No. 17, 2006, pp. 2543-2548. doi:10.1080/00397910600781448
- 28. C. Praveen, K. H. Kumar, D. Muralidharan and P. T. Perumal, “Oxidative Cyclization of Thiophenolic and Phenolic Schiff’s Bases Promoted by PCC: A New Oxidant for 2-Substituted Benzothiazoles and Benzoxazoles,” Tetrahedron, Vol. 64, No. 10, 2008, pp. 2369-2374. doi:10.1016/j.tet.2008.01.004
- 29. G. J. Brink, W. C. E. Arends and R. A. Sheldon, “The Baeyer-Villiger Reaction: New Developments toward Greener Procedures,” Chemical Reviews, Vol. 104, No. 9, 2004, pp. 4105-4124. doi:10.1021/cr030011l
- 30. M. Beller, “The Current Status and Future Trends in Oxidation Chemistry,” Advanced Synthesis & Catalysis, Vol. 346, No. 2-3, 2004, pp. 107-108. doi:10.1002/adsc.200404008
- 31. Y. Kawashita, N. Nakamichi, H. Kawabata and M. Hayashi, “Direct and Practical Synthesis of 2-Arylbenzoxazoles Promoted by Activated Carbon,” Organic Letters, Vol. 5, No. 20, 2003, pp. 3713-3715. doi:10.1021/ol035393w
- 32. Y. X. Chen, L. F. Qian, W. Zhang and B. Han, “Efficient Aerobic Oxidative Synthesis of 2-Substituted Benzoxazoles, Benzothiazoles, and Benzimidazoles Catalyzed by 4-Methoxy-TEMPO,” Angewandte Chemie International Edition, Vol. 47, No. 48, 2008, pp. 9330-9333. doi:10.1002/anie.200803381
- 33. L. Songnian and Y. Lihu, “A Simple and Efficient Procedure for the Synthesis of Benzimidazoles Using Air as the Oxidant,” Tetrahedron Letters, Vol. 46, No. 25, 2005, pp. 4315-4319. doi:10.1016/j.tetlet.2005.04.101
- 34. Y. Riadi, R. Mamouni, R. Azzalou M.El Haddad, S. Routier, G. Guillaumet and S. Lazar, “An Efficient and Reusable Heterogeneous Catalyst Animal Bone Meal for Facile Synthesis of Benzimidazoles, Benzoxazoles, and Benzothiazoles,” Tetrahedron Letters, Vol. 52, No. 27, 2011, pp. 3492-3495. doi:10.1016/j.tetlet.2011.04.121
- 35. Y. Riadi, R. Mamouni, Y. Abrouki, M. El Haddad, N. Saffaj, S. El Antri, S. Routier, G. Guillaumet and S. Lazar, “Animal Bone Meal (ABM): A Novel Natural Catalyst for Thia-Michael Addition,” Letters in Organic Chemistry, Vol. 7, No. 3, 2010, pp. 269-271. doi:10.2174/157017810791112397
- 36. Y. Riadi, Y. Abrouki, R. Mamouni, M. El Haddad, S. Routier, G. Guillaumet and S. Lazar, “New Eco-Friendly Animal Bone Meal Catalysts for Preparation of Chalcones and Aza-Michael Adducts,” Chemistry Central Journal, Vol. 6, 2012, p. 60.
- 37. R. Azzalou, R. Mamouni, Y. Riady, M. El Haddad, Y. El Mouzdahir, R. Mahboub, A. Elmchaouri, S. Lazar and G. Guillaumet, “A Mild and Efficient One-Pot Synthesis of 2-Substituted Benzimidazoles through a New Surfactant-Modified Moroccan Clay Catalyst,” Revista de Chimie (Bucharest), Vol. 61, No. 12, 2010, pp. 11551157.
- 38. R. Azzalou, R. Mamouni, M. El Haddad, M. C. ViaudMassuard, G. Guillaumet and S. Lazar, “Optimization of the Synthesis of 2-Substituted Benzimidazoles Catalyzed by Al-PILC Under Microwave Irradiation,” Revista de Chimie, Vol. 62, 2011, p. 2.
- 39. R. Glaeser, “Determination of the Base-Exchange Capacity in Montmorillonite,” Comptes Rendus de l’Académie des Sciences de Paris, Vol. 222, 1946, p. 1179.
- 40. E. P. Barrett, L. G. Joyner and P. H. Halenda, “The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms,” Journal of the American Chemical Society, Vol. 73, No. 1, 1951, pp. 373-380. doi:10.1021/ja01145a126
- 41. V. R. Devalla and K. Ethirajulu, “Synthesis of 2-Substituted Benzoxazoles and Benzimidazoles Based on Mass Spectral Ortho Interactions,” Journal of the Chemical Society, Perkin Transactions 2, No. 7, 1995, pp. 1497-1501.
- 42. H. Thakuria and G. Das, “An Expeditious One-Pot Solvent-Free Synthesis of Benzimidazole Derivatives,” Archive for Organic Chemistry, Vol. 2008, No. 15, 2008, pp. 321-328. doi:10.3998/ark.5550190.0009.f28
- 43. K. Bougrin, A. Loupy and M. Soufiaoui, “Trois Nouvelles Voies de Synthèse des Dérivés 1,3-Azoliques Sous Micro-Ondes,” Tetrahedron, Vol. 54, No. 28, 1998, pp. 8055-8064. doi:10.1016/S0040-4020(98)00431-1
- 44. S. L. Balaji, R. P. Umesh, R. M. Jyotirling and A. M. Ramrao, “Synthesis of 2-Arylbenzothiazoles Catalyzed by Biomimetic Catalyst, β-Cyclodextrin,” Bulletin of the Korean Chemical Society, Vol. 31, No. 8, 2010, pp. 23292332. doi:10.5012/bkcs.2010.31.8.2329
- 45. K. M. Khan, F. Rahim, S. A. Halim, M. Taha, M. Khan, S. Perveen, Z. Haq, M. A. Mesaik and M. I. Choudhary, “Synthesis of Novel Inhibitors of β-Glucuronidase Based on Benzothiazole Skeleton and Study of Their Binding Affinity by Molecular Docking,” Bioorganic & Medicinal Chemistry, Vol. 19, No. 14, 2011, pp. 4286-4294. doi:10.1016/j.bmc.2011.05.052
*Corresponding author.





