 Vol.1, No.3, 54-66 (201 1) doi:10.4236/scd.2011.13006 C opyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/SCD/ S tem Cell Discovery Bcr-Abl-mediated Raf kinase inhibitor protein suppression promotes chronic myeloid leukemia progenitor cells proliferation Satoki Nakamura1*#, Tomohiro Yagyu2#, Tomonari Takemura2, Lin Tan2, Yasuyuki Nagata2, Daisuke Yokota2, Isao Hirano2, Kiyoshi Shibata3, Michio Fujie3, Shinya Fujisawa4, Kazunori Ohnishi1,2 1Department of Internal Medicine III, Hamamatsu University School of Medicine, Hamamatsu, Japan; *Corresponding Author: satonaka@hama-med.ac.jp 2Cancer Center, Hamamatsu University School of Medicine, Hamamatsu, Japan; 3Equipment Center, Hamamatsu University School of Medicine, Hamamatsu, Japan; 4Division of Hematology, Hamamatsu Medical Center, Hamamatsu, Japan. Received 27 July 2011; revised 18 September 2011; accepted 30 September 2011. ABSTRACT The Ras/Raf-1/MEK/ERK pathway is constitutive- ly activated in Bcr-Abl transformed cells, and Ras activity enhances the oncogenic ability of Bcr- Abl. On the other hand, Raf kinase inhibitor protein (RKIP) inhibits activation of MEK b y Raf-1 and its downstream signal transduction, resulting in blocking the MAP kinase pathway. Moreover, Raf-1 has been reported to regu late cell cycle progres- sion. However, the mechanism by which Bcr-Abl promotes the cell cycle progression through Ra f- 1 is not completely understood. In the present study, we found that the expression of RKIP was suppressed in CML cells, and investigated the interaction between RKIP and Bcr-Abl in CML cells. In aldehyde dehydrogenase (A L D H ) hi/ CD34+ cells derived from CML patients, the inhibition of Bcr-Abl induced RKIP expression and reduced the phosphorylated-FOXM1 (pFOXM1) status, re- sulting in inhibited colony formation of Bcr-Abl+ progenitor cells. Moreover, overexpression of RKIP significantly decreased the colony num- bers, reduced the pFOXM1 status, and reduced pFOXM1 target genes such as Skp2, Cdc25B and KIS, and induced the expression of p27Kip1 and p21Cip1. Thus, Bcr-Abl represses the expression of RKIP, and continuously activates FOXM1, re- sulting in the proliferation of CML pr ogenitor cells through the c ell cycle modulati on. Keywords: RKIP; FOXM1; Bcr-Abl; Progenitor Cells; Cell Cycle 1. INTRODUCTION Chronic myeloid leukemia (CML) is a hematopoietic stem cell malignancy that is characterized by the Phila- delphia chromosome [1,2], a shortened chromosome 22 that is a by-product of a reciprocal chromosomal trans- location between the long arms of chromosomes 9 and 22 t (9; 22) (q34; q11), resulting in a chimeric Bcr-Abl oncoprotein with highly deregulated, constitutive tyro- sine kinase activity [3,4]. The most commonly occurring from of Bcr-Abl is a 210-kDa protein that is a critical role in the pathogenesis of CML [5]. Bcr-Abl activates a variety of signaling pathways, including the Ras/Raf- 1/MEK/ERK [6], PI3K/Akt [7], JAK/STAT [8], and NF- κB [9] signaling pathways. These signaling pathways play important role in Bcr-Abl-mediated leukemogenesis. The Ras pathway regulates multiple biological aspects includ- ing mitogenesis and differentiation [10,11], and is consti- tutively activated in Bcr-Abl transformed cells. Moreover, inhibition of Ras activity represses the oncogenic ability of Bcr-Abl [6]. Bcr-Abl activates the Ras pathway through the adap- tor protein Grb2, which associates with the guanine nu- cleotide exchange factor Sos and binds to the phospho- rylated tyrosine residue 177 within the Bcr region of Bcr- Abl. Sos forms a platform for recruitment of Ras, result- ing in the activation of Ras from GDP- to GTP-bound state [12,13]. The activated Ras phosphorylates Raf, and ERK is phosphorylated by MEK, which is phosphoryla- ted and activated by the activated Raf-1 [14]. ERK then translocates into the nucleus and promotes cell prolifera- tion through activation of various transcription factors as playing important roles in Bcr-Abl-mediated leukemo- genesis [15]. On the other hand, the anti-apoptotic factor #These authors contributed equally.  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 5555 of the Bcl-2 family, Mcl-1, is constitutively expressed in a Ras/Raf-1/MEK-dependent manner via Ras activation in Bcr-Abl positive cells [16]. Moreover, Raf-1 has been re- ported to have an important role in regulation of cell cycle progression [17]. Thus, in Bcr-Abl expressing cells, Ras pathway plays an important role in both cell proliferation and survival. Raf kinase inhibitor protein (RKIP), which is a mem- ber of the phosphatidylethanolamine binding protein 1 (PEBP1) family, is a ubiquitously expressed and consti- tutively conserved protein [18]. RKIP inhibits activation of MEK by Raf-1 and its downstream signal transduc- tion, resulting in blocking the MAP kinase pathway [19, 20]. It has been reported that RKIP expression is down- regulated in metastatic prostate cancers and the loss of RKIP levels promotes the metastatic potential of prostate cancer cells [21]. Moreover, RKIP has been reported to be downregulated in malignant melanoma [22], hepato- cellular carcinoma [23], breast cancer [24], and colorec- tal cancer [25,26]. These data suggest RKIP might play as a tumor and/or metastasis suppressor for solid tumors. However, it has been reported that RKIP expression has no relationship with ERK phosphorylation or the clinical course of disease in patients with melanoma or breast cancer [24,27]. These different observations suggest that RKIP contributes to the regulation of not only MAPK signaling including phosphorylated ERK but also other signal pathways. In the present study, we investigated the role of RKIP in CML cell lines and the Bcr-Abl+ hematopoietic pro- genitor cells derived from CML patients. We found that Bcr-Abl suppressed the expression of RKIP in CML cells, and detected changes of the cell cycle regulator proteins in CML-derived hematopoietic progenitor cells and CML cell lines. Interestingly, we also found the up- regulation of p27Kip1 and p21Cip1 through FOXM1 inac- tivation in RKIP overexpressed-CML cells. 2. MATERIALS AND METHODS 2.1. Reagents Imatinib mesylate (STI571) and AMN107 were kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). BMS354825 was kindly provided by Bristol-Myers Sq- uibb (New York, NY). Each compound was prepared as a 10 mM stock solution in dimethyl sulfoxide and stored at –20˚C. Experiments were performed with 1000-fold dilu- tions of the stock solutions into reaction mixtures. 2.2. Cells and Cell Cultures Human CML cell lines, K562 and Meg01, and human acute myeloblastic leukemia (AML) cell lines, U937 and HL60, were purchased from American Type Culture Collection (Manassas, VA). We established SHG3 cells from the bone marrow of a patient with CML (chronic phase), and YRK2 cells from the bone marrow of patient with AML M5b (French-American-British (FAB) classi- fication). These cells were cultured in RPMI 1640 con- taining 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 g/mL streptomycin, and 200 U/mL penicillin (GIBCO-BRL, Gaithersburg, MD) and main- tained in a humidified 5% CO2 atmosphere at 37˚C. 2.3. Bone Marrow Samples Prior to participation in the study, patients gave infor- med consent according to the Declaration of Helsinki. Samples of normal bone marrow were obtained from 6 healthy volunteers. Bone marrow was also obtained from 12 patients with CML in the first chronic phase. Mo- nonuclear cells (MNCs) were isolated from bone mar- row samples by Ficoll-Hypaque density gradient centri- fuga- tion. CML cells were obtained from patients before they began treatment with Abl kinase inhibitors. 2.4. Leukemic Blast Cells Purified by ALDH Activity and CD34 Expression from Bone Marrow Derived MNCs For 2-color staining, MNCs were stained with anti- CD34-phycoerythrin (PE) conjugated antibody (Becton Dickinson, San Jose, CA) and Aldefluor reagent (Stem- Co Biomedical, Durham, NC) according to the manu- facturer’s specifications and separated using fluoresce- nce-activated cell sorting (FACS) (Becton Dickinson). Aldefluor substrate (0.625 g/mL) was added to 2 to 7 × 106 cells/mL suspended in Aldefluor assay buffer and in- cubated for 20 to 30 min at 37˚C to allow the conversion of Aldefluor substrate to a fluorescent product that is re- tained within the cell because of its negative charge. The ALDHhi cells were gated, and the CD34+ and CD34- ce- lls in the gated ALDHhi population were sorted on a FACS. Sorted ALDHhi/CD34+ and ALDHhi/CD34– cell popula- tions were collected in methylcellulose media (Methocut H4435; Stem Cell Technologies). 2.5. RT-PCR and Quantitative Real-Time PCR (QRT-PCR) Total RNA was extracted from cells by using an RNeasy system (Qiagen, Tokyo, Japan), and 1 g RNA was reverse transcribed by using a 1st strand cDNA sy- nthesis kit (Roche, Indianapolis, IN). PCR was perfor- med with a DNA thermal cycler (model PTC 200; MJ Research, Watertown, MA). Oligonucleotide sequences for each primer were as follows: RKIP, sense 5’-ATG- CCGGTGGACCTCAGC-3’, antisense 5’-GCTGCTC-  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 56 GTACAGTTTGGGA-3’; FOXM1, sense 5’-GGAGGA- AA- TGCCACACTTAGCG-3’, antisense 5’-TAGGAC- TTCTTGGGTCTTGGGGTG-3’; Bcr-Abl, sense 5’-CGA- GCGGCTTCACTCAGA-3’, antisense 5’-ACAGCATTC- CGCTGACCAT-3’, and GAPDH; sense 5’-GAACGGGA- AGCTCACTGGCATGGC-3’, antisense 5’-TGAGGTCC- ACCACCCTGTTGCTG-3’. PCR conditions for RKIP, FOXM1, Bcr-Abl and GAPDH were 28 cycles of denatura- tion at 96˚C for 30 sec, annealing at 56˚C for 30 sec, and extension at 72˚C for 30 sec. PCR products were electro- phoresed in a 1.5% agarose gel containing 500 g/L ethidium bromide and visualized with UV light. In each experiment, RT-PCR was performed in duplicate. The quantitative real-time PCR was performed by using SY- BER-Green dye on an ABI PRISM 7700 Sequence detec- tor (Perkin-Elmer/Applied Biosystems, Foster City, CA). 2.6. Plasmid and RNA Interference The full-length cDNA coding human RKIP and FOXM1 was obtained by RT-PCR from human bone marrow cDNA (BD Biosciences Clontech, Palo Alto, CA) and cloned into the eukaryotic expression vector pcDNA3.1/V5-His (In- vitrogen, Carlsbad, CA). Sequences of recombinant RKIP and FOXM1 cDNAs were verified by automated sequ- encing. The vectors for RNA interference (RNAi) specific to human RKIP and Bcr-Abl were constructed based on the piGENE PUR hU6 vector (iGENE Therapeutics, Tsu- kuba, Japan), according to the manufacturer’s instruc- tions. We used the following targeting sequences and oligonucleotides: RKIP shRNA; 5’-AAGGTGGCCTC- CTTCCGTAAA-3’, Bcr-Abl shRNA #1: sense 5’-GC- AGAGUUCAAAAGCCCUUdTdT-3’ and antisense 5’- AAGGGCUUUUGAACUCUGCdTdT-3’ and Bcr-Abl shRNA #2, sense 5’-AGCAGAGUUCAAAAGCCCU- dTdT-3’ and antisense 5’-AGGGCUUUUGAACUCU- GCUdTdT -3’. A scrambled shRNA sequence was sense 5`-UUGUACGGCAUC AGCGUUAdTdT and antisense 5`-UUACGUGAUGCCGUA CAAdTdT and used as a control. The vector was transfected into cells by using the Lipofectamine 2000 kit (Life Technologies, Gaithersburg, MD), according to the manufacturer’s instructions. After 12 h, the same transfection procedure was repeated, and cells were harvested 48 and 72 h after the initial transfe- ction. Transfection efficiency was consistently 50% to 60%, as determined by the RT-PCR measurement of RKIP, FOXM1, and Bcr-Abl mRNA. 2.7. Lentivirus Construction and Production The full-length RKIP, FOXM1, and b3a2 Bcr-Abl cDNA (a kind gift from Dr. J. Y. Wang, University of California, San Diego, CA) was cloned upstream from the internal ribosomal entry site of replication-deficient, self-inacti- vating lentiviral vector, pRRLsin-IRES-EGFP. The RKIP, FOXM1, and Bcr-Abl—containing vectors were termed LV-RKIP, LV-FOXM1, and LV-Bcr-Abl, respectively, and the control vector was termed LV-Con. All vector particles, pseudotyped with the vesicular stomatitis virus G glyco- protein, were produced using a three-plasmid expression system as previously described [28]. The cell-free su- pernatants containing virus particles, LV-RKIP, LV-FOX- M1, LV-Bcr-Abl, and LV-Con, were separately concentr- ated by ultracentrifugation at 28,000 rpm for 2 h at 4˚C in an SW28 rotor. Virus vector stocks were resuspended in complete DMEM and stored at –80˚C. The virus vector titer of pRRLsin-IRES-EGFP was derived on a fluores- cence-activated cell sorter (Becton Dickinson) using transfected HeLa cells. To calculate titers, the number of target cells was multiplied by the percentage of eGFP- positive cells divided by the volume of the input virus and the titer of each concentrated lentivirus vector stock (LV-RKIP, LV-FOXM1, LV-Bcr-Abl and LV-Con), which were 3.2 × 108, 2.4 × 108, 3.1 × 108 and 4.7 × 108 gene- transducing units per milliter, respectively. 2.8. Immunoprecipitation and Western Blot Analysis For immunoprecipitation and immunoblotting, total cell lysates were prepared from the Abl kinase inhibitor treated-, DNA-transfected, and shRNA-transfected-cells. Cell lysates were incubated with the FOXM1, Rb and Abl-specific monoclonal antibody for 1 hour at 4C, washed Protein G agarose beads (sc-2002, Santa Cruz, CA) were added, and incubated overnight at 4C. The immunoprecipitates were washed 3 times in the lysis buffer, and proteins were eluted with the SDS sample loading buffer. Western blot analysis was performed as previously described [28] using the following antibodies: anti-RKIP goat polyclonal antibody (sc-5426, Santa Cruz), anti-FOXM1 rabbit polyclonal antibody (sc-500, Santa Cruz), anti-c-Abl mouse monoclonal antibody (sc-52990, Santa Cruz), anti-KIS rabbit polyclonal antibody (AB- GENT, San Diego, CA), anti-Rb mouse monoclonal an- tibody (sc-74562, Santa Cruz), anti-phosphotyrosine (p- Tyr) mouse monoclonal antibody (sc-508, Santa Cruz), anti-p27Kip1 rabbit polyclonal antibody (sc-527, Santa Cruz), and anti-p21Cip1 mouse monoclonal antibody (sc- 469, Santa Cruz). To assure equal protein loading, simi- lar experiments were performed with a mouse mono- clonal anti-Actin antibody (C-4; ICN, Aurora, OH) as an internal control. After being washed in Tris-buffered sa- line Tween, blots were incubated with horseradish peroxi- dase-conjugated anti-mouse, anti-rabbit, or anti-goat IgG (Amersham, Arlington Heights, IL) for 1 h and exposed to X-ray film at room temperature. The signal was de- tected by chemiluminescence with an ECL detection kit  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 5757 (Amersham). 2.9. Cell Proliferation and Viability Assay To assess cell proliferation, cells (1 × 104) were see- ded in 24-well plates and incubated with STI571 (1 M) at 37˚C. Cells were then transfected with RKIP DNA. After 24, 48, and 72 hours incubation, cell proliferation was measured by counting cells using a hemocytometer. To assess cell viability, the numbers of nonviable cells were determined by counting the cells that showed try- pan blue uptake in a hemocytometer and reported as the percentage of untreated control cells. Each data point was performed in triplicate, and the results are reported as mean counts SD. 2.10. Cell Cycle Analysis DNA content analysis was performed using propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO) staining. In brief, cells were transfected with RKIP DNA. Cells were cultured in 2 ml complete medium containing 1 × 106 cells at 37˚C. After 7 days incubation, the cells were wa- shed twice with cold PBS, fixed with 70% ethanol over- night before treatment with 100 g/ml RNase A, and then stained with 50 g/ml PI. The relative DNA content per cell was measured by flow cytometry using an Epics Elite flow cytometer (Coulter Immunotech, Marseille, France). The percentage of cells in G1, S, and G2/M ph- ases, was calculated using ModFit program (Becton Dic- kinson, San Jose, CA). 2.11. Colony Forming Cell Assa y ALDHhi/CD34+ cells from CML bone marrow cells were treated the Abl kinase inhibitors, or transfected with scrambled shRNA, RKIP shRNA, Bcr-Abl shRNA #1, RKIP DNA and FOXM1 DNA. Human clonogenic pro- genitor assays were performed by plating purified popula- tions of cells at concentrations ranging from 2 × 102 to 7 × 102 (ALDHhi/CD34+) into methylcellulose media (Metho- cut H4435). Colonies were enumerated under light mi- croscopy (Zeiss, Muenchen, Germany) following incuba- tion at 37˚C, 5% CO2, for 7 to 10 days. 2.12. Collection of Progenitor Cells, and QRT-PCR and Western Blot Analysis in the Progenitor Cells After the colony forming assays, each colony was har- vested by glass syringe, pooled, and washed. Immunopr- ecipitation and western blotting were performed using the interested antibody. An RNeasy system was used to extract total RNA from approximately 2 × 104 cells from each colony, and then RT-PCR and QRT-PCR were also performed using RKIP, FOXM1, Bcr-Abl, and GAPDH primers. 2.13. Statistical Analysis Data are representative of at least three experiments with essentially similar results. These results are expre- ssed as the means ± SD from three independent experi- ments. The means were compared by using the Student’s t test. P values less than 0.01 were considered statisti- cally significant. 3. RESULTS 3.1. The Abl kinase Inhibitors Induce RKIP mRNA Expression in CML Cell Lines RKIP mRNA were constitutively expressed in CML cell lines (K562, Meg01, and SHG3 cells) (Figure 1(a)) and AML cell lines (U937, HL60, and YRK2 cells) (Figure 1(b)). In CML cell lines, the expression of RKIP mRNA was inhibited as compared to AML cell lines. Interestingly, we found that the expression of RKIP mRNA increased in the three CML cell lines treated with Abl kinase inhibitors (STI571, AMN107, or BMS35482 5) for 24 h as compared to untreated cells. However, the Abl kinase inhibitors did not affect the RKIP mRNA expression in the AML cell lines, which did not express Bcr-Abl mRNA. On the other hand, in the CML cell lines transfected with Bcr-Abl shRNA #1, the RKIP mRNA expression was significantly increased as compared to control cells. In the AML cell lines transfected with LV- Bcr-Abl, the RKIP mRNA expression was reduced as compared to control cells. As shown in Figure 1(c), RKIP protein was also con- stitutively expressed in CML cell line (K562, Meg01 and SHG3 cells) and in AML cell line (HL60, U937 and YRK2 cells) (data not shown). The treatment with Abl kinase inhibitors increased the protein levels of RKIP in CML cells. Moreover, the RKIP protein level was in- creased in K562 and Meg01 cells transfected with Bcr- Abl shRNA #1. Thus, Abl kinase inhibitors or the knock- down of Bcr-Abl protein induced RKIP protein expres- sion. The expression of RKIP protein in CML cell lines was at a lower level compared to that in AML cell lines. On the other hand, in the AML cell lines, the Abl kinase inhibitors did not affect the levels of RKIP protein (data not shown). The Abl kinase inhibitors and knockdown of Bcr-Abl using shRNA also inhibited the levels of tyro- sine phosphorylated Bcr-Abl (p-Bcr-Abl) in CML cells. Thus, Bcr-Abl expression regulates RKIP mRNA and protein expression. 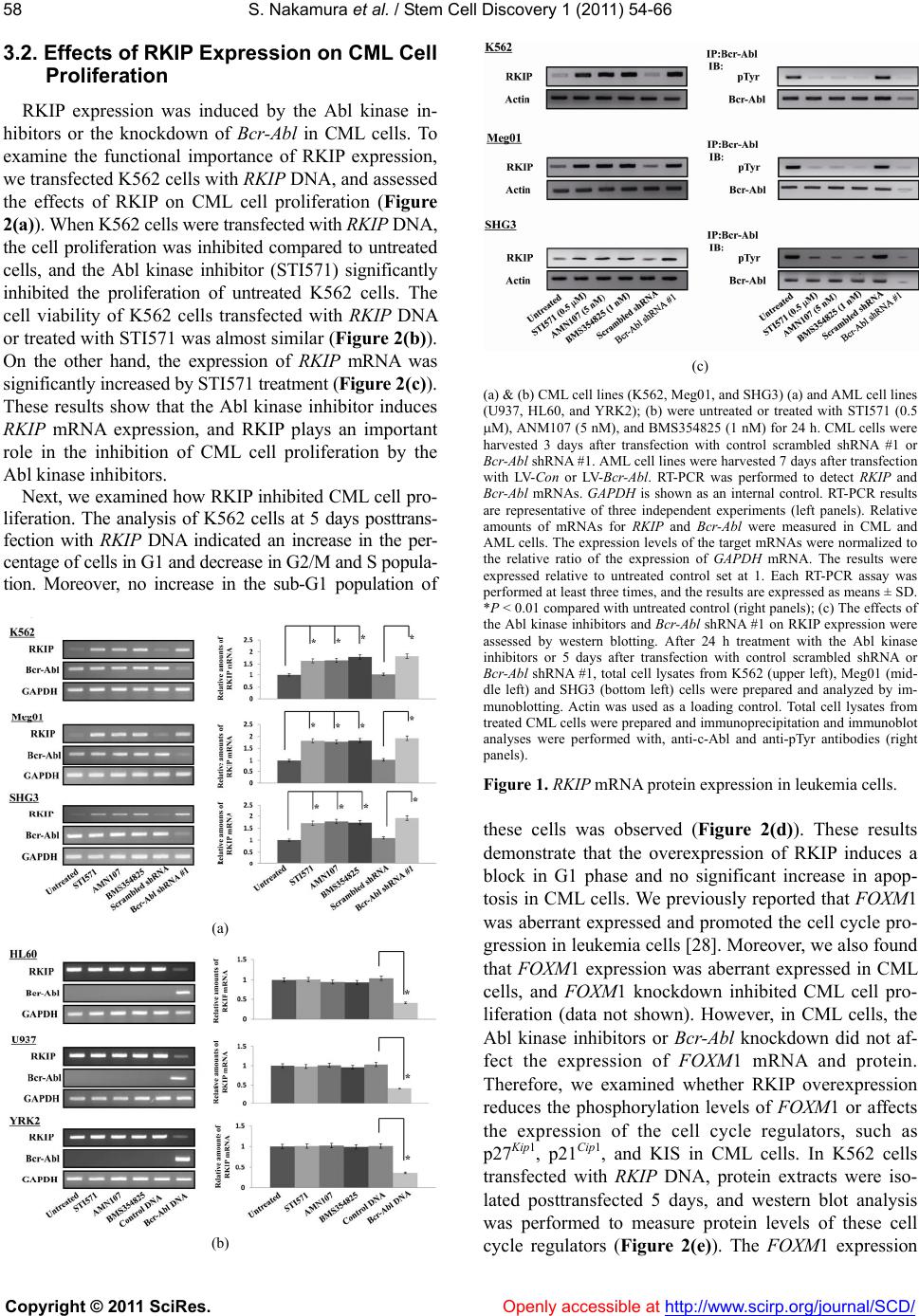 S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 58 3.2. Effects of RKIP Expression on CML C ell Proliferation RKIP expression was induced by the Abl kinase in- hibitors or the knockdown of Bcr-Abl in CML cells. To examine the functional importance of RKIP expression, we transfected K562 cells with RKIP DNA, and assessed the effects of RKIP on CML cell proliferation (Figure 2(a)). When K562 cells were transfected with RKIP DNA, the cell proliferation was inhibited compared to untreated cells, and the Abl kinase inhibitor (STI571) significantly inhibited the proliferation of untreated K562 cells. The cell viability of K562 cells transfected with RKIP DNA or treated with STI571 was almost similar (Figure 2(b)). On the other hand, the expression of RKIP mRNA was significantly increased by STI571 treatment (Figure 2(c)). These results show that the Abl kinase inhibitor induces RKIP mRNA expression, and RKIP plays an important role in the inhibition of CML cell proliferation by the Abl kinase inhibitors. Next, we examined how RKIP inhibited CML cell pro- liferation. The analysis of K562 cells at 5 days posttrans- fection with RKIP DNA indicated an increase in the per- centage of cells in G1 and decrease in G2/M and S popula- tion. Moreover, no increase in the sub-G1 population of (a) (b) (c) (a) & (b) CML cell lines (K562, Meg01, and SHG3) (a) and AML cell lines (U937, HL60, and YRK2); (b) were untreated or treated with STI571 (0.5 M), ANM107 (5 nM), and BMS354825 (1 nM) for 24 h. CML cells were harvested 3 days after transfection with control scrambled shRNA #1 or Bcr-Abl shRNA #1. AML cell lines were harvested 7 days after transfection with LV-Con or LV-Bcr-Abl. RT-PCR was performed to detect RKIP and Bcr-Abl mRNAs. GAPDH is shown as an internal control. RT-PCR results are representative of three independent experiments (left panels). Relative amounts of mRNAs for RKIP and Bcr-Abl were measured in CML and AML cells. The expression levels of the target mRNAs were normalized to the relative ratio of the expression of GAPDH mRNA. The results were expressed relative to untreated control set at 1. Each RT-PCR assay was performed at least three times, and the results are expressed as means ± SD. *P < 0.01 compared with untreated control (right panels); (c) The effects of the Abl kinase inhibitors and Bcr-Abl shRNA #1 on RKIP expression were assessed by western blotting. After 24 h treatment with the Abl kinase inhibitors or 5 days after transfection with control scrambled shRNA or Bcr-Abl shRNA #1, total cell lysates from K562 (upper left), Meg01 (mid- dle left) and SHG3 (bottom left) cells were prepared and analyzed by im- munoblotting. Actin was used as a loading control. Total cell lysates from treated CML cells were prepared and immunoprecipitation and immunoblot analyses were performed with, anti-c-Abl and anti-pTyr antibodies (right panels). Figure 1. RKIP mRNA protein expression in leukemia cells. these cells was observed (Figure 2(d)). These results demonstrate that the overexpression of RKIP induces a block in G1 phase and no significant increase in apop- tosis in CML cells. We previously reported that FOX M1 was aberrant expressed and promoted the cell cycle pro- gression in leukemia cells [28]. Moreover, we also found that FOXM1 expression was aberrant expressed in CML cells, and FOXM1 knockdown inhibited CML cell pro- liferation (data not shown). However, in CML cells, the Abl kinase inhibitors or Bcr-Abl knockdown did not af- fect the expression of FOXM1 mRNA and protein. Therefore, we examined whether RKIP overexpression reduces the phosphorylation levels of FOXM1 or affects the expression of the cell cycle regulators, such as p27Kip1, p21Cip1, and KIS in CML cells. In K562 cells transfected with RKIP DNA, protein extracts were iso- lated posttransfected 5 days, and western blot analysis was performed to measure protein levels of these cell cycle regulators (Figure 2(e)). The FOXM1 expression 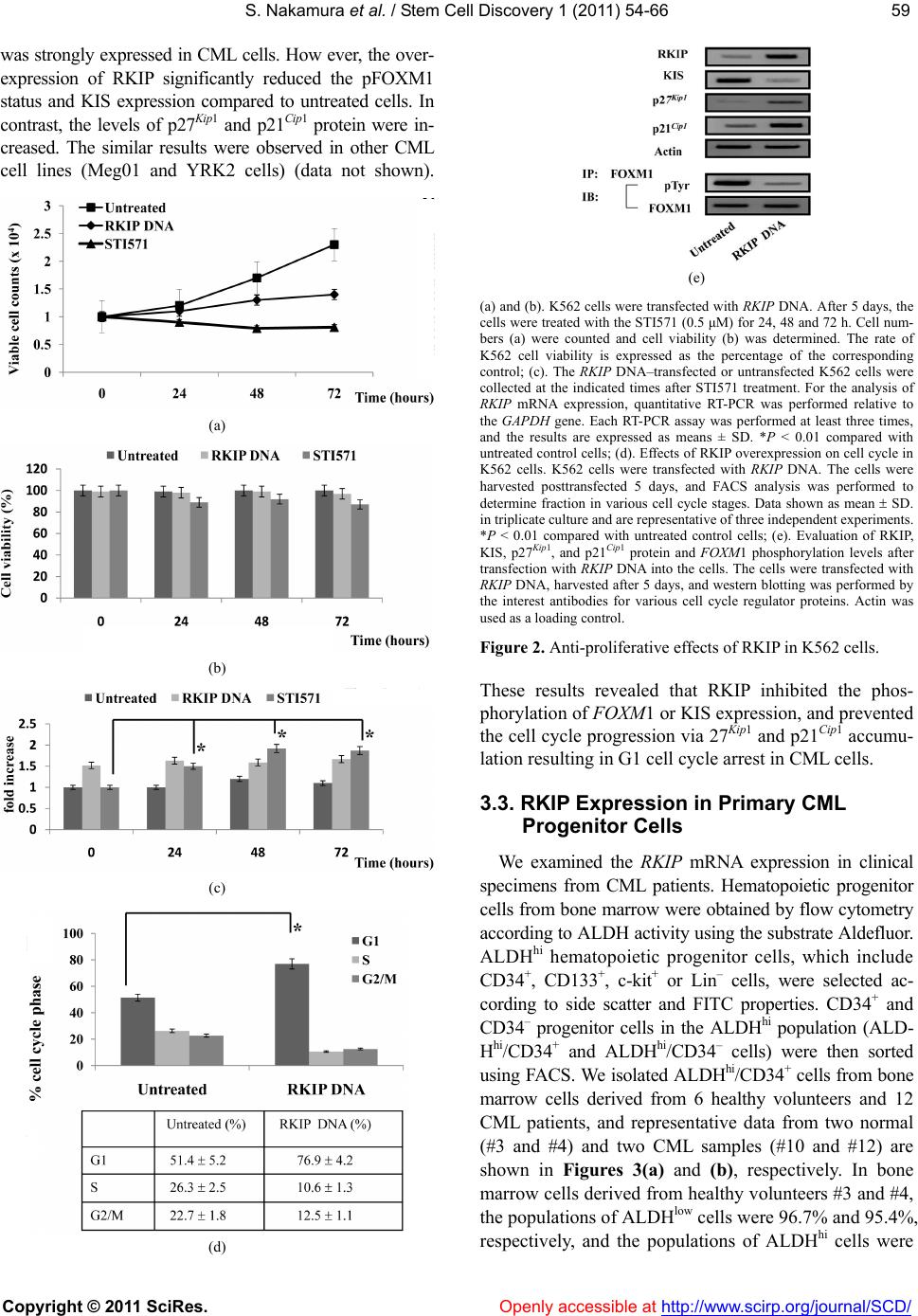 S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 5959 was strongly expressed in CML cells. How ever, the over- expression of RKIP significantly reduced the pFOXM1 status and KIS expression compared to untreated cells. In contrast, the levels of p27Kip1 and p21Cip1 protein were in- creased. The similar results were observed in other CML cell lines (Meg01 and YRK2 cells) (data not shown). (a) (b) (c) (d) (e) (a) and (b). K562 cells were transfected with RKIP DNA. After 5 days, the cells were treated with the STI571 (0.5 μM) for 24, 48 and 72 h. Cell num- bers (a) were counted and cell viability (b) was determined. The rate of K562 cell viability is expressed as the percentage of the corresponding control; (c). The RKIP DNA–transfected or untransfected K562 cells were collected at the indicated times after STI571 treatment. For the analysis of RKIP mRNA expression, quantitative RT-PCR was performed relative to the GAP DH gene. Each RT-PCR assay was performed at least three times, and the results are expressed as means ± SD. *P < 0.01 compared with untreated control cells; (d). Effects of RKIP overexpression on cell cycle in K562 cells. K562 cells were transfected with RKIP DNA. The cells were harvested posttransfected 5 days, and FACS analysis was performed to determine fraction in various cell cycle stages. Data shown as mean SD. in triplicate culture and are representative of three independent experiments. *P < 0.01 compared with untreated control cells; (e). Evaluation of RKIP, KIS, p27Kip 1, and p21Cip1 protein and FOXM1 phosphorylation levels after transfection with RKIP DNA into the cells. The cells were transfected with RKIP DNA, harvested after 5 days, and western blotting was performed by the interest antibodies for various cell cycle regulator proteins. Actin was used as a loading control. Figure 2. Anti-proliferative effects of RKIP in K562 cells. These results revealed that RKIP inhibited the phos- phorylation of FOXM1 or KIS expression, and prevented the cell cycle progression via 27Kip1 and p21Cip1 accumu- lation resulting in G1 cell cycle arrest in CML cells. 3.3. RKIP Expression in Primary CML Progenitor Cells We examined the RKIP mRNA expression in clinical specimens from CML patients. Hematopoietic progenitor cells from bone marrow were obtained by flow cytometry according to ALDH activity using the substrate Aldefluor. ALDHhi hematopoietic progenitor cells, which include CD34+, CD133+, c-kit+ or Lin– cells, were selected ac- cording to side scatter and FITC properties. CD34+ and CD34– progenitor cells in the ALDHhi population (ALD- Hhi/CD34+ and ALDHhi/CD34– cells) were then sorted using FACS. We isolated ALDHhi/CD34+ cells from bone marrow cells derived from 6 healthy volunteers and 12 CML patients, and representative data from two normal (#3 and #4) and two CML samples (#10 and #12) are shown in Figures 3(a) and (b), respectively. In bone marrow cells derived from healthy volunteers #3 and #4, the populations of ALDHlow cells were 96.7% and 95.4%, respectively, and the populations of ALDHhi cells were  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 60 0.09% and 0.07%, respectively. Within these ALDHhi ce- lls, the CD34+ cell populations were 78.1% and 80.7%, and CD34- cell populations were 20.5% and 17.4%, re- spectively (Figure 3(a)). There were no significant dif- ferences in the population rates among 6 normal healthy volunteers. On the other hand, in bone marrow cells de- rived from CML patients #10 and #12, the populations of ALDHlow cells were 89.3% and 91.4%, respectively, and the populations of ALDHhi cells were 4.7% and 2.6%, respectively. Within these ALDHhi cells, the CD 34+ cell populations were 82.6% and 84.1%, and CD34- cell population were 14.8% and 11.7%, respectively (Figure 3(b)). There were no significant differences in the population rates among 12 CML specimens. In ALDHlow, ALDHhi, ALDHhi/CD34- and ALDHhi/CD34+ populations derived from CML patients, RKIP mRNA and protein were slightly expressed as compared to normal ALDHlow, ALDHhi, ALDHhi/CD34– and ALDHhi/CD34+ cells (data not shown). Interestingly, in CML (#10 and #12)-derived ALDHhi/CD34+ cells treated with STI571 or transfected Bcr-Abl shRNA #1 or #2, RKIP mRNA was strongly induced compared to untreated cells (Figure 3(c)). In normal (#3 and #4) ALDHhi/CD34+ cells, the STI571 treatment did not affect the expression levels of RKIP mRNA. In contrast, the overexpression of Bcr-Abl re- duced the RKIP mRNA expression. The effects of STI571 on RKIP and Bcr-Abl mRNA levels in ALDHhi/CD34+ cells from CML patients (#10 and #12) were assessed by quantitative RT-PCR. In ALDHhi/CD34+ cells derived from all healthy volunteers (n = 6) and all CML patients (n = 12), there were no significant differences in the lev- els of RKIP mRNA induction among the other Abl ki- nase inhibitors (AMN107 or BMS354825). These results demonstrate that the Bcr-Abl inhibits the RKIP expres- sion in ALDHhi/CD34+ cells derived from CML pa- tients.c. (a) (b) (c) (a) & (b). Selection of ALDHhi/CD34+ hematopoietic progenitor cells from the bone marrow of two healthy volunteer (#3 and #4) (a) and from two CML patient (#10 and #12) (b). Region P and Q denote populations of ALDHhi and ALDHlow cells, respectively (left panels). Region R and S denote populations CD34+ and CD34- cells in the ALDHhi population (Re- gion P), respectively. Negative control (light grey region) and CD34-PE staining (dark grey region) (right panels); (c). The expression of RKIP and Bcr-Abl mRNA in sorted ALDHhi/CD34+ cells from two healthy volunteers (#3 and #4) and from two CML patients (#10 and #12) were analyzed after treatment with STI571 (0.5 μM). After 3 days STI571 treatment or 5 days after transfection with Bcr-Abl shRNA #1 and #2, the cells were harvested. The effects of STI571 treatment or Bcr-Abl knockdown on RKIP and Bcr-Abl mRNA levels in ALDHhi/CD34+ cells were assessed by QRT-PCR. Representative data are shown (upper panels). The levels of target mRNAs were normalized to the relative ratio of the expression of GAPDH mRNA (middle and bottom panels). The results are expressed relative to the un- treated control, which is set at a value of 1. Each RT-PCR assay was per- formed at least three times and the results are expressed as means ± SD. *P < 0.01 compared with untreated control cells. Figure 3. RKIP expression in hematopoietic progenitor ALDHhi/ CD34+ cells derived from CML patients. 3.4. Induction of RKIP Expression Inhibited the Colony Formation in CML Derived ALDHhi/CD34+ Progenitor Cells We examined the effect of Abl kinase inhibitors or kno- ckdown of Bcr-Abl on the colony formation of ALD- Hhi/CD34+ hematopoietic progenitor cells from pretreat-  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 6161 ment a CML patient #10 (Figure 4). The ALDHhi/CD34+ cells, which were untransfected or transfected with Bcr- Abl shRNA #1, were treated with Abl kinase inhibitors for 7 to 10 days, and then the colony numbers were cou- nted. When untreated, the mean colony number was 116 (range, 98 - 127). When treated with STI571, AMN107, BMS354825, or Bcr-Abl shRNA #1 transfection, the me- an colony numbers were 11 (6 - 17), 8 (1 - 14), 5 (2 - 11), 4 (0 - 7), respectively (Figure 4(a)). The colony numbers were remarkably reduced when the cells were cultured with STI571, AMN107, or BMS354825 and transfected with Bcr- Abl shRNA #1. The difference between the ef- fects of Abl kinase inhibitors and the knockdown of Bcr- Abl was not significant in CML-derived ALDHhi/CD34+ cells. Moreover, in CML-derived ALDHhi/CD-34+ cells tr- eated with the Abl kinase inhibitors or transfected with Bcr-Abl shRNA #1, the RKIP protein expression was in- creased and the phosphorylation levels of FOXM1 were decreased compared to untreated or untransfected cells. On the other hand, the Abl kinase inhibitors or Bcr-Abl knockdown did not affect the expression of FOXM1 mRNA and protein. To assess the function of RKIP ex- pression on colony formation of the CML derived AL- DHhi/CD34+ cells, we investigated whether the overex- pression or suppression of RKIP expression affect the activity of colony formation. As shown in Figures 4(b) and 4(c), the overexpression of RKIP significantly de- creased the colony numbers, and reduced the phosphory- lation levels of FOXM1. On the other hand, the overex- pression did not affect the expression of FOXM1 mRNA and protein. The reduction of RKIP expression slightly increased the colony numbers by STI571 treatment com- pared to when not transfected with RKIP shRNA #1. Moreover, in the RKIP shRNA-transfected ALDHhi/CD34+ cells, the induction of RKIP expression by STI571 was weakened. There were no significant differences in the levels of RKIP mRNA induction among CML specimens. These results demonstrate that the inhibition for Bcr-Abl expression induced the RKIP expression and reduced the phosphorylation levels of FOXM1, and the overexpres- sion of RKIP inhibited the colony formation of ALD- Hhi/CD34+ cells from CML specimens through the redu- ction of the pFOXM1 status. 3.5. Induction of RKIP Expression Reduced the Phosphorylation Levels of FOXM1 in CML-Derived ALDHhi/CD34+ Progenitor Cells Finally, we examined whether overexpression of RKIP reduced the phosphorylation levels of FOXM1 in CML (#10)-derived ALDHhi/CD34+ cells (Figure 5(a)). When the CML-derived ALDHhi/CD34+ cells were treated with STI 571 or transfected with RKIP DNA, RKIP expres- sion was increased, and the phosphorylation levels of FOXM1 were decreased. Moreover, the phosphorylation levels of Rb were also decreased. We found that the in- duction of RKIP expression decreased phosphorylated FOXM1 and Rb levels in CML derived ALDHhi/CD34+ cells. These results show that activated Rb inhibited the phosphorylation of FOXM1. Next, we examined whe- ther the FOXM1 overexpression rescued the effects of cell cycle genes essential for G1-S progression, includ- ing Skp2, Cdc25B, KIS, p27Kip1, and p21Cip1 by treat- ment with STI571 in CML derived ALDHhi/CD34+ cells (Figure 5(b)). When the CML derived ALDHhi/CD34+ cells were treated with STI 571 or transfected with RKIP DNA, the phosphorylation levels of FOXM1 and the expression of Skp2, Cdc25B and KIS protein were de- creased, and the expression of CDKIs (p27Kip1 and p21Cip1) protein was increased. On the other hand, in FOXM1 DNA transfected-ALDHhi/CD34+ cells, the reduction of Skp2, Cdc25B and KIS expression by STI571 treatment was inhibited, and the induction of p27Kip1 and p21Cip1 expression by STI571 treatment was inhibited. These results indicate that RKIP promotes cell cycle progress- sion through the reduction of the pFOXM1 status, and has an important role in ALDHhi/CD34+ CML cell pro- liferation. 4. DISCUSSION We investigated the role of RKIP expression on the cell growth inhibition of CML cells and CML-derived proge- nitor cells. In this study, we demonstrated that Bcr-Abl in- hibited the expression of RKIP and promoted the CML cell proliferation through the FOXM1 activation. The Ras/Raf-1/MEK/ERK signaling pathway is known to be essential for cellular proliferation, differentiation, apoptosis, survival and migration [29,30]. The deregula- tion of them leads to various diseases including malig- nancies [20,31] and developmental disorders [32]. How- ever, the regulation of Ras/Raf-1/MEK/ERK pathway is very complex and not clearly understood. It has been reported that RKIP binds to Raf-1 and disrupt down- stream of MAP kinase pathway [19], and the overex- pression of RKIP can act as a competitive inhibitor MEK [20]. Moreover, RKIP expression has been shown to downregulated in metastatic prostate cancers and the loss RKIP levels was suggested to promotes the metas- tatic potential of prostate cancer cells [21], and it has been shown to be a decrease of RKIP expression in malignant melanoma and breast cancer cells [24,33], and the absence of RKIP expression in melanoma metastases [22]. In cell cycle effects, ERK/MAPK-independent functions of Raf kinases have been reported. Raf-1 activates the Cdk25 phosphatase, which results in CDK activation [17].  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. http://www.scirp.org/journal/SCD/ 62 (a) (b) (c) (a) The purified ALDHhi/CD34+ cells (3 × 102 to 5 × 102 cells/plate) from a CML patient (#10) were isolated and cultured in semisolid methylcellulose media (Methocult; H4435). The ALDHhi/CD34+ cells, which were untransfected or transfected with Bcr-Abl shRNA #1, were treated with the Abl kinase inhibitors, and were enumerated after 7 to 10 days of in vitro cul- ture. The rate of the progenitor cells was evaluated as the percentage of the corresponding control (top panel). To detect RKIP and Bcr-Abl protein, western blot was performed. Actinis shown as a loading control. Representative data are shown (middle panels). For the analysis of RKIP mRNA expression, QRT-PCR was performed relative to the GAPDH gene (middle panels). The densitometric analysis of pFOXM1 levels in ALDHhi/CD34+ cells from the CML patient (#10). Immunoprecipi- tation and western blot analysis were performed by using anti-pTyr antibodies and anti-FOXM1 antibodies. Results are rep- resentative of three independent experiments (bottom panels); (b) ALDHhi/CD34+ cells were transfected with RKIP DNA or RKIP shRNA and then treated with STI571 (0.5 μM) for 7 to 10 days. The numbers of colony were counted. The rate of the progenitor cells was evaluated as a percentage of the corresponding control. To detect RKIP and Bcr-Abl protein, western blot was performed. Actin is shown as a loading control. Representative data are shown (middle panels). For the analysis of RKIP mRNA expression, QRT-PCR was performed relative to the GAPDH gene (middle panels). The densitometric analysis of pFOXM1 levels in ALDHhi/CD34+ cells from the CML patient (#10). Immunoprecipitation and western blot analysis were performed by using anti-pTyr antibodies and anti-FOXM1 antibodies. Results are representative of three independent ex- periments (bottom panels). Data are shown as the mean SD in triplicate culture and are representative of three independent experiments. Data are shown as the mean SD in triplicate culture and are representative of three independent experiments. *P < 0.01 compared with untreated control cells; (c) The cells transfected with RKIP DNA or shRNA were viewed using phase-contrast microscopy after 14 days culture with or without STI571 (0.5 μM). Original magnification 4. Figure 4. RKIP regulates FOXM1 activation on ALDHhi/CD34+ hematopoietic progenitor cells derived from CML patients. Openly accessible at 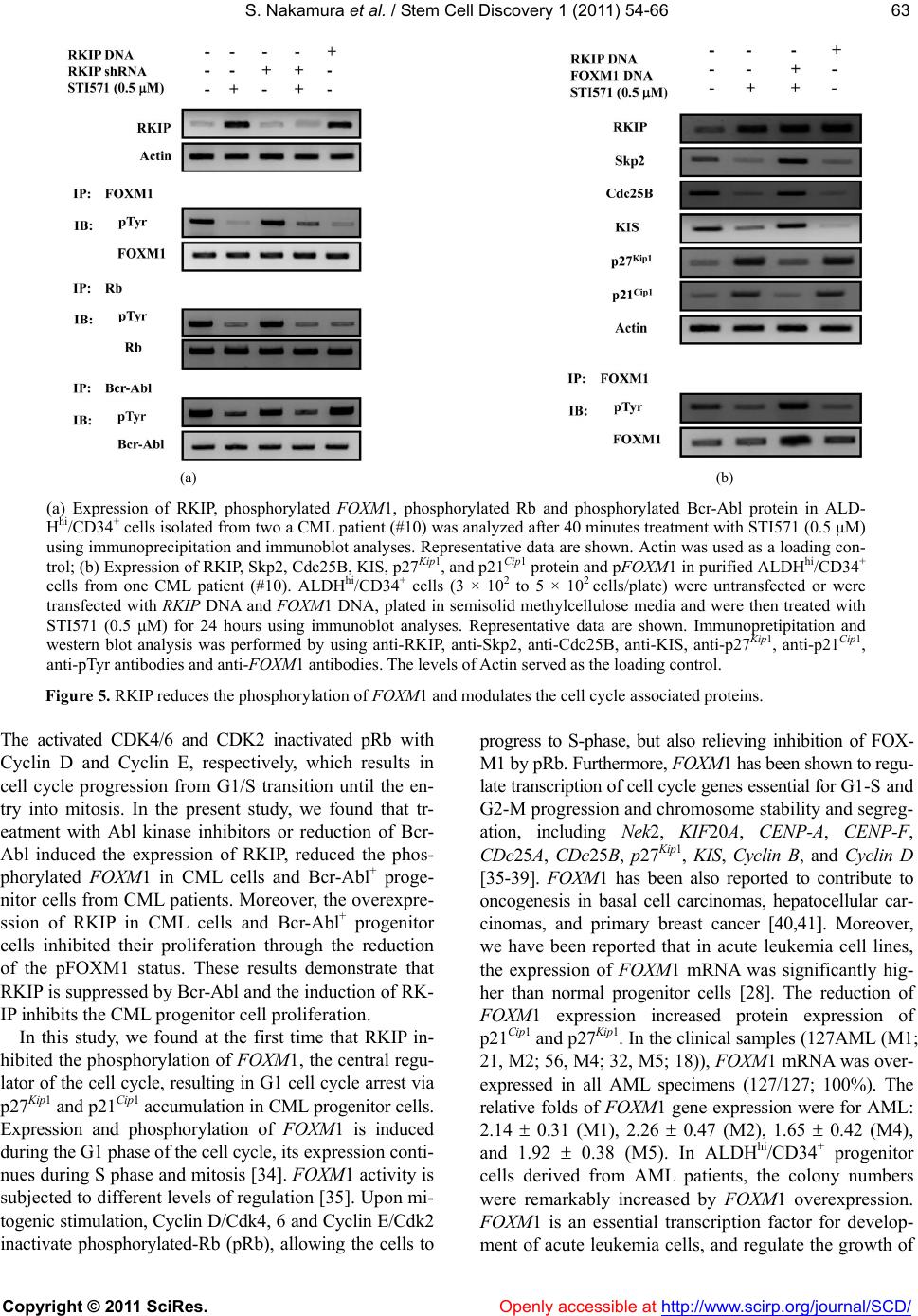 S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 6363 (a) (b) (a) Expression of RKIP, phosphorylated FOXM1, phosphorylated Rb and phosphorylated Bcr-Abl protein in ALD- Hhi/CD34+ cells isolated from two a CML patient (#10) was analyzed after 40 minutes treatment with STI571 (0.5 μM) using immunoprecipitation and immunoblot analyses. Representative data are shown. Actin was used as a loading con- trol; (b) Expression of RKIP, Skp2, Cdc25B, KIS, p27Kip1, and p21Cip1 protein and pFOXM1 in purified ALDHhi/CD34+ cells from one CML patient (#10). ALDHhi/CD34+ cells (3 × 102 to 5 × 102 cells/plate) were untransfected or were transfected with RKIP DNA and FOXM1 DNA, plated in semisolid methylcellulose media and were then treated with STI571 (0.5 μM) for 24 hours using immunoblot analyses. Representative data are shown. Immunopretipitation and western blot analysis was performed by using anti-RKIP, anti-Skp2, anti-Cdc25B, anti-KIS, anti-p27Kip1, anti-p21Cip1, anti-pTyr antibodies and anti-FOXM1 antibodies. The levels of Actin served as the loading control. Figure 5. RKIP reduces the phosphorylation of FOXM1 and modulates the cell cycle associated proteins. The activated CDK4/6 and CDK2 inactivated pRb with Cyclin D and Cyclin E, respectively, which results in cell cycle progression from G1/S transition until the en- try into mitosis. In the present study, we found that tr- eatment with Abl kinase inhibitors or reduction of Bcr- Abl induced the expression of RKIP, reduced the phos- phorylated FOXM1 in CML cells and Bcr-Abl+ proge- nitor cells from CML patients. Moreover, the overexpre- ssion of RKIP in CML cells and Bcr-Abl+ progenitor cells inhibited their proliferation through the reduction of the pFOXM1 status. These results demonstrate that RKIP is suppressed by Bcr-Abl and the induction of RK- IP inhibits the CML progenitor cell proliferation. In this study, we found at the first time that RKIP in- hibited the phosphorylation of FOXM1, the central regu- lator of the cell cycle, resulting in G1 cell cycle arrest via p27Kip1 and p21Cip1 accumulation in CML progenitor cells. Expression and phosphorylation of FOXM1 is induced during the G1 phase of the cell cycle, its expression conti- nues during S phase and mitosis [34]. FOXM1 activity is subjected to different levels of regulation [35]. Upon mi- togenic stimulation, Cyclin D/Cdk4, 6 and Cyclin E/Cdk2 inactivate phosphorylated-Rb (pRb), allowing the cells to progress to S-phase, but also relieving inhibition of FOX- M1 by pRb. Furthermore, FOXM1 has been shown to regu- late transcription of cell cycle genes essential for G1-S and G2-M progression and chromosome stability and segreg- ation, including Nek2, KIF20A, CENP-A, CENP-F, CDc25A, CDc25B, p27Kip1, KIS, Cyclin B, and Cyclin D [35-39]. FOXM1 has been also reported to contribute to oncogenesis in basal cell carcinomas, hepatocellular car- cinomas, and primary breast cancer [40,41]. Moreover, we have been reported that in acute leukemia cell lines, the expression of FOXM1 mRNA was significantly hig- her than normal progenitor cells [28]. The reduction of FOXM1 expression increased protein expression of p21Cip1 and p27Kip1. In the clinical samples (127AML (M1; 21, M2; 56, M4; 32, M5; 18)), FOXM1 mRNA was over- expressed in all AML specimens (127/127; 100%). The relative folds of FOXM1 gene expression were for AML: 2.14 0.31 (M1), 2.26 0.47 (M2), 1.65 0.42 (M4), and 1.92 0.38 (M5). In ALDHhi/CD34+ progenitor cells derived from AML patients, the colony numbers were remarkably increased by FOXM1 overexpression. FOXM1 is an essential transcription factor for develop- ment of acute leukemia cells, and regulate the growth of  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 64 ALDHhi/CD34+ progenitor cells derived from AML pa- tients. In this study, we also showed that FOXM1 ex- pression in CML cells was increased compared to nor- mal ALDHhi/CD34+ cells. However, the Abl kinase inhi- bitors or Bcr-Abl knockdown did not affect the expres- sion of FOXM1 mRNA and protein. On the other hand, the overexpression of RKIP or Bcr-Abl knockdown re- duced the pFOXM1 status and KIS expression, and in- duced p27Kip1 and p21Cip1 expression resulting in inhibition of CML cells. These results demonstrate that FOXM1 may be one of target proteins for RKIP. The ALDHhi/CD34+ progenitor cells derived from CML patients were significantly reduced when they were treated with STI571, AMN107, and BMS354825 or tran- sfected with Bcr-Abl shRNA #1. Moreover, the relative ex- pression of RKIP mRNA was increased and the pFOX- M1 status was decreased in the progenitor cells treated with the Abl kinase inhibitors or transfected with Bcr-Abl shRNA #1. In ALDHhi/CD34 + progenitor cells transfected with RKIP DNA, the colony forming activity was inhibited, and the combination with the Abl kinase inhibitor showed more strong effects. In contrast, in ALDHhi/ CD34+ progenitor cells transfected with RKIP shRNA, the inhibition effects of colony formation activ- ity by the Abl kinase inhibitors were weakened. These results indicate that the expression of RKIP induced by the Abl kinase reduce the pFOXM1 status and col- ony-forming cells derived from CML progenitor cells. Moreover, the RKIP overexpression decreased the pho- sphorylation levels of FOXM1 and Rb proteins, pro- moted the cell cycle progression via reduction Skp2, Cdc25B, and KIS proteins expression and induction of p27Kip1 and p21Cip1 expression in CML-derived ALD- Hhi/CD34+ progenitor cells. These findings indicate that Bcr-Abl inhibited the expression of RKIP, and reduced the phosphorylated FOXM1, resulting in the promotion of Bcr-Abl+ progenitor cell proliferation. This study shows for the first time that Bcr-Abl re- presses the expression of RKIP and that the Abl kinase inhibitors induced RKIP expression. Moreover, RKIP reduces the pFOXM1 status. These induced RKIP plays an important role in cell growth inhibition in CML pro- genitor cells in vitro. 5. CONFLICT OF INTEREST None of the authors have competing financial interests in relation to the work described in this article. 6. ACKNOWLEDGEMENTS This work was supported by a grant-in-aid for scientific research (#17590987) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. REFERENCES [1] Kurzrock, R., Gutterman, J.U. and Talpaz, M. (1988) The molecular genetics of Philadelphia chromosome-positive leukemias. New England Journal of Medicine, 319, 990- 998. doi:10.1056/NEJM198810133191506 [2] Rudkin, C.T., Nowell, P.C. and Hungerford, D.A. (1964) DNA contents of chromosome ph1 and chromosome 21 in human cronic granulocytic leukemia. Science, 144, 1229-1231. doi:10.1126/science.144.3623.1229 [3] Shtivelman, E., Lifshitz, B., Gale, R.P. and Ganaani, E. (1985) Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature, 315, 550-554. doi:10.1038/315550a0 [4] Lugo, T.G., Pendergast, A.M., Muller, A.J. and Witte, O.N. (1990) Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science, 247, 1079-1082. doi:10.1126/science.2408149 [5] Daley, G.Q., Van, Etten, R.A. and Baltimore, D. (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science, 247, 824-830. doi:10.1126/science.2406902 [6] Sawyers, C.L., McLaughlin, J. and Witte, O.N. (1995) Genetic requirement for Ras in the transformation of fi- broblasts and hematopoietic cells by the Bcr-Abl onco- gene. Journal of Experimental Medicine, 181, 307- 313.doi:10.1084/jem.181.1.307 [7] Skorski, T., Bellacosa, A., Nieborowska-Skorska, M., Majewski, M., Martinez, R., Choi, J.K., Trotta, R., Wlo- darski, P., Perrotti, D., Chan, T.O., Wasik, M.A., Tsichlis, P.N. and Calabretta, B. (1997) Transformation of hema- topoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO Journal, 16, 6151- 6161.doi:10.1093/emboj/16.20.6151 [8] Carlesso, N., Frank, D.A. and Griffin, J.D. (1996) Tyro- syl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) pro- teins in hematopoietic cell lines transformed by Bcr/Abl. Journal of Experimental Medicine, 183, 811-820. doi:10.1084/jem.183.3.811 [9] Reuther, J.Y., Reuther, G.W., Cortez, D., Pendergast, A.M. and Baldwin Jr., A.S. (1998) A requirement for NF- kappa B activation in Bcr-Abl-mediated transformation. Genes and Development, 12, 968-981. doi:10.1101/gad.12.7.968 [10] Nottage, M. and Siu, L.L. (2002) Rationale for Ras and raf-kinase as a target for cancer therapeutics. Current Pharmaceutical Design, 8, 2231-2241. doi:10.2174/1381612023393107 [11] O’Neill, E. and Kolch, W. (2004) Conferring specificity on the ubiquitous Raf/MEK signalling pathway. British Journal of Cancer, 90, 283-288. doi:10.1038/sj.bjc.6601488 [12] Ren, R. (2005) Mechanisms of BCR-ABL in the patho- genesis of chronic myelogenous leukaemia. Nature Re- views Cancer, 5, 172-183.doi:10.1038/nrc1567 [13] Pendergast, A.M., Quilliam, L.A., Cripe, L.D., Bassing, C.H., Dai, Z., Li, N., Batzer, A., Rabun, K.M., Der, C.J. and Schlessinger, J. (1993) BCR-ABL-induced onco- genesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell, 75, 175-185. [14] Chang, F., Steelman, L.S., Lee, J.T., Shelton, J.G., Navo-  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 6565 lanic, P.M., Blalock, W.L., Franklin, R.A. and McCubrey, J.A. (2003) Signal transduction mediated by the Ras/Raf/ MEK/ERK pathway from cytokine receptors to trans- cription factors: Potential targeting for therapeutic inter- vention. Leukemia, 17, 1263-1293. doi:10.1038/sj.leu.2402945 [15] Sattler, M., Mohi, M.G., Pride, Y.B., Quinnan, L.R., Ma- louf, N.A., Podar, K., Gesbert, F., Iwasaki, H., Li, S., Van Etten, R.A., Gu, H., Griffin, J.D. and Neel, B.G. (2002) Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell, 1, 479-492. doi:10.1016/S1535-6108(02)00074-0 [16] Aichberger, K.J., Mayerhofer, M., Krauth, M.T., Skvara, H., Florian, S., Sonneck, K., Akgul, C., Derdak, S., Pickl, W. F., Wacheck, V., Selzer, E., Monia, B.P., Moriggl, R., Valent, P. and Sillaber, C. (2005) Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leu- kemia (CML): Evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood, 105, 3303-3311. doi:10.1182/blood-2004-02-0749 [17] Hindley, A. and Kolch, W. (2002) Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. Journal of Cell Science, 115, 1575-1581. [18] Serre, L., Pereira, de Jesus, K., Zelwer, C., Bureaud, N., Schoentgen, F. and Benedetti, H. (2001) Crystal struc- tures of YBHB and YBCL from Escherichia coli, two bacterial homologues to a Raf kinase inhibitor protein. Journal of Molecular Biology, 310, 617-634. doi:10.1006/jmbi.2001.4784 [19] Yeung, K., Seitz, T., Li, S., Janosch, P., McFerran, B., Kaiser, C., Fee, F., Katsanakis, K.D., Rose, D.W., Mis- chak, H., Sedivy, J.M. and Kolch, W. (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature, 401, 173-177. doi:10.1038/43686 [20] Yeung, K., Janosch, P., McFerran, B., Rose, D.W., Mis- chak, H., Sedivy, J. M. and Kolch, W. (2000) Mechanism of suppression of the Raf/MEK/extracellular signal- regulated kinase pathway by the Raf kinase inhibitor protein. Molecular Cell Biology, 20, 3079-3085. doi:10.1128/MCB.20.9.3079-3085.2000 [21] Fu, Z., Smith, P.C., Zhang, L., Rubin, M.A., Dunn, R.L., Yao, Z. and Keller, E.T. (2003) Effects of raf kinase in- hibitor protein expression on suppression of prostate cancer metastasis. Journal of the National Cancer Insti- tute, 95, 878-889. doi:10.1093/jnci/95.12.878 [22] Schuierer, M.M., Bataille, F., Hagan, S., Kolch, W. and Bosserhoff, A.K. (2004) Reduction in Raf kinase inhibi- tor protein expression is associated with increased Ras- extracellular signal-regulated kinase signaling in mela- noma cell lines. Cancer Research, 64, 5186-5192. doi:10.1158/0008-5472.CAN-03-3861 [23] Schuierer, M.M., Bataille, F., Weiss, T., Hellerbrand, C. and Bosserhoff, A.K. (2006) Raf kinase inhibitor protein is downregulated in hepatocellular carcinoma. Oncology Reports, 16, 451-456. [24] Hagan, S., Al-Mulla, F., Mallon, E., Oien, K., Ferrier, R., Gusterson, B., García, J.J. and Kolch, W. (2005) Reduc- tion of Raf-1 kinase inhibitor protein expression corre- lates with breast cancer metastasis. Clinical Cancer Re- search, 11, 7392-7397. doi:10.1158/1078-0432.CCR-05-0283 [25] Al-Mulla, F., Hagan, S., Behbehani, A.I., Bitar, M.S., George, S.S., Going, J.J., García, J.J., Scott, L., Fyfe, N., Murray, G.I. and Kolch, W. (2006) Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. Journal of Clinical Oncology, 24, 5672- 5679. doi:10.1200/JCO.2006.07.5499 [26] Zlobec, I., Baker, K., Minoo, P., Jass, J.R., Terracciano, L. and Lugli, A. (2008) Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein exoression. Clinical Can- cer Research, 14, 143-148. doi:10.1158/1078-0432.CCR-07-1380 [27] Houben, R., Vetter-Kauczok, C.S., Ortmann, S., Rapp, U.R., Broecker, E.B. and Becker, J.C. (2008) Phospho- ERK staining is a poor indicator of the mutational status of BRAF and NRAS in human melanoma. Journal of Investigative Dermatology, 128, 2003-2012. doi:10.1038/jid.2008.30 [28] Nakamura, S., Hirano, I., Okinaka, K., Takemura, T., Yokota, D., Ono, T., Shigeno, K., Shibata, K., Fujisawa, S. and Ohnishi, K. (2010) The FOXM1 transcriptional factor promotes the proliferation of leukemia cells thr- ough modulation of cell cycle progression in acute mye- loid leukemia. Carcinogenesis, 31, 2012-2021. doi:10.1093/carcin/bgq185 [29] Wellbrock, C., Karasarides, M. and Marais, R. (2004) The RAF proteins take centre stage. Nature Reviews Mole- cular Cell Biology, 5, 875-885. doi:10.1038/nrm1498 [30] Dhillon, A.S., Hagan, S., Rath, O. and Kolch, W. (2007) MAP kinase signaling pathways in cancer. Oncogene, 26, 3279-3290. doi:10.1038/sj.onc.1210421 [31] Roberts, P.J. and Der, C.J. (2007) Targeting the Raf-MEK- ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene, 26, 3291-3310. doi:10.1038/sj.onc.1210422 [32] Schubbert, S., Shannon, K. and Bollag, G. (2007) Hy- peractive Ras in developmental disorders and cancer. Na- ture Reviews Cancer, 7, 295-308. doi:10.1038/nrc2109 [33] Li, H.Z., Gao, Y., Zhao, L.X., Liu, Y.X., Sun, B. C., Yang, J. and Yao, Z. (2009) Effects of Raf kinase inhibitor pro- tein expression on metastasis and progression of human breast cancer. Molecular Cancer Research, 7, 832-840. doi:10.1158/1541-7786.MCR-08-0403 [34] Ye, H., Holterman, A.X., Yoo, K.W., Franks, R.R. and Costa, R.H. (1999) Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S-phase. Molecular Cell Biology, 19, 8570-8580. [35] Laoukili, J., Kooistra, M.R., Bras, A., Kauw, J., Kerkho- ven, R.M., Morrison, A., Clevers, H. and Medema, R.H. (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nature Cell Biol- ogy, 7, 126-136. doi:10.1038/ncb1217 [36] Wonsey, D.R. and Follettie, M.T. (2005) Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Research, 65, 5181-5189. doi:10.1158/0008-5472.CAN-04-4059 [37] Leung, T.W., Lin, S.S., Tsang, A.C., Tong, C.S., Ching, J.C., Leung, W.Y, Gimlich, R., Wong, G.G. and Yao, K.M. (2001) Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Letters, 507, 59-66.  S. Nakamura et al. / Stem Cell Discover y 1 (2011) 54-66 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/SCD/ 66 doi:10.1016/S0014-5793(01)02915-5 [38] Wang, X., Kiyokawa, H., Dennewitz, M.B. and Costa, R.H. (2002) The forkhead box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proceedings of the Na- tional Academy Scie nce of the USA, 99, 16881-16886. doi:10.1073/pnas.252570299 [39] Petrovic, V., Costa, R.H., Lau, L.F., Raychaudhuri, P. and Tyner, A.L. (2008) FoxM1 regulates growth factor in- duced expression of the KIS kinase to promote cell cycle progression. Journal of Biological Chemistry, 104, 453- 460. [40] Teh, M.T., Wong, S.T., Neill, G.W., Ghali, L.R., Philpott, M.P. and Quinn, A.G. (2002) FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Research, 62, 4773-4780. [41] Kalinichenko, V.V., Major, M.L., Wang, X., Petrovic, V., Kuechle, J., Yoder, H.M., Dennewitz, M.B., Shin, B., Datta, A., Raychaudhuri, P. and Costa, R.H. (2004) Fox- m1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Development, 18, 830-850. doi:10.1101/gad.1200704
|