A Comparative Study of Bisphenol-A, Hydantoin and Cyanuric Acid Based Epoxy Resins Using XRD1517
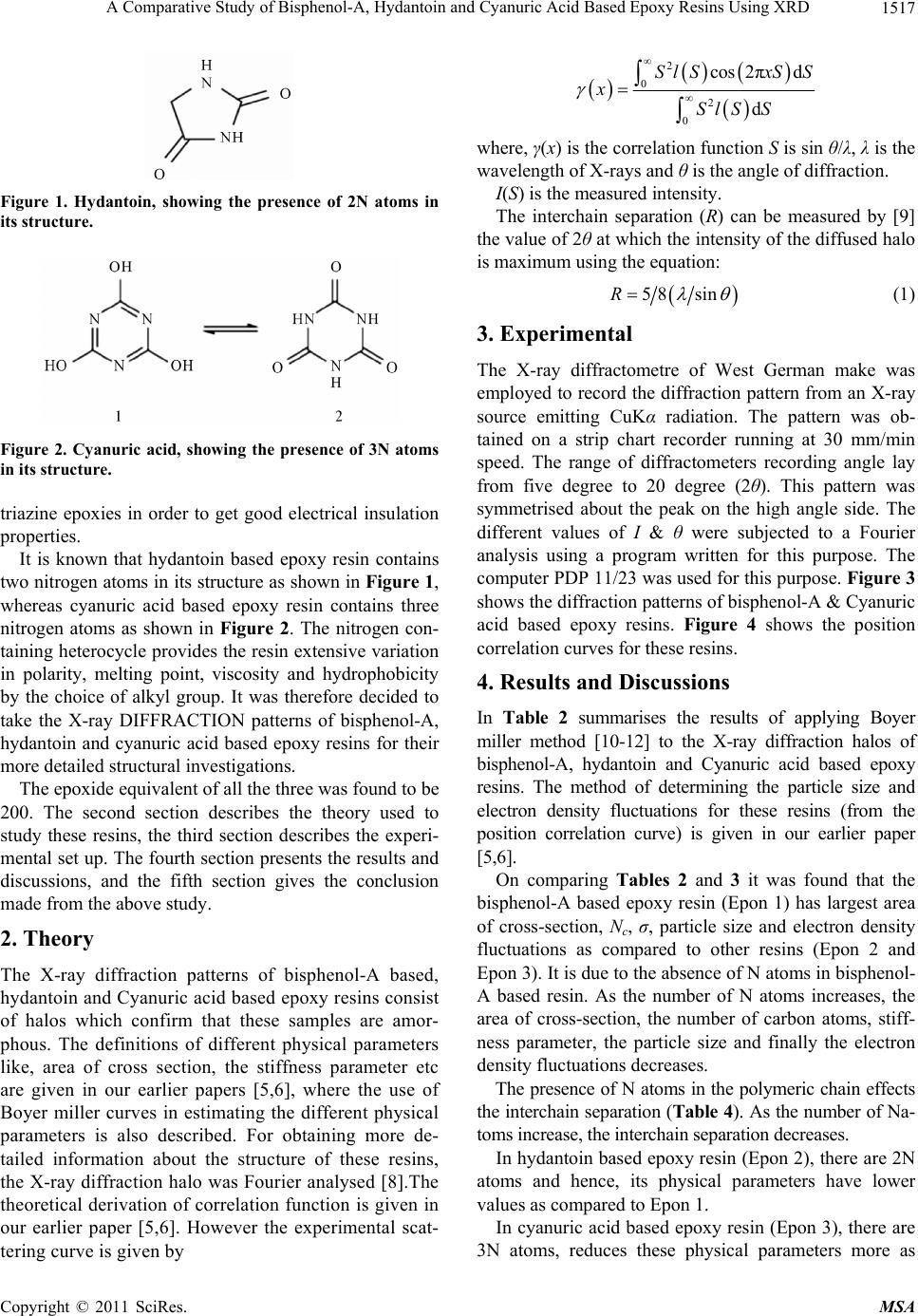
Figure 1. Hydantoin, showing the presence of 2N atoms in
its structure.
Figure 2. Cyanuric acid, showing the presence of 3N atoms
in its structure.
triazine epoxies in order to get good electrical insulation
properties.
It is known that hydantoin based epoxy resin contains
two nitrogen atoms in its structure as shown in Figure 1,
whereas cyanuric acid based epoxy resin contains three
nitrogen atoms as shown in Figure 2. The nitrogen con-
taining heterocycle provides the resin extensive variation
in polarity, melting point, viscosity and hydrophobicity
by the choice of alkyl group. It was therefore decided to
take the X-ray DIFFRACTION patterns of bisphenol-A,
hydantoin and cyanuric acid based epoxy resins for their
more detailed structural investigations.
The epoxide equivalent of all the three was found to be
200. The second section describes the theory used to
study these resins, the third section describes the experi-
mental set up. The fourth section presents the results and
discussions, and the fifth section gives the conclusion
made from the above study.
2. Theory
The X-ray diffraction patterns of bisphenol-A based,
hydantoin and Cyanuric acid based epoxy resins consist
of halos which confirm that these samples are amor-
phous. The definitions of different physical parameters
like, area of cross section, the stiffness parameter etc
are given in our earlier papers [5,6], where the use of
Boyer miller curves in estimating the different physical
parameters is also described. For obtaining more de-
tailed information about the structure of these resins,
the X-ray diffraction halo was Fourier analysed [8].The
theoretical derivation of correlation function is given in
our earlier paper [5,6]. However the experimental scat-
tering curve is given by
2
0
2
0
cos 2πd
d
Sl SxSS
x
Sl SS
where, γ(x) is the correlation function S is sin θ/λ, λ is the
wavelength of X-rays and θ is the angle of diffraction.
I(S) is the measured intensity.
The interchain separation (R) can be measured by [9]
the value of 2θ at which the intensity of the diffused halo
is maximum using the equation:
58 sinR
(1)
3. Experimental
The X-ray diffractometre of West German make was
employed to record the diffraction pattern from an X-ray
source emitting CuKα radiation. The pattern was ob-
tained on a strip chart recorder running at 30 mm/min
speed. The range of diffractometers recording angle lay
from five degree to 20 degree (2θ). This pattern was
symmetrised about the peak on the high angle side. The
different values of I & θ were subjected to a Fourier
analysis using a program written for this purpose. The
computer PDP 11/23 was used for this purpose. Figure 3
shows the diffraction patterns of bisphenol-A & Cyanuric
acid based epoxy resins. Figure 4 shows the position
correlation curves for these resins.
4. Results and Discussions
In Table 2 summarises the results of applying Boyer
miller method [10-12] to the X-ray diffraction halos of
bisphenol-A, hydantoin and Cyanuric acid based epoxy
resins. The method of determining the particle size and
electron density fluctuations for these resins (from the
position correlation curve) is given in our earlier paper
[5,6].
On comparing Tables 2 and 3 it was found that the
bisphenol-A based epoxy resin (Epon 1) has largest area
of cross-section, Nc, σ, particle size and electron density
fluctuations as compared to other resins (Epon 2 and
Epon 3). It is due to the absence of N atoms in bisphenol-
A based resin. As the number of N atoms increases, the
area of cross-section, the number of carbon atoms, stiff-
ness parameter, the particle size and finally the electron
density fluctuations decreases.
The presence of N atoms in the polymeric chain effects
the interchain separation (Table 4). As the number of Na-
toms increase, the interchain separation decreases.
In hydantoin based epoxy resin (Epon 2), there are 2N
atoms and hence, its physical parameters have lower
values as compared to Epon 1.
In cyanuric acid based epoxy resin (Epon 3), there are
3N atoms, reduces these physical parameters more as
Copyright © 2011 SciRes. MSA