Open Access Library Journal
Vol.04 No.08(2017), Article ID:78828,9 pages
10.4236/oalib.1103715
Evaluation of Hexadate Ligand 1, 3-bis(2,2’:6’,2’’-Terpyridyl-5-Ylmethylsulfany l)Propane in the Determination of Iron(II) in Solution by Spectrophotometric and Fluoremetric Methods of Analysis
Philip K. Maritim
Department of Environmental Earth Sciences, University of Eldoret, Eldoret, Kenya

Copyright © 2017 by author and Open Access Library Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 6, 2017; Accepted: August 28, 2017; Published: August 31, 2017
ABSTRACT
Uv-visible and fluorescence spectra of the ligand 1,3-bis(2,2’:6’,2’’-ter- pyridyl-5-ymethylsulfanyl)propane L and it’s iron(II) complex have been investigated for analytical purposes. The two spectra of L and terpy are very similar which confirmed the ability of L to co-ordinate through the six N atoms of L with minimum distortion of the metal ion’s octahedral geometry. The ligand-based absorption band of L is shifted to the longer wavelength. It was found that L is able to displace the two terpyridine groups in the complex to give [FeL]2+. The high stability of the complex makes it good in spectrophotometry analysis of metals ions in solution. The fluorescence of L was progressively quenched with an increasing concentration of iron(II). This makes L a possible reagent for the quantitative analysis of metal by measuring fluorescence quenching.
Subject Areas:
Analytical Chemistry
Keywords:
Ligand, Fluorescence, Spectrophotometry

1. Introduction
Spectrophotometric and fluorescence methods are sensitive for metal ion analysis. The equipment is compact and easy to use. This has made the two methods popular, and they are used in routine laboratories all over the world. Any attempt to improve on detection limits of this type of equipment is valuable.
Spectroscopy analysis is based on the relationship between the degree of absorption and the concentration of the absorbing materials. Some ligands formed coloured complex with metals. The intense of the colour of the metal complexes can make spectrophotometer instruments to be able to detect very low concentration of metal, hence improving the detection limit.
Fluorescence is one of the several mechanisms by which a molecule can return to the ground state after being exited by absorption of radiation. All molecules have a potential to fluoresce but most do not, because their structure provides a radiationless pathway at a greater rate than the fluorescence emissions. Compounds that can fluoresce are often rigid molecules such as aromatics, compounds with conjugated double bonds, and heterocycles.
Inorganic fluorimetry is often based upon the reaction of an analyte with a chelating agent to form a complex that fluoresces or upon measurement of fluorescence quenching as a result of the analyte co-ordination. The fluorescence is measured at 90˚ to the excitation light. Fluorescent spectroscopy can often give high sensitivity and specificity. In favourable cases, it can measure as low as 10−9 mg/cm3 of analyte. The intense colour of the terpyridine metal complexes can make such instruments capable of detecting very low concentration of metal, hence improving the detection limit [1] .
It has been recognised that metals sometimes react with ligands to form intensely coloured compounds. These ligands have been used for the determination of metal ions in solution at very low concentration. There are a number of ligands that are available for analysis of metals. Those for determining low concentration of iron(II) include 2.2’-bipyridyl, 4,7-diphenyl-10-phenanthroline, and 2,4,6-tri(2-pyridyl)-1,3,5-triazine; they react with iron(II) to form Fe(II) complexes with their molar extinction coefficient (εmax) of 800, 2240, 2260 dm3 mol−1∙cm−1 respectively as demonstrated by R. C. Denny et al. [2] . Another known ligand which is used for the determination of iron(II) by spectrophotometry is 2,2’:6’,2’’-terpyridine. 2,2’:6’,2’’-terpyridine was first isolated by Morgan and Burstall [3] in 1937 in low yield. Since then, varied and exciting co-ordination chemistry of 2,2’:6’2’’-terpyridine has been established as demonstrated by E. C. Constable et al. [4] . This has led to the development of synthetic strategies enabling a wide range of its substituted analogues to be prepared in good yield. Today the parent ligand 1 is commercially available [5] .
This ligand 1 has been recognised as a useful ligand in the fields of organic, organometallic and co-ordination chemistry. It is a good ligand for analytical chemistry since it forms bis(terpyridine) metal complexes with many metal ions e.g. the iron(II) complex 2 which has blood red colour. 2,2’:6’,2’’-Terpyridine co- ordinates strongly through the three nitrogen atoms, and remains planar in a meridonal mode of co-ordination.


Terpyridine ligands (terpy), and their metal complexes, can have very intense colours which arise from electronic transitions p®p* and metal to ligand charge transfer. The terpyridine ligand possess low-lying p* orbital which are able to accept electrons density from the metal as earlier explain by G. T. Morgan et al. [3] . The strong colour which results from this metal to ligand charge transfer band is useful for trace metal analysis because even in small quantities metal ions can produce compounds with high absorbance.
It has been reported that 2.2’;6’2’’-terpyridine (terpy) and its substituted derivative form stable complexes with transition metals such as iron(II) and nickel (II) [6] [7] [11] .
For example bis(2,2’:6’,2’’-terpyridine) iron(II) complex which is stable over a wide range of pH, and has a high molar extinction coefficient (ε552 12 500 dm3∙mol?1∙cm?1) as earlier reported by R. L. Morris [8] . Therefore, pyridine is a highly sensitive reagent for Fe2+ determination. It has been used to determine Fe2+ in water in the present of heavy metals [9] .
The detection limit in the use of these ligands depends on the stability of the complexes that are formed, and on the magnitude of the molar extinction coefficients of the complexes.
In search for even more ligand that forms more stable complexes with metals we synthesis and characterise a new ligand (1,3-bis(2,2’:6’,2’’-terpyridyl-5-yl- methylsulfanyl) propane L as reported by Gleb et al. [12] . In the study, two terpyridines derivatives (3) were linked together through the reaction of a dithiol with sython 3. The 5-substituted bromomethyl derivative 3 is ideal for the proposed complex in order to avoid the sulphurs at the “bridge” from taking part in co-ordination with the metal ions. The aim here was to sythesis a complex of a single metal by co-ordination with the two bridged terpyridines to form octahedral complex. The use of 1,3-propanedithiol has advantages in this case because sulphur atoms of the product L, unlike nitrogen atoms, do not undergo protonation in aqueous solutions. Therefore, not pH dependent like nitrogen atoms. The ligand was synthesised from 5-bromomethy-2,2’:6’2’’-terpyridine 3 obtained by method of P. Sheldon [10] . The ligand was found to form stable complexes nickel(II).
Therefore the objective of this study is to react ligand L with iron(II) and evaluate whether can be used in determining iron in solution by UV/Vis spectroscopy and Fluorescence method of analysis.


2. Results and Discussion
The ligand L was synthesis using the methods reported by Gleb et al. 2000 [12] from Bromo-terpy 3 as shown on the scheme provided in Scheme 1.
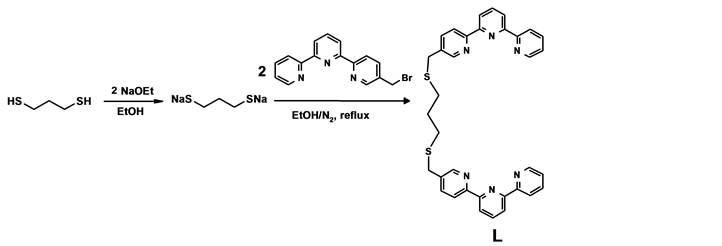
2.1. Spectrophotometric Analysis
2.1.1. Comparison of uv Spectra of L and Terpy
The electronic absorption spectra of L and terpy (3) are shown in Figure 1.
L and terpy show lmax at 238 nm and 280 nm respectively. Their molar extinction coefficients (εmax) are 46,000 dm3∙mol−1∙cm−1 and 18,000 dm3∙mol−1∙cm−1 respectively. It was noted that both terpy and L exhibit similar electronic transitions. Curve (L) indicates that one linked terpyridine has a higher absorbance than terpyridine alone, and the longest wavelength peak is shifted to a longer wavelength.
2.1.2. Comparison of uv-Visble Spectra of [FeL]2+ and [Fe(Terpy)2]2+
A uv/vis spectra of methanolic solutions of [Fe(terpy)2]2+ and [FeL]2+ was obtaine at concentrations of 1.43 × 10−5 mol∙dm−3 and 1.19 × 10−5 mol∙dm−3 respec-
Figure 1. Comparison of UV spectra of (L) and (terpy).
tively and then the normalised. The two spectra were very similar which confirmed the ability of L to co-ordinate through the six N atoms of L with minimum distortion of the metal ion’s octahedral geometry. The ligand-based absorption band of L is shifted to the longer wavelength. They both exhibit a metal to ligand charge-transfer band at 552 nm of molar extinction coefficients.
(ε552 nm) of 11,500 dm3∙mol−1∙cm−1 [FeL]2+ and 9050 dm3∙mol−1∙cm−1 [Fe(terpy)2]2+.
The two complexes show two ligand-based absorption bands [FeL]2+ at 276 and 328 nm, and [Fe(terpy)2]2+ at 273 and 319 nm. The maximum molar extinction coefficients for each of the complexes are (ε328 nm) 41,800 dm3∙mol−1∙cm−1 [FeL]2+ and (ε319 nm) 41,200 dm3∙mol−1∙cm−1 [Fe(terpy)2]2+.
2.2. Fluorescence Measurements
A solution of L (4.92 × 10−6 mol∙dm−3) was reacted with increasing concentrations of standardised Fe2+ ions of concentration between (2 × 10−6 - 2 × 10−5 mol∙dm−3) to form [FeL]2+. The fluorescence of these solutions was scanned in a 1 cm fluorescence cuvette from 350 - 600 nm with an excitation at 276 nm.
L is luminescent with a maximum in the emission spectrum at 350 nm.
Figure 2 shows the effect on the luminescence of L with added increasing amount of Fe2+. The luminescence of L is quenched progressively with increase in the concentration of iron(II). It can be noticed that at concentration e fluorescence intensity as reached the lowest hence its detection limits. This makes L a good reagent for the qualitative analysis of metal ions by measuring the fluorescence quenching.
3. Experimental
3.1 Synthesise of [FeL](PF6)2
A solution of iron(II) tetrafluoroborate (28.8 mg, 0.0835 mmol) in methanol (30
Figure 2. Change of Flourecence spectra of methanolic solutions of L (4.92 × 106 mol∙dm3) with increasing [Fe2+] (exicitation at 276 nm).
cm3) was added dropwise to a solution of L (50 mg, 0.0835 mmol) in methanol (refluxed to dissolve), and the resulting deep purple solution was stirred for 30 minutes. Excess methanolic ammonium hexafluorophosphate was added, and the volume reduced with a rotary evaporator to precipitate [FeL](PF6)2. The precipitate was filtered and washed with ether to give the complex as fine purple solid, (yield 41 mg, 53%).
13C NMR (100 MHz,CDCl3): d/p.p.m 161.26, 160.86, 158.92, 156.44, 155.45, 153.61, 141.82, 139.54, 139.47, 138.94, 128.14, 124.4, 124.04, 123.74, 118.14, 33.06, 31.05, 29.08.Elemental Analysis; observed C 42.1, H 3.85, N 8.06 and calculated for C35H30N6S2P2F12Fe 2.5H2O is C 42.5, H 3.56, N 8.49% FAB mass spectrum; calculated for C35H30N6S2P2F12Fe i.e. [FeL][PF6]2 m/z 944.6.
Found no molecular ion but daugther ion at m/z 799, 673, and 654.
3.2. Standardisation of FeSO4∙(NH4)2SO4.6H2O with Standard 0.1 mol∙dm−3 Cerium(IV) Sulphate
Ferroin indicator was first prepared by dissolving FeSO4∙(NH4)2SO4∙6H2O (784 mg, 0.2 mmol) in deionised water (10 cm3). To this solution, 1,10-phenathroline monohydrate (120 mg, 0.6 mmol) was added to form [Fe(phen)3]2+ (ferroin) which has a red blood colour [13] . FeSO4∙(NH4)2SO4∙6H2O 1.961 gm, (5.0 mmol) was dissolved in N2 scrubbed deionised water and made to 50 cm3 in a volumetric flask. 5.0 ± 0.1 cm3 of this solution was transferred into a conical flask, 0.5 cm3 of 0.5 mol∙dm−3 sulphuric acid and a drop of ferroin was added. This solution was titrated with standard 0.1 mol∙dm−3 cerium(IV) sulphate solution to the end point red to green. Titration was repeated four times and an average of the volume of Ce4+ taken. The four titre values obtained were 5.04, 5.03, 5.29 and 5.03 cm3. The average is 5.03 ± 0.01. The concentration of the Fe2+ was found to be 0.101 ± 0.002 mol∙dm3.
3.3. Fluorescence Analysis
Table 1 demonstrates how Fe2+ solution of original concentration of A 2 × 10−4 mol∙dm−3 were diluted. A volume of 49.5 microliters of the standardised solution of 0.101 mol∙dm3 FeSO4∙NH4)2SO4∙6H2O was taken and diluted to 25 cm3 in a 25 cm3 volumetric flask with spectrophotometric methanol to give a solution of concentration 2 × 10−4 mol∙dm−3. A solution of L (4.915 mg, 8.1 × 10−6 moles) was prepared with methanol in a 50 cm3 volumetric flask to give a solution of concentration 1.62 × 10−4 mol∙dm3. This solution was diluted and mixed as shown in the table below. The concentration of L was kept constant at 4.92 ×10−6 mol∙dm−3 whilst that of Fe2+ was varied.
Each solution was transferred in turn to a quartz fluorescence cuvette and fluorescence emission spectrum was scanned between 200 - 450 nm with a Perkin Elmer LS50 luminescence spectrometer. The excitation wavelength was 276 nm (exit slit 5 nm and em. slit 10 nm). The spectrum was processed using a spread sheet.
4. Conclusions
The electronic absorption spectra of L and terpy (3) are shown in Figure 1.
L and terpy show lmax at 238 nm and 280 nm respectively. Their molar extinction coefficients (εmax) are 46,000 dm3∙mol−1∙cm−1 and 18,000 dm3∙mol−1∙cm−1 respectively. It was noted that both terpy and L exhibit similar electronic transitions. The Uv/Vis spectra of both ligand L and Terpy are very similar which confirmed the ability of L to co-ordinate through the six N atoms of L with minimum distortion of the metal ion’s octahedral geometry. The ligand-based absorption band of L is shifted to the longer wavelength. They both exhibit a metal to ligand charge-transfer band at 552 nm of molar extinction coefficients (ε552 nm) of 11,500 dm3∙mol−1∙cm−1 and 9050 dm3∙mol−1∙cm−1 respectively.
The stability of [FeL]2+ is demonstrated by the ability of L to displace both terpy ligands in [Fe(terpy)2]2+ which was as earlier discussed by Gleb et al. [12] . The equilibrium formation constant b2 of [Fe(terpy)2]2+ is 1020.9 [11] [12] and from the above observation, b1 of [FeL]2+ is more than this. The exact value of b1 for [FeL]2+ could be established by competition experiments with other multidentate ligands of known donor strength.
Table 1. Preparation of L and Fe2+ solutions for fluorescence analysis.
Uv spectra of both L and terpy are very similar.
Iron(II) is low spin when complex with L, which contributes to the high stability of the complex, therefore, making L a good reagent for the determination of iron(II) by spectrophotometric method of analysis.
The ligand L is fluorescent. From the results in Figure 2, the fluorescence is progressively quenched by increased concentration of the iron(II). Therefore, L can be used for the determination of some metal ions by measuring the fluorescence quenching.
The use of L for the analysis of other Fe(II) ions in the presence of several other metal ions, e.g. in industrial waste water, needs further investigation.
Cite this paper
Maritim, P.K. (2017) Evaluation of Hexadate Ligand 1, 3-bis(2,2’:6’,2’’-Terpyridyl-5-Ylmethylsulfany l) Propane in the Determination of Iron(II) in Solution by Spectrophotometric and Fluoremetric Methods of Analysis. Open Access Library Journal, 4: e3715. https://doi.org/10.4236/oalib.1103715
References
- 1. Vogel, A.I. (1978) Text Book of Quantitative Inorganic Analysis. 4th Edition, Longman, London, New York, 318.
- 2. Denny, R.C. and Sinclair, R. (1987) Textbook of uv/Visible Spectroscopy. John Wiley and Sons, Chichester, New York, Toronto, Singabore, 70.
- 3. Morgan, G.T. and Burstall, F.H. (1937) Researches on Residual Affinity and Co-Ordination. Part XXXVII. Complex Metallic Salts Containing 2:6-di-2’-Pyridylpyridine (2:2’:2’’-Tripyridyl). Journal of the Chemical Society, 1649-1655.
https://doi.org/10.1039/JR9370001649 - 4. Constable, E.C. and Ward, M.D. (1988) A Convenient, High Yield Synthesis of 2,2’:6’,2’’-Terpyridine and Its Iron(II) Complex. Inorganica Chimica Acta, 141, 201-203.
- 5. Alcock, N.W., Clarke, A.J., Moore, P., Crawle, S., Sheldon, P., Smith, S.M. and Turonek, M.L. (1995) 4,8,11-Tetrakis{(2,2’-Bipyridyl-5’-Ylmethyl)-bis(2,2’-bipy- ridyl)Ruthenium(II)}-1,4,8,11-Tetraazacyclotetradecane (L1), a Macrocyclic pH and Transition Metal Ion Fluorescence Sensor. Equilibrium, and Stopped-Flow Kinetic, Fluorimetric Studies of the Reactions of L1 with Nickel(II) and Copper(II) Ions in Aqueous Solution at Neutral pH. Inorganica Chimica Acta, 240, 159.
- 6. Constable, E.C. and Thomson, A.M.W.G. (1992) Ligand Reactivity in Iron(II) Complexes of 4’-(4’’’-Pyridyl)-2,2’:6’,2’’-Terpyridine. Journal of the Chemical Society, Dalton Transactions, No. 20, 2947-2950.
https://doi.org/10.1039/DT9920002947 - 7. Joscean, A.M. and Moore, P. (1998) Comparison of the Rates and Mechanisms of Formation and Solvolysis of [Fe(bipy)3]2+ (Bipy = 2,2’-Bipyridine) and [FeL]2+ [L = 1,4,7-tris(2,2’-Bipyridyl-5-Ylmethyl)-1,4,7-Triazacyclononane] and Their Stabilities in Dimethylformamide Solution. J. Chem. Soc. Dalton. Tans., 369-374.
- 8. Morris, R.L. (1952) Determination of Iron in Water in Presence of Heavy Metals. Analytical Chemistry, 24, 1376-1378.
https://doi.org/10.1021/ac60068a039 - 9. Douglas, J.E. and Wilkins, C.J. (1969) Terpyridyl Complexes of Zinc, Cadmium, and Mercury. Inorganica Chimica Acta, 3, 635.
- 10. Sheldon, P. (1994) Coordination Chemistry of Azamacrocycles Functionalised with N-Pendant Pyridyl, Bipyridyl and Terpyridyl Arms. PhD Thesis, University of Warwick, War-wick.
- 11. Constable, E.C., Lewis, J., Liprot, M.C. and Raithy, P.R. (1990) The Coordination Chemistry of 4’-Phenyl-2,2’:6’, 2’’-Terpyridine; the Synthesis, Crystal and Molecular Structures of 4’-Phenyl-2,2’:6’,2’’-Terpyridine and bis(4’-Phenyl-2,2’:6’,2’’-Terpyridine)Nickel(II) Chloride Decahydrate. Inorganica Chimica Acta, 178, 47-54.
- 12. Priimov, G.U., Moore, P., Maritim, P.K., Butalanyi, P.K. and Alcock, N.W. (2000) Synthesis of Two Covalently Linked Bis(2,2’:6’,2’’-Terpyridine) (Terpy) Chelating Ligands with Different Length Spacers, Comparison of the Crystal Structures of Their Mononuclear Nickel(II) Complexes, and Kinetic and Mechanistic Studies of the Reaction of One Ligand with [Fe(terpy)2]2+. Journal of the Chemical Society, Dalton Transactions, No. 4, 445-449.
- 13. Moore, P. (1972) Analysis of Kinetic Data for a First-Order Reaction with Unknown Initial and Final Readings by the Method of Non-Linear Least Squares. Journal of the Chemical Society, Faraday Transaction I, 68, 1890-1893.
https://doi.org/10.1039/f19726801890




