 Vol.1, No.3, 121-127 (201 doi:10.4236/ojas.2011.13016 C opyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 1) Op en Journal of Anim al Sciences Potential benefits of quinoxaline 1, 4-dioxides in aldosterone dysmetabolism disease —A medical hypothesis Da-Jiang Zou1, Qiao-Feng Zheng1, Xian-Ju Huang1,*, Xu Wang2, Awais Ihsan2 1College of Pharmacy, South-Central University for Nationalities, Wuhan, China; *Corresponding Author: huangxianju1972@yahoo.cn 2National Reference Laboratory of Veterinary Drug Residues and MOA Key Laboratory of Food Safety Evaluation, Huazhong Agri- culture University, Wuhan, China. Received 13 June 2011; revised 20 July 2011; accepted 22 August 2011. ABSTRACT Quinoxaline 1, 4-dioxides (QdNOs) are quinoxa- line derivatives which have been used as an- timicrobial agents and growth promoters in animals widely. They are also assumed to cure human disease such as anticancer, antituber- cular and inhibiting parasite. QdNOs such as carbadox and their major metabolites induced a special decline of aldosterone production from the swine adrenal in vivo and in vitro, and thus cause hypovolemia, hyponatremia and hyper- kalemia. This can also be expected to be the case for human. As a mainly physiological hormone and a novel steroid w ith potent miner- alocorticoid activity, aldosterone plays an im- portant role in the pathophysiological process of brain, renal and heart disease progression and may be a renal and vascular risk factor. Here, we provide evidence to support the hy- pothesis that QdNOs may lead potential benefit s in aldosterone dysmetabolism disease via the synthesis deficiency of aldosterone in adrenal and/or the cardiovascular tissues. If the hy- pothesis is true, it may provide a new option into the therapy for aldosterone dysmetabolism disease, especially in cardiovascular system, and thus assume a broader application of QdNOs. Keywords: Quinoxaline 1; 4-Dioxides; Aldosterone; Adrenal Gland; Dysmetabolism; Cardiovascular Tissues 1. INTRODUCTION Since 1940s, Quinoxaline-di-N-oxides (QdNOs) (Fig- ure 1) are known as potent antibacterial agents which act on several gram-positive and -negative species [1-4]. For this reason they have been used as growth promoters in agricultural stock farming to promote growth of pigs, cattle, fish and poultry when added to the feed during rearing in dosages 25 - 100 mg/kg [5-8]. Recently, extensive study has been carried out on QdNOs which indicates that they can be also applied for treatment of human diseases. For example, QdNOs have shown a selective cytotoxicity against hypoxic cells pre- sented in solid tumors [9-11]. It was [12] demonstrated a dose-dependent inhibition of the proliferation of T-84 human colon cancer cells by QdNOs. Other studies have put in evidence that QdNOs are endowed with antitu- bercular activities in vitro. They have inhibitor activity of clinical isolates of Mycobacterium tuberculosis [13] or Mycobacterium tuberculosis H37Rv [14-15]. Fur- thermore, QdNOs presented good in vitro inhibitor ac- tivity of Trypanosoma cruzi, the causative agent of Cha- gas’ disease affects around 20 million people in Central and South America [16]. The endocrinological effects of these compounds have long been recognized. Besides their influence on meta- bolic hormones and epidermal growth factor in swine [17] QdNOs such as carbadox and its major metabolites induced a special decline of aldosterone production from the adrenal in vivo and in vitro, and thus cause hypo- volemia, hyponatremia and hyperkalemia [18-21] Al- dosterone is a steroid hormone and the most important mineralocorticoid in the human body. Clinically, excess aldosterone is associated with increased stroke risk [22]; progressive renal disease [23], higher morbidity and mortality in cardiovascular diseases such as myocardial infarction (MI) and heart failure (HF) [24-25]. Here, we provide evidence to support the hypothesis that QdNOs can lead potential benefits in cardiovascular or other diseases caused by metabolism dysfunction of aldoster- on via the synthesis deficiency of aldosterone. e 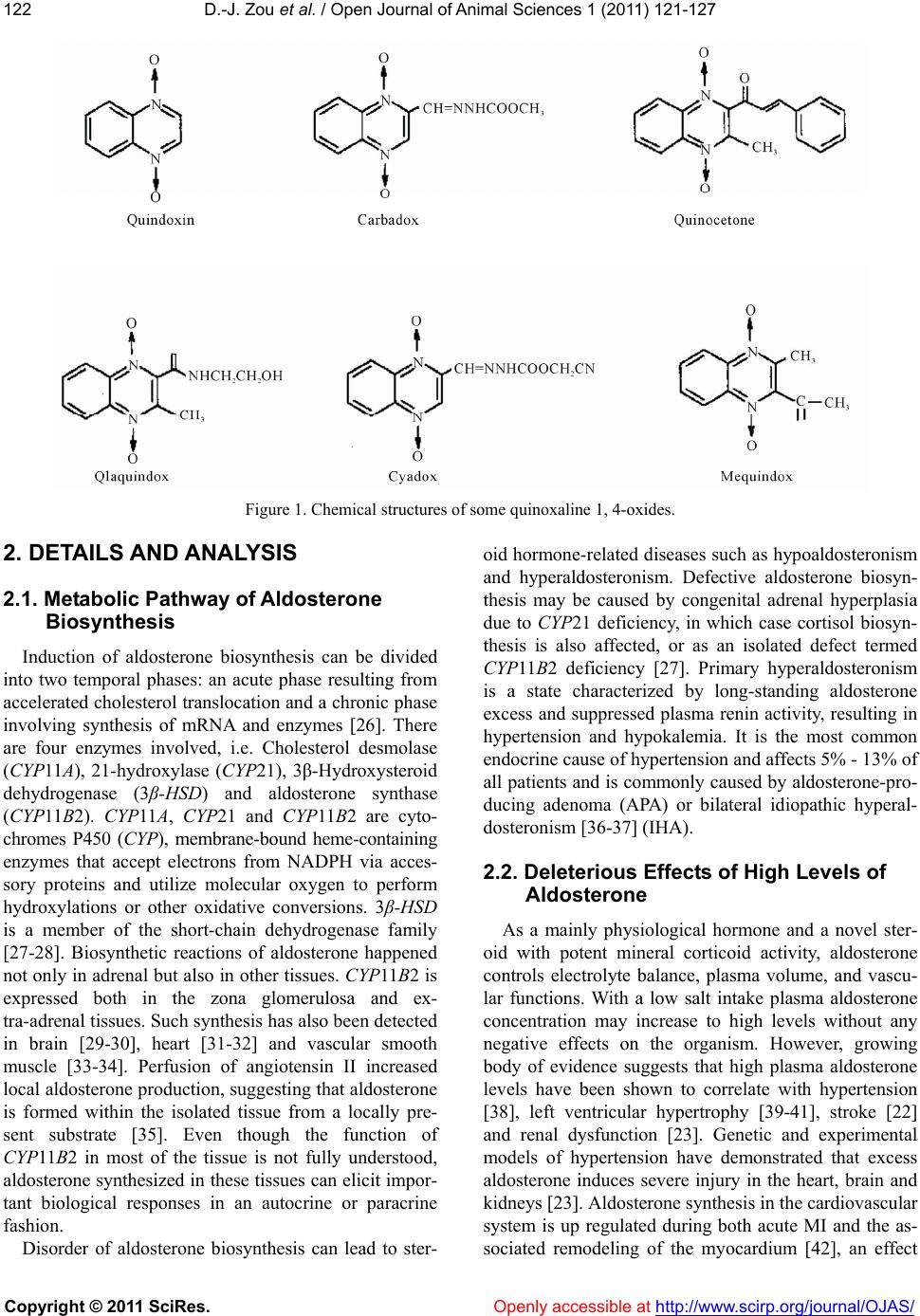 D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 122 Figure 1. Chemical structures of some quinoxaline 1, 4-oxides. 2. DETAILS AND ANALYSIS 2.1. Metabolic Pathway of Aldosterone Biosynthesis Induction of aldosterone biosynthesis can be divided into two temporal phases: an acute phase resulting from accelerated cholesterol translocation and a chronic phase involving synthesis of mRNA and enzymes [26]. There are four enzymes involved, i.e. Cholesterol desmolase (CYP11A), 21-hydroxylase (CYP21), 3β-Hydroxysteroid dehydrogenase (3β-HSD) and aldosterone synthase (CYP11B2). CYP11A, CYP21 and CYP11B2 are cyto- chromes P450 (CYP), membrane-bound heme-containing enzymes that accept electrons from NADPH via acces- sory proteins and utilize molecular oxygen to perform hydroxylations or other oxidative conversions. 3β-HSD is a member of the short-chain dehydrogenase family [27-28]. Biosynthetic reactions of aldosterone happened not only in adrenal but also in other tissues. CYP11B2 is expressed both in the zona glomerulosa and ex- tra-adrenal tissues. Such synthesis has also been detected in brain [29-30], heart [31-32] and vascular smooth muscle [33-34]. Perfusion of angiotensin II increased local aldosterone production, suggesting that aldosterone is formed within the isolated tissue from a locally pre- sent substrate [35]. Even though the function of CYP11B2 in most of the tissue is not fully understood, aldosterone synthesized in these tissues can elicit impor- tant biological responses in an autocrine or paracrine fashion. Disorder of aldosterone biosynthesis can lead to ster- oid hormone-related diseases such as hypoaldosteronism and hyperaldosteronism. Defective aldosterone biosyn- thesis may be caused by congenital adrenal hyperplasia due to CYP21 deficiency, in which case cortisol biosyn- thesis is also affected, or as an isolated defect termed CYP11B2 deficiency [27]. Primary hyperaldosteronism is a state characterized by long-standing aldosterone excess and suppressed plasma renin activity, resulting in hypertension and hypokalemia. It is the most common endocrine cause of hypertension and affects 5% - 13% of all patients and is commonly caused by aldosterone-pro- ducing adenoma (APA) or bilateral idiopathic hyperal- dosteronism [36-37] (IHA). 2.2. Deleterious Effects of High Levels of Aldosterone As a mainly physiological hormone and a novel ster- oid with potent mineral corticoid activity, aldosterone controls electrolyte balance, plasma volume, and vascu- lar functions. With a low salt intake plasma aldosterone concentration may increase to high levels without any negative effects on the organism. However, growing body of evidence suggests that high plasma aldosterone levels have been shown to correlate with hypertension [38], left ventricular hypertrophy [39-41], stroke [22] and renal dysfunction [23]. Genetic and experimental models of hypertension have demonstrated that excess aldosterone induces severe injury in the heart, brain and kidneys [23]. Aldosterone synthesis in the cardiovascular system is up regulated during both acute MI and the as- sociated remodeling of the myocardium [42], an effect  D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 123 that is believed to be due to local angiotensin II produc- tion [43], suggesting that tissue-specific effects of al- dosterone contribute to pathophysiological changes. The neurohormonal actions of aldosterone influence the prognosis of patients with HF and ischemic heart disease through two main electrolyte homeostatic actions. Firstly, overproduction of aldosterone leads to inappro- priate levels of sodium and water retention, which sets up a vicious cycle whereby the kidney attempts to com- pensate for impaired cardiac output by releasing angio- tensin II, leading to further rises in aldosterone and volume overload. Secondly, aldosterone increases the urinary excretion of magnesium and potassium leading to electrolyte imbalance, increasing the risks of ven- tricular arrhythmias—which in the presence of myocar- dial remodelling following MI or secondary to HF, in- creases risk of sudden death [44]. In several pathological conditions aldosterone promotes vascular damage by formation of reactive oxygen species [45]. Moreover, clinical and experimental data indicate that aldosterone and its receptor are implicated in various non-renal loca- tions [41], in particular the heart [35], brain tissue [46], and within the vasculature [47], suggesting that aldos- terone also exerts local effects in tissue levels. Aldosterone exerts most of its biological effects on cells by occupying an intracellular receptor, termed the type I or mineralocorticoid receptor (MR), which then binds DNA and thereby influences transcription of vari- ous genes [38-49]. However, not all of the actions of aldosterone are mediated by the classic genomic path- way involving transcription and translation. There is experimental evidence that aldosterone in a number of circumstances can stimulate an increase in intracellular calcium concentration and cause vasoconstriction by a mechanism which involves protein kinase C (PKC), and which may be independent of the classical MR. Despite pharmacological antagonism of aldosterone, which is nowadays part of a commonly applied standard therapy, could markedly reduce myocardial injury, cerebral hem- orrhage and renal vascular disease, most of the non-classical effects are insensitive to inhibitors of the classical cytosolic mineralocorticoid receptor [50]. Moreover, this type of chemical also has some adverse effect such as breast tenderness with or without gyneco- mastia. It occurs more commonly in men but not exclu- sively. Studies indicate that approximately 10% of men will complain of breast tenderness with use spironolac- tone at the 25 mg dose. Other adverse effects that are associated with spironolactone include sexual dysfunc- tion and menstrual irregularities [51]. 2.3. Evidences of Quinoxaline 1, 4-Dioxides in Aldosterone Production Experimental studies in pigs fed with carbadox, olaquindox, and cyadox had revealed obvious decreases in aldosterone and sodium concentrations in blood, with increases in potassium levels at 150 mg/kg or above [52-53]. As these drugs are given continuously, the bio- genesis of aldosterone in vivo is likely to be seriously impaired, even when used in the advised dosages. Pigs treated with 100 and 200 mg/kg carbadox showed a sig- nificant decline of aldosterone after five and three weeks, respectively. With olaquindox a continuous, significant decline was found from 50 mg/kg and above after five weeks. In the cyadox groups, a significant decline was measured after six weeks in the 50, 200 and 400 mg/kg groups [52]. The animals might compensate with en- hanced production via the alternative pathway and/or by enhanced release of other corticosteroids with mineralo- corticosteroid activity. The rapid shutdown of the mito- chondrial biogenesis of corticosteroids by QdNOs will then lead to hypoaldosteronism [51,54]. Moreover, the aldosterone decline effect was also seen in vitro. Spier- enburg et al. investigated that the carbadox had the abil- ity to inhibition of aldosterone production by pig adrenal slices. A dose dependent decrease in aldosterone produc- tion was reported at the 1~40 µg/ml [55]. In a study us- ing a suspension of porcine adrenocortical cells, QdNOs were found to reduce the output of aldosterone. The car- badox and their major metabolites induced a slowly de- veloping but virtually irreversible inhibition of the C18 transformations from corticosterone to aldosterone. But these compounds hardly affected the alternative pathway from deoxycortisol [17-18]. Obviously, the information obtained above is rather insufficient to interpret the mechanisms of QdNOs on aldosterone production related gene expression and en- zymes interactions. More detailed investigation is re- quired to delineate the pathophysiological linkage among QdNOs exposure, aldosterone synthesis, and aldosterone dysmetabolism diseases. Recently, our pre- vious work in vivo [56-57] and in vitro [57] have sug- gested that high dose of QdNOs could lead to adrnal gland impaiment and aldosterone down-regulation, combined with sodium decrease and potassium increase in rat plasma. It’s demonstrated that a complex crosstalk between ROS-generating system and aldosterone secre- tion. The induction of oxidative stress following expo- sure to mequindox (MEQ) could result in a membrane damage and dysfunction of adrenal mitochondrion, and then cause the contents loss of steroid hormone includ- ing CYP11A1, CYP11B1 and CYP11B2, finally leading to the deregulation of steroid hormone secretion and unbalance of ion concentration [55]. Moreover, Higher doses of MEQ showed similar depressive responsibility for most of renin-angiotensin-aldosterone system  D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 124 (RAAS) components in adrenal and kidney, indicating a down-regulation of both intra- and extra-RAAS, the up- stream of aldosterone production [58]. 2.4. Nucleotide Similarity of Aldosterone Biosynthesis Enzymes in Human and Pig Although exposure to QdNOs would lead to deregula- tion of aldosterone production in pigs, little information is known regarding their influence on human body. To evaluate the potential role of QdNOs in human aldos- terone production, a multiple sequence alignment of the full length nucleotide sequence of CYP11A (GeneID: Sus scrofa 403329; Homo sapiens 1583), CYP21 (GeneID: Sus scrofa 403337; Homo sapiens 1589)and 3β-HSD (GeneID: Sus scrofa 445539; Homo sapiens 3284) gene were identified by performing BLASTN searches in GenBankTM. Sus scrofa and Homo sapiens shared 84% identity with CYP11A1, 84% identity with CYP21A and 79% identity with 3β-HSD, respectively. The great simi- larity of aldosterone biosynthesis enzymes between hu- man and pig confirmed the possibility that QdNOs in- fluence aldosterone synthesis enzymes in human body. As QdNOs and their major metabolites induced a special decline of aldosterone production from the swine adrenal in vivo and in vitro, this can also be expected to be the case for human. 3. DISCUSSION Based on above information, there is plenty of evi- dence to support the notion that aldosterone is an inde- pendent risk factor for brain, renal and heart disease. QdNOs may be helpful for the diseases caused by ele- vated levels of aldosterone. Thus we focus on the hy- pothesis that QdNOs could also cause aldosterone defi- ciency in human body and be beneficial for aldosterone dysmetabolism disease, which may suggest a newly candidate remedy for cerebrovascular system, renal and cardiovascular disease. Although treatment with a mineralocorticoid receptor antagonist promotes blood pressure (BP) reduction and regresses target organ damage, they may be useless for non-genomic aldosterone action in clinical studies par- ticularly in the cardiovascular system [49]. QdNOs have an effect independent of MR and perhaps act on the up- stream cascade of aldosterone production, which is to- tally different with mineralocorticoid receptor antagonist. Further research on QdNOs may improve the under- standing of their participation in the pathogenesis of aldosterone related diseases and eventually enhance the options for therapy. There are at least three proposed mechanisms of QdNOs mediated in aldosterone deficiency and heart disease. Firstly, we postulate that the mechanism by which QdNOs inhibit the production of aldosterone, mainly by regulating some key enzymes necessary for aldosterone biosynthesis. To make known whether it is true, interaction of QdNOs with aldosterone associated physiological regulators, enzymes and cellular second messengers should be investigate to further discuss their mechanisms. Secondly, as most of enzymes involved in the biogenesis of adrenal steroids are also present in other steroid hormone producing organs, aldosterone produced within brain or heart was suggested to be similarly influenced by QdNOs. A comparable differen- tial effect of QdNOs on the biogenesis and release of steroid hormones is to be expected. Lastly and most im- portantly, the chronic and continuously down regulation of aldosterone level in plasma and/or heart tissue caused by QdNOs would lead a directly shutdown of MR affini- ties, which may be an important direction in the therapy for heart diseases. Future pre-clinical and clinical studies associated with the precise mechanisms for QdNOs on heart diseases are anticipated. These studies should cir- cumvent (1) appropriate pathological models in vivo and in vitro, (2) secure evaluation of QdNOs for their toxic- ity and adverse effects on human or other animals, (3) comparison between QdNOs and aldosterone antagonists such as eplerenone,K+-canrenoate or spironolactone, the agents being currently used clinically [59]. All of the above hypothesis are testable in lab or in clinical. Although QdNOs are known to their adrenal or other toxicities [19,21,60], their adverse effects can be avoided or decreased by controlling the dose. This aldosterone inhibition effect of QdNOs occurs in concentrations well below toxicity concentration found in vivo treatment. Moreover, some QdNOs such as cyadox is relatively less toxic and effective member of this family. Cyadox has been shown to be a very safe drug when used as a feed additive, and thus should be taken more attention than others. 4. CONCLUSIONS It is meaningful to investigate the profitable role of QdNOs in cardiovascular or other diseases caused by metabolism dysfunction of aldosterone, which will lead to a further study in this kind of chemical. If the hy- pothesis is true, it may provide a new option into the therapy for aldosterone dysmetabolism disease, espe- cially in cardiovascular system, and thus assume a broader application of QdNOs. REFERENCES [1] Suter, W., Rosselet, A. and Knusel, F. (1978) Mode of  D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 125 action of quindoxin and substituted quinoxaline-di-N- oxides on Escherichia coli. Antimicrob Agents Che- mother, 13, 770-783. [2] Bertschinger, H.U. (1976) Die chemotherapeutische Wirksamkeit von Olaquindox bei Ferkeln mit experi- menteller Colidiarhoe und Colienterotoxamie. Schweiz Arch Tierheilkd, 118, 397-407. [3] Coulthard, C.E. and Hale, L.J. (1955) The treatment of experimental bacillary infections of mice with quinoxa- line 1:4 di-N-oxide. British Journal of Pharmacol, 10, 394-396. [4] Hennessy, T.D. and Edwards, J.R. (1972) Antibacterial properties of quindoxin: A new growth promoting agent. Veterinary Record, 90, 187-191. doi:10.1136/vr.90.7.187 [5] Carta, A., Corona, P. and Loriga, M. (2005) Quinoxaline 1, 4-dioxide: A versatile scaffold endowed with manifold activities. Current Medical Chemistry , 12, 2259-2272. doi:10.2174/0929867054864831 [6] Hoskin, B.D. and Lyon, P.M. (1972) The effect of quin- doxin on the growth of broiler chickens. Veterinary Re- cord, 90, 396-399. doi:10.1136/vr.90.14.396 [7] Kirchgessner, M. and Roth, F.X. (1977) Olaquindoxein neuer Wachstumspromotor in der Tierernahrung. III. Zur Wirksamkeit in der Kalbermast. Z. Tierphysiol. Tierer- naehr Futtermittelkd, 38, 23-28. doi:10.1111/j.1439-0396.1977.tb00204.x [8] Qiu, Y.S., Yuan, Z.H., Fan, S.X., Liu, D.C., Huang, L.L. and Yin, J.Y. (2003) Development of liquid chroma- tographic methods for determination of cyadox and its main metabolites in edible tissues and serum of swine. Chinese Journal of Veterinary Science, 23, 363-365. [9] Gali-Muhtasib, H.U., Diab-Assaf, M. and Haddadin, M.J. (2005) Quinoxaline 1, 4-dioxides induce G2/M cell cycle arrest and apoptosis in human colon cancer cells. Cancer Chemother Pharmacol, 55, 369-378. doi:10.1007/s00280-004-0907-x [10] KangAe, L., obert, A.R and John, J.L. (2007) Hypoxia, drug therapy and toxicity. Pharmacology Therapeutics, 113, 229-246. doi:10.1016/j.pharmthera.2006.08.001 [11] Amin, K.M., Ismail, M.M.F., Noaman, E., Soliman, D.H. and Ammar, Y.A. (2006) New quinoxaline 1, 4-di-N-oxi- des, Part 1: Hypoxia-selective cytotoxins and anticancer agents derived from quinoxaline 1, 4-di-N-oxides. Bio- organic Medicinal Chemistry, 14, 6917-6923. doi:10.1016/j.bmc.2006.06.038 [12] Gali-Muhtasib, H.U., Haddadin, M.J., Rahhal, D.N. and Younes, I. (2001) Quinoxaline 1, 4-dioxides as anticancer and hypoxiaselective drugs. Oncology Reports, 8, 6- 79-684. [13] Zanetti, S., Sechi, L.A., Molicotti, P., Cannas, S., Bua, A., Deriu, A., Carta, A. and Paglietti, G. (2005) In vitro ac- tivity of new quinoxalin 1, 4-dioxide derivatives against strains of Mycobacterium tuberculosis and other myco- bacteria. International Journal of Antimicrob Agents, 25, 179-181. doi:10.1016/j.ijantimicag.2004.11.003 [14] Montoya, M.E., Sainz, Y., Ortega, M.A., Lopez, D.e., Cerain, A. and Monge, A. (1998) Synthesis and anti-tuberculosis activity of some new 2-quinoxaline carbonitriles. Farmaco, 53, 570-573. doi:10.1016/S0014-827X(98)00067-6 [15] Ortega, M.A., Sainz, Y., Montoya, M.E., Jaso, A., Zarranz, B., Aldana, I. and Monge, A. (2002) Anti- Mycobacterium tuberculosis agents derived from quin- oxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1, 4-di-N-oxide. Arzneimittelforschung, 52, 113-119. [16] Aguirre, G., Cerecetto, H., Maio, R.D., González, M., Alfaro, M.E.M., Jaso, A., et al. (2004) Quinoxaline N, N′-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity rela- tionships. Bioorganic Medicinal Chemistry Letters, 14, 3835-3839. doi:10.1016/j.bmcl.2004.04.088 [17] Jager, L.P., de Graaf, G.J., Widjaja-Greefkes, H.C.A. (1996) Screening for drug-induced alterations in the production and release of steroid hormones by porcine adrenocortical cells in vitro. Toxicol in vitro, 10, 595-608. doi:10.1016/S0887-2333(96)00047-1 [18] Jager, L.P., de Graaf, G.J., Widjaja-Greefkes, H.C.A., Accord-Burleson, C.C.F., Van, den Dungen, H.M. and Baars, A.J. (1994) Effects of feed additives and veteri- nary drugs on aldosterone production and release by por- cine adrenal cells in vitro. Journal of Veterinary Phar- macology and Therapeutics, 17, 175-185. doi:10.1111/j.1365-2885.1994.tb00231.x doi:10.1016/0021-9975(88)90030-8 [20] Spierenburg, T.J., Baars, A.J., de Graaf, G.J., Jager, L.P. (1988) Carbadox-induced inhibition of aldosterone pro- duction in porcine adrenals in vitro. Toxicol in vitro, 2, 1- 41-143. doi:10.1016/0887-2333(88)90026-4 [21] van de Kerk, P. (1985) Drug toxicity in piglets following long-term consumption of medicated feed. Tijdschr Dier- geneeskd, 11 0, 181-184. [22] Rigsby, C.S., Cannady, W.E. and Dorrance, A.M. (2005) Aldosterone: Good guy or bad guy in cerebrovascular di- sease? Trends Endocrinol Metab, 16, 401-406. doi:10.1016/j.tem.2005.09.002 [23] Epstein, M. (2001) Aldosterone as a mediator of progres- sive renal disease: Pathogenetic and clinical implications. American Jorunal of Kidney Diseases, 37, 677-688. doi:10.1016/S0272-6386(01)80115-3 [24] Tan, L.B., Schlosshan, D. and Barker, D. (2004) Fiftieth anniversary of aldosterone: From discovery to cardio- vascular therapy. International Journal of Cardiology, 96, 321-333. doi:10.1016/j.ijcard.2004.05.004 [25] Cohn, J.N. and Colucci, W. (2006) Cardiovascular Ef- fects of Aldosterone and Post-Acute Myocardial Infarc- tion Pathophysiology. American Jorunal of Cardiology, 97, 4-12. doi:10.1016/j.amjcard.2006.03.004 [26] Stocco, D.M. (2001) StAR protein and the regulation of steroid hormone biosynthesis. Annual Review of Physi- ology, 63, 193-213. doi:10.1146/annurev.physiol.63.1.193 [27] White, P.C. (2004) Aldosterone synthase deficiency and related disorders. Molecular and Cellular Endocrinology, 217, 81-87. doi:10.1016/j.mce.2003.10.013 [28] Lisurek, M., Bernhardt, R. (2004) Modulation of aldos- terone and cortisol synthesis on the molecular level. Mo- lecular Cellular Endocrinology, 25, 149-159. doi:10.1016/j.mce.2003.11.008 [29] Erdmann, B., Gerst, H., Lippoldt, A., Bülow, H., Ganten, D., Fuxe, K., et al. (1996) Expression of cytochrome P45011B1 in the brain of normal hypertensive transgenic  D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 126 rats. Brain Research, 733, 73-82. doi:10.1016/0006-8993(96)00540-9 [30] MacKenzie, S.M., Clark, C.J., Fraser, R., Gome- z-Sanchez, C.E., Connell, J.M. and Miller, W.L. (1988) Molecular biology of steroid hormone synthesis. Endo- crine Reviews, 9, 295-318. doi:10.1210/edrv-9-3-295 [31] Rudolph, A.E., Blasi, E.R. and Delyani, J.A. (2000). Tissue-specific corticosteroidogenesis in the rat. Mo- lecular and Cellular Endocrinology, 165, 221-224. doi:10.1016/S0303-7207(00)00258-6 [32] Yoshimura, M., Nakamura, S., Ito, T., Nakayama, M., Harada, E., Mizuno, Y., et al. (2002) Expression of al- dosterone synthase gene in failing human heart: Quanti- tative analysis using modified real-time polymerase chain reaction. Journal of Clinical Endocrinology and Metabolism, 87, 3936-3940. doi:10.1210/jc.87.8.3936 [33] Xiao, F., Puddefoot, J.R. and Vinson, G.P. (2000) Aldos- terone mediates angiotensin Ⅱ-stimulated rat vascular smooth muscule cell proliferation. Journal of Endocri- nology, 165, 533-536. doi:10.1677/joe.0.1650533 [34] Slight, S.H., Joseph, J., Ganjam, V.K. and Weber, K.T. (1999) Extra-adrenal mineralocorticoids and cardiovas- cular tissue. Journal of Molecular Cellular Cardiology, 31, 1175-1184. doi:10.1006/jmcc.1999.0963 [35] Silvestre, J.S., Robert, V., Heymes, C., AupetitFaisant, B., Mouas, C., Moalic, J.M., et al. (1998) Myocardial pro- duction of aldosterone and corticosterone in the rat. Physiological regulation, Journal of Biology Chemistry, 273, 4883-4891. doi:10.1074/jbc.273.9.4883 [36] Ishibashi, T., Satoh, F., Yamada, T., Sato, A., Matsuhashi, T. and Takase, K. (2007) Primary aldosteronism: A picto- rial essay. Abdom Imaging, 32, 504-514. doi:10.1007/s00261-006-9151-7 [37] Young, W.F. (2003) Minireview: Primary aldostero- nism-changing concepts in diagnosis and treatment. En- docrinology, 144, 2208-2213. doi:10.1210/en.2003-0279 [38] Calhoun, D.A. (2006) Aldosteronism and Hypertension. Clinical Journal of the American Society of Nephrology, 1, 1039-1045. doi:10.2215/CJN.01060306 [39] Cohn, J.N. and Colucci, W. (2006) Cardiovascular Ef- fects of Aldosterone and Post-Acute Myocardial Infarc- tion Pathophysiology. American Journal of Cardiology, 97, 4-12. doi:10.1016/j.amjcard.2006.03.004 [40] Brown, N.J. (2005) Aldosterone and end-organ damage. Current Opinion in Nephrology and Hypertension, 14, 235-241. doi:10.1097/01.mnh.0000165889.60254.98 [41] Rocha, R.C.T., Stier, Jr. (2001) Pathophysiological ef- fects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab, 12, 308-314. doi:10.1016/S1043-2760(01)00432-5 [42] Danser, A.H., van Kesteren, C.A., Bax, W.A., Tavenier, M., Derkx, F.H.M. and Saxena, P.R., et al. (1997) Pror- enin, renin, angiotensinogen, and angio-tensin-converting enzyme in normal and failing human hearts. Evidence for renin binding Circulation, 96, 220-226. [43] DahlÖf, B. (1995) Effect of angiotensin II receptor blockade on cardiac hypertrophy and remodelling: A re- view. Journal of Human Hypertension, 9, 37-44. [44] Tan, L.B., Schlosshan, D. and Barker, D. (2004) Fiftieth anniversary of aldosterone: From discovery to cardio- vascular therapy. International Journal of Cardiology, 96, 321-333. doi:10.1016/j.ijcard.2004.05.004 [45] Skøtt, O., Uhrenholt, T.R., Schjerning, J., Hansen, P.B.L., Rasmussen, L.E. and Jensen, B.L. (2006) Rapid actions of aldosterone in vascular health and disease—friend or foe? Pharmacology Therapeutics, 111, 495-507. [46] Patel, P.D., Sherman, T.G., Goldman, D.J., Watson, S.J. (1989) Molecular cloning of a mineralocorticoid (type I) receptor complementary DNA from rat hippocampus. Molecular Endocrinology, 3, 1877-1185. doi:10.1210/mend-3-11-1877 [47] Takeda, Y., Miyamori, I., Inaba, S., Furukawa, K., Hata- keyama, H., Yoneda, T., et al. (1997) Vascular aldoster- one in genetically hypertensive rats. Hypertension, 29, 45-48. [48] Loffing, J., Summa, V., Zecevic, M. and Verrey, F. (2001) Mediators of aldosterone action in the renal tubule. Cur- rent Opinion in Nephrology and Hypertension, 10, 667-675. doi:10.1097/00041552-200109000-00019 [49] Arriza, J.L., Weinberger, C., Cerelli, G., Glaser, T.M., Handelin, B.L., Housman, D.E., et al. (1987) Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science, 237, 268-275. doi:10.1126/science.3037703 [50] Lösel, R., Schultz, A. and Wehling, M. (2004) A quick glance at rapid aldosterone action. Molecular Cellular Endocrinology, 217, 137-141. doi:10.1016/j.mce.2003.10.018 [51] Nishizaka, M.K., Zaman, M.A. and Calhoun, D.A. (2003) Efficacy of low-dose spironolactone in subjects with re- sistant hypertension. American Journal of Hypertens, 6, 925-930. doi:10.1016/S0895-7061(03)01032-X [52] van der Molen, E.J., De Graaf, G.J., Spierenburg, T.J., Nabuurs, M.J.A., Baars, A.J. and Jager, L.P. (1986) Hyp- oaldosteronism in piglets induced by carbadox. Expe- rientia, 42, 1247-1249. doi:10.1007/BF01946407 [53] van der Molen, E.J., Baars, A.J., De Graaf ,G.J. and Jager, L.P. (1989) Comparative study of the effect of carbadox, olaquindox and cyadox on aldosterone, sodium and po- tassium plasma levels in weaned pigs. Research in Vet- erinary Science, 47, 11-16. [54] van der Molen, E.J., de Graaf, G.J. and Baars, A.J. (1989) Persistence of carbadox-induced adrenal lesions in pigs following drug withdrawal and recovery of aldosterone plasma concentrations. Journal of Comparative Path ology, 100, 295-300. doi:10.1016/0021-9975(89)90107-2 [55] Spierenburg, T.J., Baars, A.J., de Graaf, G.J. and Jager, L.P. (1988) Carbadox-induced inhibition of aldosterone production in porcine adrenals in vitro. Toxicol in vitro, 2, 141-143. doi:10.1016/0887-2333(88)90026-4 [56] Huang, X.J., Ihsan, A., Wang, X., Dai, M.H., Wang, Y.L., Su, S.J., Xue, X.J. and Yuan, Z.H. (2009) Long-term dose-dependent response of Mequindox on aldosterone, corticosterone and five steroidogenic enzyme mRNA in the adrenal of male rats. Toxicology Letters, 191, 167-173. doi:10.1016/j.toxlet.2009.08.021 [57] Huang, X.J., Zhang, H.H., Wang, X., Huang ,L.L., Zhang, L.Y., Yan, C.X. and Yuan, Z.H. (2010) ROS mediated cytotoxicity of porcine adrenocortical cells induced by QdNOs derivatives in vitro. Chemico-Biologicol Interac- tions, 185, 227-234. doi:10.1016/j.cbi.2010.02.030 [58] Huang, X.J., Wang, X., Ihsan, A., Liu, Q., Xue, X.J., Su, S.J., Yang, C.H. and Yuan, Z.H. (2010) Interactions of  D.-J. Zou et al. / Open Journal of Animal Sciences 1 (2011) 121-127 Copyright © 2011 SciRes. http://www.scirp.org/journal/OJAS/ Openly accessible at 127 NADPH oxidase, renin-angiotensin-aldosterone system and reactive oxigen species in mequindox-mediated al- dosterone secretion in wistar. Toxicology Letters, 198, 112-118. doi:10.1016/j.toxlet.2010.05.013 [59] Ajani, A.E., Marwick, T.H. and Krum, H. (2006) Aldos- terone antagonists: A new treatment option for patients with post-myocardial infarction heart failure. Cardio- vascular Revascularization Medicine, 7, 234-236. doi:10.1016/j.carrev.2006.07.002 [60] FAO/WHO (1990) Joint Expert Committee on Food Ad- ditives: Evaluation of certain veterinary drug residues in food. Technology Services, 799, 45.
|