 Vol.1, No.3, 75-88 (2011) doi:10.4236/ojas.2011.13010 C opyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ Open Journal of Animal Sciences Meat quality in “in door” and “out door” production systems of poultry and swine W agner A. G. Araújo1*, Luiz F. T. Albino1, Nilva K. Sakomura2, Pedro V. R. Paulino1, Anastacia M. Campos1 1Animal Science Department/Departamento de Zootecnia, Federal University of Viçosa (UFV), Viçosa, MG., Brazil; *Corresponding Author: azisoo@yahoo.com.br 2Animal Science Department/Departamento de Zootecnia, State University of São Paulo (UNESP), Jaboticabal, SP., Brazil. Received 6 June 2011; revised 11 July 2011; accepted 2 August 2011. ABSTRACT The meat quality can be influenced by many interacting factors before and af ter the slaughter. Currently more sustainable production systems are t argeted in general, whether or not they have any effect on meat quality. The sustainability is a condition of agroecology and necessarily im- plies on the animal and plant association and succession. A condition for sustainability is to minimize or even eliminate the use of inputs from processes of chemical synthesis. In the case of pigs and poultry, this is feasible by adopting production systems that allows nutria- ents recycle directly on the soil at levels that do not involve pollution. Although we have the understanding that the general principles of sustainability to be observed are universal, the solution is not simple. For each situation a vi- able alternative must be sought, depending on the social, economic, ecological and cultural realities. In tropical and subtropical climates the production of pigs and poultry outdoors can be an appropriate option. This also leads to nutria- ents recycle and promotes a better energy bal- ance of the system. Among the alternatives that can be taken to introduce differentiating factors in meat production as food is the type of pro- duction system, due to its direct impact on the meat quality. These systems have a direct in- fluence through the consumed food, by the conditions of animal wellbeing, physical activity and the environment provided. The performance and meat quality depend on the interaction of genotypes, rearing conditions, pre-slaughter handling and processing of the meat and the carcass. The influence of the rearing system on the animal performance, on the carcass and finally on t he m eat is th e res ult of t he inter ac tive effects among facilities, feeding level and genotype used in the production systems. The production of poultry and pigs more exten sively tend to get a final product with its own or- ganoleptic characteristics, changing the meat default color and content, place of fat deposi- tion and the fatty acid profile deposited on the carcass. Keywords: Alternative Systems; Meat Quality; Poultry; Swine 1. INTRODUCTION The technological, nutritional and sensorial meat quality can be influenced by many interacting factors before and after the slaughter. Currently more sustain- able production systems are targeted in general, whether or not they have any effect on meat quality. Following the example of what happens in the world, the alternative rearing of swine and poultry in Brazil has found more supporters among the business makers, which is also an alternative for small and medium pro- ducers who aim at a more profitable market niche (De- mattê Filho & Mendes, 2001).The main desired charac- teristics in these farming systems are: health security, the organoleptic quality of the product, the growing concern over the environment, animal welfare and consumers’ health (Bastianelli, 2001). The sustainability is a condition of agroecology and necessarily implies on the animal and plant association and succession. A condition for sustainability is to minimize or even eliminate the use of inputs from proc- esses of chemical synthesis. In the case of pigs and poul- try, this is feasible by adopting production systems that allows nutrients recycle directly on the soil at levels that do not involve pollution.  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 76 Although we have the understanding that the general principles of sustainability to be observed are universal, the solution is not simple. For each situation a viable alternative must be sought, depending on the social, economic, ecological and cultural realities. In tropical and subtropical climates, such as in Brazil, the produc- tion of pigs and poultry outdoors can be an appropriate option. This also leads to nutrients recycle and promotes a better energy balance of the system. In the past few years it has been consolidated, in many countries, a consumer market willing to pay more for products with “ethical quality” (Warriss, 2000). In Brit- ain, for example, organic pork is twice the price of con- ventional and the demand is greater than the supply (Edwards, 1999). Also in Brazil there are indications that at least a portion of the consuming public is willing to pay more for organic beef and pork (Machado Filho, 2000). Among the alternatives that can be taken to introduce differentiating factors in meat production as food is the type of production system, due to its direct impact on the meat quality. These systems have a direct influence through the consumed food, by the conditions of animal wellbeing, physical activity and the environment pro- vided (Dal Bosco et al., 2002). The performance and meat quality depend on the in- teraction of genotypes, rearing conditions, pre-slaughter handling and processing of the meat and the carcass. The influence of the rearing system on the animal perform- ance, on the carcass and finally on the meat is the result of the interactive effects among facilities (type of floor, the space, environmental temperature, physical activity), feeding level and genotype used in the production sys- tems (Lebret, 2008). Some studies indicate increased water retention ca- pacity in meat produced outdoors. On the other hand, the outdoor production may further increase the meat shear force compared to conventional systems (Olsson & Pickova, 2005). The color can be affected in different ways, generating more pigmented or pale meat (depend- ing on the system), structurally affecting the meat (Ols- son & Pickova, 2005). The greater unsaturated lipid pro- file of meat produced in a system that includes foods containing polyunsaturated fatty acids (fodder) is favor- able, in relation to the nutritional meat quality, when compared to conventional total confinement (Olsson & Pickova, 2005). 1.1. Implications So the best knowledge of the organoleptic changes in pork and chicken, through alternative systems of pro- duction, could determine the encouragement of agricul- tural activities that would ensure better welfare of ani- mals through the rationale of improving the quality of the final product. From another viewpoint, these systems would fit better on little farms with family labor, making them more competitive with differentiated products of high added value. 1.2. Muscle Fiber Types and Meat Quality Understanding the growth and muscle development is one of the most important aspects in animal science, since this is the end product of systems aimed to meat production. There are two types of skeletal muscles, the red and the white. The red is composed predominantly of oxidative fibers and the white muscle is composed pre- dominantly of glycolytic fibers (Lawrie, 2005). According to Rehfeldt et al. (2000), muscle mass is mostly determined by the number of muscle fibers and the size of these fibers. Animals with more moderately sized muscle fibers produce better quality meat. During myogenesis, the number of muscle fibers is determined by genetic and environmental factors. The same authors state that after birth the total number of fibers remains constant in mammals and birds and that the increased skeletal muscle mass is mainly due to hypertrophy of fibers, together with the proliferative activity of satellite cells, which are source of new cores that are going to be embedded in the muscle fiber. According Lefaucheur & Gerrard (2000), the muscle’s weight is a function of the fiber type, relative size and length of the fiber. The growth capacity is related to the number of muscle fibers. The glycolytic fibers have higher relative volume and the increase in the proportion of these could lead to an increase in muscle weight. To understand the muscle development is essential studying the mechanisms that regulate the number, type and size of muscle fibers. These variations of the muscle fibers are related to several factors that interfere with muscle development in the prenatal and postnatal de- velopment periods (Dauncey & Gilmour, 1996). Among these factors it can be cited those intrinsic to the animals (genetic, growth regulators, transcription activators, en- docrine status, muscle proteinases and innervation ), environmental factors (diet and temperature), motor ac- tivity, agents splitters of nutrients (ractopamine in pig feed), age, sex, disease, type and location of muscle fi- bers in the muscle (Gonzales & Sartori, 2002). To understand the importance of the factors that affect growth and protein deposition in muscle, one must con- sider the impact of these factors on the quality of the muscle that will be transformed into food for human. But the meat quality is not only determined by the intrinsic (physiological) and extrinsic (environment) influences on the bird’s muscle in the period preceding his death. Once the muscle tissue is dynamic, it also responds to  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 77 environmental influences during and after the animal’s death. The type of processing, considering the slaughter and the carcass treatment, interacts with the muscle’s characteristics and, together, will determine the meat quality (Pearson & Young, 1989). Both in birds and mammals, three types of muscle fi- bers can be identified based on their metabolic and con- tractile characteristics: Type I - slow twitch and oxida- tive (SO), type IIA-fast twitch oxidative (FOG) and type IIB / X- fast twitch glycolytic (FG) (Macari et al. 2002; Lawrie, 2005). The type I fibers are small, have many mitochondria, myoglobin pigment in abundance and are well vascular- ized, which gives them their red color. The mitochondria are large and have numerous cristae. The storage of oxygen by myoglobin prevents fatigue. The type IIA fibers are medium sized with oxidative and glycolytic energy metabolism, having numerous mitochondria and myoglobin, high vascularization and are resistant to fa- tigue, while the type IIB fibers are large, with few mito- chondria and myoglobin, with glycolytic energy metabo- lism and poor vascularization , besides being easily fati- gable and easily accumulate lactic acid (Macari et al. 2002; Lawrie, 2005). The predominant muscle fiber type is especially im- portant for the carcass quality of pigs. Due to the gener- ally higher content of glycogen in type II fibers com- pared to type I, there would be a great anaerobic glycol- lytic potential on those during the post mortem. This results in sharp drop in pH, resulting in a meat called PSE (pale, soft and exudative) (Lawrie, 2005). Swines of the Piétrain breed have greater genetic ten- dency to higher prevalence of such a failure, both due to the presence of the halothane gene and the predominance of type II fibers (Lawrie, 2005). However other factors may influence the formation of the fibers, thereby changing their characteristics, such as exercise and the animal’s age (Lawrie, 2005). In general physical training (long term) may increase the myoglobin content of mus- cle fibers. In this way there is a change in their initial physical characteristics, making the fibers more likely to express aerobics features, since myoglobin becomes an intracellular oxygen reserve (Lawrie, 2005). Also Sosnicki et al. (1996) reported the importance of the presence of white fibers, affecting the meat quality, due to change in meat pH, making it acidic after slaugh- ter, due to the glycogen content of the cells that form the white muscle fibers. This implies on a loss in industrial yield from certain carcass cuts of pork, especially ham, when cooked without the addition of phosphate. Another determining factor in swine carcass quality is the content of intramuscle fat (marbling), affecting the meat appearance and taste. This has been identified as an important factor in determining the consumer preference for the consumption of pork (Plastow, 2000). In birds the chest muscle, white colored, has pre- dominantly FG and FOG fibers and histologically pre- sents low density of blood capillaries and contain few mitochondria. The red muscles, from the thigh and drumstick, on the other hand, are richly vascularized and contain a large number of mitochondria in the fibers which are mainly of the SO and FOG types (Macari et al., 2002). According to Gonzales & Sartori (2002), the distribu- tion of muscle fibers in a certain type of muscle is a functional adaptation from its various kinds of activities. Thus, the rate of different muscle fibers types in a mus- cle is directly correlated with its function in the bird’s body. In agreement, Remignon et al. (1994) reported that the frequency of different types of muscle fibers in broilers is primarily related to the muscle function. The breeding of broiler chickens for increasing muscle mass and speeding growth reduced the muscle’s oxidative capacity, resulting in a more anaerobic muscle (Soike & Bergmann, 1997). Therefore, the meat of broiler chick- ens has lighter color due to the muscles being more an- aerobics. According to Macari et al. (2002), after the bird’s death, the tissues are deprived of oxygen, resulting in anoxia. In this situation, with the continuity of their metabolic activity, the muscle fibers rely solely on the anaerobic mechanisms for the ATP production. Thus, using the glycogen that is quickly consumed. Concomi- tantly, occurs an accumulation of lactic acid, the end product of anaerobic metabolism, due to its non-removal via the bloodstream. The anaerobic metabolism reduces sarcoplasmic pH level which inhibits glycolysis, ceasing the ATP production. But the ATP consumption continues, reaching critical mass, so that the dissociation of the actin-myosin system is not performed. Due to the forma- tion of the actin-miosin complex, the muscle loses its plasticity and gets unable to relax; hence the rigor mortis takes place (Gonzales & Sartori, 2002). The time to develop the process of rigor mortis de- pends on the muscle type, the frequency of fibers types in the muscle, the speed of pH tissue fall and the envi- ronmental factors before and after slaughter that the animals were submitted (Gonzales & Sartori, 2002). Changes in the establishment of the rigor mortis may increase the meat pallor and its ability to reduce fluid retention, worsening the quality of the processed prod- ucts (Dransfield & Sosnicki, 1999). 2. FORAGES The replacement of part of the concentrate feed (diets based on corn and soybean meal) for fodder (forages), is  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 78 one of the causes of the production system influence on pigs’ and poultry’s carcass. The animals when begin to eat forage have a greater development of the gastrointes- tinal tract as an adaptation to increased dietary fiber. This is due mainly to the production of volatile fatty acids by allozyme processes (bacterial activity), espe- cially in the cecum of these animals. However only a small portion of the energy required for both mainte- nance and production is resulting from such sources, being essential the concentrate feeding to not compro- mise the production index. Also due to the low protein content of these sources and low nutritional contribution of bacterial protein in the nutrition of monogastric ani- mals, it becomes necessary the protein nutrition almost in its entirety coming from the concentrated feed. One of the main influences is the lipid type contained in these forages and how they are deposited on the car- cass. The total lipids content in forages is variable (4% - 12% of the dry matter), being greater the younger they are, with the highest proportion in the leaves, and from these the majority are chloroplast lipids. The latter may have an impact mainly on aspects related to the meat color. The fatty acid composition of forages is characterized by a high percentage of polyunsaturated acids, particu- larly linolenic acid (C18: 3 Ω-3), which represents over 50% of total fatty acids, followed by linoleic acid (C18: 2 Ω-6, 10% - 20%).They present a relationship Ω-6/Ω-3 of 0.20, whereas the content of saturated fatty acids (10% - 20%) and monounsaturated fatty acids (1% - 17%) is low. The forages are rich in linoleic acid and low in satu- rated lipids when young and rich in oleic acids in vege- tative stages more advanced (Morand-Fehr & Tran, 2001). Moreover, the quality of the adipose tissue in relation to its nutritional value, organoleptic characteris- tics and conservation is related to its fatty acid composi- tion (Lizardo et al., 2002). The composition of lipids in the carcass of pigs and poultry is influenced by several factors, such as geno- type, sex, age, animal’s weight (Girard et al., 1988), the location of fat deposition in the carcass (Miller et al. 1990), environmental temperature (Katsumata et al. 1995) and especially nutrition. The latter is mainly in- fluenced by the energy and fat content in the diet, fatty acid composition and daily fatty acids consumption (Wiseman & Agunbiade, 1998). In their study, Ponte et al. (2008), broilers of the Red- Bro Cou Nu X RedBro M genotype were fed on a ce- real-based diet in portable floorless pens located either on subterranean clover (Trifolium subterraneum) pas- tures and without access to pasture. Unlike the imagined, the pasture intake had a low impact on the fatty acid and vitamin E homologue profiles of meat from free-range broilers. However, breast meat from birds with free ac- cess to pasture presented lower levels of the n-6 and n-3 fatty acid precursors linoleic acid (18:2n-6) and α-lino- lenic acid (18:3n-3), respectively. In the same work, con- trary to other results, the levels of eicosapentaenoic acid (20:5n-3) in breast meat were significantly greater in birds consuming pastures, which suggests greater con- version of α-linolenic acid into eicosapentaenoic acid in these birds. 3. SWINE PRODUCTION The production of pigs outdoors offers a greater vari- ety of environments for animals and greater freedom of behavior, but poses challenges for the breed adaptation, management control, biosafety and ambience. Each of these has potential implications for the real and per- ceived quality of the product. According to Edwards (2005) in most conventional production systems of northern Europe only adult ani- mals and infants remain in the pasture. However, in the Mediterranean traditional systems and in organic pro- duction systems, animals can be kept throughout his life outdoors. Influences on the organoleptic quality of products derive from the choice of breeds better suited to outdoor systems, changes to the rate of growth and in- creased proportion of roughage on the diet. According to Honeyman (2005) recently in the United States the production of pigs outdoors using tents has been used for finishing pigs and gestating sows. The seasonal outdoor lambing and indoor (winter/summer) in some systems, along with lactation group has also been used, as the example of the French system (plein air). Most of the meat markets, mainly in Europe, has re- quired adjustments in order to encourage the use of out- door systems or straw, the withdrawal of subtherapeutic doses of antibiotic growth promoters and there are still a encourage to a family environment agricultural produc- tion (Honeyman, 2005). Indirect consequences would have positive and nega- tive influences on the physiological responses to stress during pre-slaughter (Edwards, 2005). Although some aspects of animal health and hygiene can be improved, in broader conditions, the exposure to parasites and con- tact with wild animals may increase the risk of zoonotic infection (Edwards, 2005). Product quality attributes can be influenced positively or negatively, depending on the consumers’ opinion, the quality of management and the creation of units of pigs outdoors visible to the public as marketing, reiterating the argument of the ethical quality of the product. 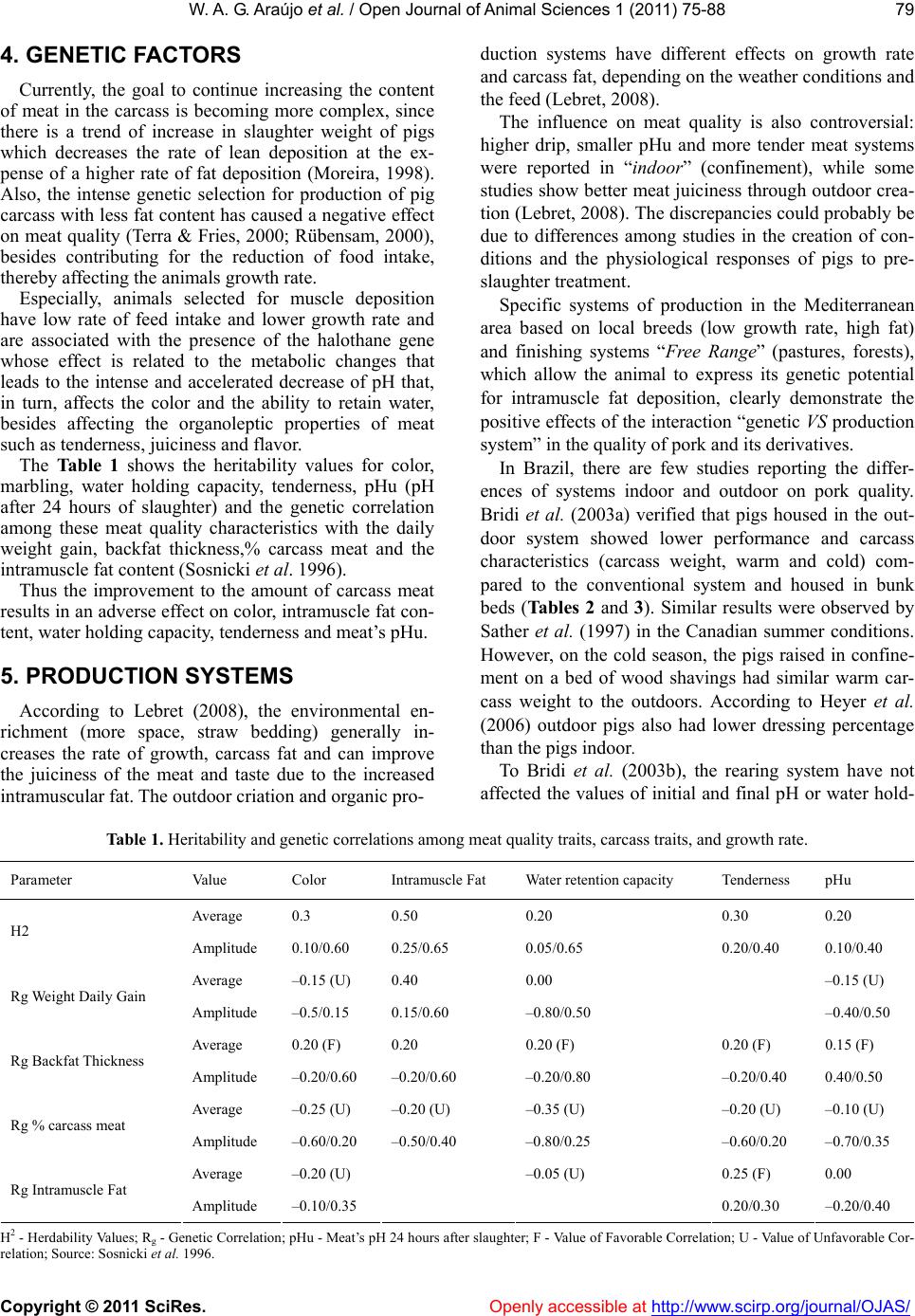 W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. http://www.scirp.org/journal/OJAS/Openly accessible at 79 4. GENETIC FACTORS Currently, the goal to continue increasing the content of meat in the carcass is becoming more complex, since there is a trend of increase in slaughter weight of pigs which decreases the rate of lean deposition at the ex- pense of a higher rate of fat deposition (Moreira, 1998). Also, the intense genetic selection for production of pig carcass with less fat content has caused a negative effect on meat quality (Terra & Fries, 2000; Rübensam, 2000), besides contributing for the reduction of food intake, thereby affecting the animals growth rate. Especially, animals selected for muscle deposition have low rate of feed intake and lower growth rate and are associated with the presence of the halothane gene whose effect is related to the metabolic changes that leads to the intense and accelerated decrease of pH that, in turn, affects the color and the ability to retain water, besides affecting the organoleptic properties of meat such as tenderness, juiciness and flavor. The Table 1 shows the heritability values for color, marbling, water holding capacity, tenderness, pHu (pH after 24 hours of slaughter) and the genetic correlation among these meat quality characteristics with the daily weight gain, backfat thickness,% carcass meat and the intramuscle fat content (Sosnicki et al. 1996). Thus the improvement to the amount of carcass meat results in an adverse effect on color, intramuscle fat con- tent, water holding capacity, tenderness and meat’s pHu. 5. PRODUCTION SYSTEMS According to Lebret (2008), the environmental en- richment (more space, straw bedding) generally in- creases the rate of growth, carcass fat and can improve the juiciness of the meat and taste due to the increased intramuscular fat. The outdoor criation and organic pro- duction systems have different effects on growth rate and carcass fat, depending on the weather conditions and the feed (Lebret, 2008). The influence on meat quality is also controversial: higher drip, smaller pHu and more tender meat systems were reported in “indoor” (confinement), while some studies show better meat juiciness through outdoor crea- tion (Lebret, 2008). The discrepancies could probably be due to differences among studies in the creation of con- ditions and the physiological responses of pigs to pre- slaughter treatment. Specific systems of production in the Mediterranean area based on local breeds (low growth rate, high fat) and finishing systems “Free Range” (pastures, forests), which allow the animal to express its genetic potential for intramuscle fat deposition, clearly demonstrate the positive effects of the interaction “genetic VS production system” in the quality of pork and its derivatives. In Brazil, there are few studies reporting the differ- ences of systems indoor and outdoor on pork quality. Bridi et al. (2003a) verified that pigs housed in the out- door system showed lower performance and carcass characteristics (carcass weight, warm and cold) com- pared to the conventional system and housed in bunk beds (Tables 2 and 3). Similar results were observed by Sather et al. (1997) in the Canadian summer conditions. However, on the cold season, the pigs raised in confine- ment on a bed of wood shavings had similar warm car- cass weight to the outdoors. According to Heyer et al. (2006) outdoor pigs also had lower dressing percentage than the pigs indoor. To Bridi et al. (2003b), the rearing system have not affected the values of initial and final pH or water hold- Table 1. Heritability and genetic correlations among meat quality traits, carcass traits, and growth rate. Parameter Value Color Intramuscle Fat Water retention capacity Tenderness pHu Average 0.3 0.50 0.20 0.30 0.20 H2 Amplitude 0.10/0.60 0.25/0.65 0.05/0.65 0.20/0.40 0.10/0.40 Average –0.15 (U) 0.40 0.00 –0.15 (U) Rg Weight Daily Gain Amplitude –0.5/0.15 0.15/0.60 –0.80/0.50 –0.40/0.50 Average 0.20 (F) 0.20 0.20 (F) 0.20 (F) 0.15 (F) Rg Backfat Thickness Amplitude –0.20/0.60 –0.20/0.60 –0.20/0.80 –0.20/0.40 0.40/0.50 Average –0.25 (U) –0.20 (U) –0.35 (U) –0.20 (U) –0.10 (U) Rg % carcass meat Amplitude –0.60/0.20 –0.50/0.40 –0.80/0.25 –0.60/0.20 –0.70/0.35 Average –0.20 (U) –0.05 (U) 0.25 (F) 0.00 Rg Intramuscle Fat Amplitude –0.10/0.35 0.20/0.30 –0.20/0.40 H2 - Herdability Values; Rg - Genetic Correlation; pHu - Meat’s pH 24 hours after slaughter; F - Value of Favorable Correlation; U - Value of Unfavorable Cor- relation; Source: Sosnicki et al. 1996. 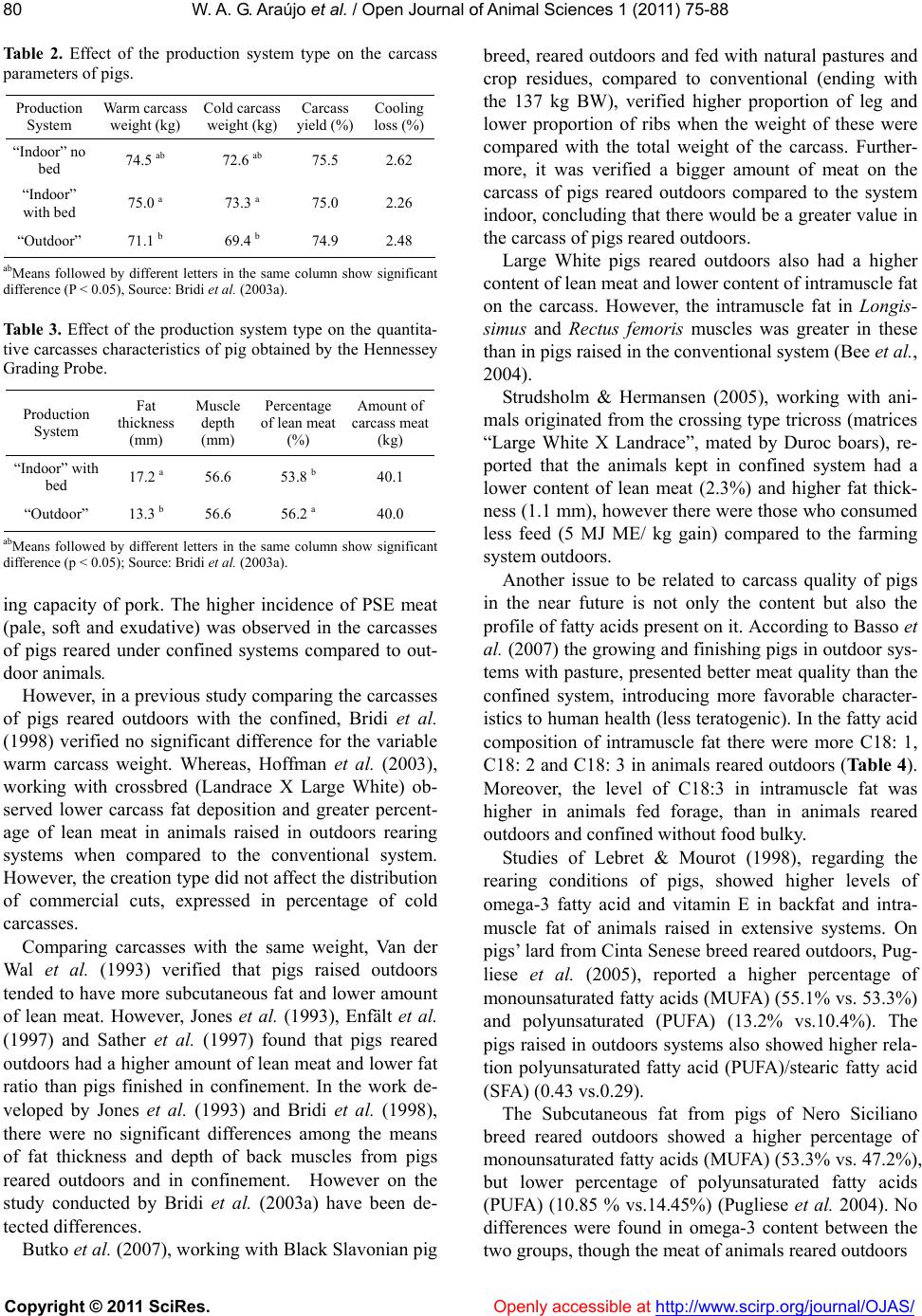 W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 80 Table 2. Effect of the production system type on the carcass parameters of pigs. Production System Warm carcass weight (kg) Cold carcass weight (kg) Carcass yield (%) Cooling loss (%) “Indoor” no bed 74.5 ab 72.6 ab 75.5 2.62 “Indoor” with bed 75.0 a 73.3 a 75.0 2.26 “Outdoor” 71.1 b 69.4 b 74.9 2.48 abMeans followed by different letters in the same column show significant difference (P < 0.05), Source: Bridi et al. (2003a). Table 3. Effect of the production system type on the quantita- tive carcasses characteristics of pig obtained by the Hennessey Grading Probe. Production System Fat thickness (mm) Muscle depth (mm) Percentage of lean meat (%) Amount of carcass meat (kg) “Indoor” with bed 17.2 a 56.6 53.8 b 40.1 “Outdoor” 13.3 b 56.6 56.2 a 40.0 abMeans followed by different letters in the same column show significant difference (p < 0.05); Source: Bridi et al. (2003a). ing capacity of pork. The higher incidence of PSE meat (pale, soft and exudative) was observed in the carcasses of pigs reared under confined systems compared to out- door animals. However, in a previous study comparing the carcasses of pigs reared outdoors with the confined, Bridi et al. (1998) verified no significant difference for the variable warm carcass weight. Whereas, Hoffman et al. (2003), working with crossbred (Landrace X Large White) ob- served lower carcass fat deposition and greater percent- age of lean meat in animals raised in outdoors rearing systems when compared to the conventional system. However, the creation type did not affect the distribution of commercial cuts, expressed in percentage of cold carcasses. Comparing carcasses with the same weight, Van der Wal et al. (1993) verified that pigs raised outdoors tended to have more subcutaneous fat and lower amount of lean meat. However, Jones et al. (1993), Enfält et al. (1997) and Sather et al. (1997) found that pigs reared outdoors had a higher amount of lean meat and lower fat ratio than pigs finished in confinement. In the work de- veloped by Jones et al. (1993) and Bridi et al. (1998), there were no significant differences among the means of fat thickness and depth of back muscles from pigs reared outdoors and in confinement. However on the study conducted by Bridi et al. (2003a) have been de- tected differences. Butko et al. (2007), working with Black Slavonian pig breed, reared outdoors and fed with natural pastures and crop residues, compared to conventional (ending with the 137 kg BW), verified higher proportion of leg and lower proportion of ribs when the weight of these were compared with the total weight of the carcass. Further- more, it was verified a bigger amount of meat on the carcass of pigs reared outdoors compared to the system indoor, concluding that there would be a greater value in the carcass of pigs reared outdoors. Large White pigs reared outdoors also had a higher content of lean meat and lower content of intramuscle fat on the carcass. However, the intramuscle fat in Longis- simus and Rectus femoris muscles was greater in these than in pigs raised in the conventional system (Bee et al., 2004). Strudsholm & Hermansen (2005), working with ani- mals originated from the crossing type tricross (matrices “Large White X Landrace”, mated by Duroc boars), re- ported that the animals kept in confined system had a lower content of lean meat (2.3%) and higher fat thick- ness (1.1 mm), however there were those who consumed less feed (5 MJ ME/ kg gain) compared to the farming system outdoors. Another issue to be related to carcass quality of pigs in the near future is not only the content but also the profile of fatty acids present on it. According to Basso et al. (2007) the growing and finishing pigs in outdoor sys- tems with pasture, presented better meat quality than the confined system, introducing more favorable character- istics to human health (less teratogenic). In the fatty acid composition of intramuscle fat there were more C18: 1, C18: 2 and C18: 3 in animals reared outdoors (Table 4). Moreover, the level of C18:3 in intramuscle fat was higher in animals fed forage, than in animals reared outdoors and confined without food bulky. Studies of Lebret & Mourot (1998), regarding the rearing conditions of pigs, showed higher levels of omega-3 fatty acid and vitamin E in backfat and intra- muscle fat of animals raised in extensive systems. On pigs’ lard from Cinta Senese breed reared outdoors, Pug- liese et al. (2005), reported a higher percentage of monounsaturated fatty acids (MUFA) (55.1% vs. 53.3%) and polyunsaturated (PUFA) (13.2% vs.10.4%). The pigs raised in outdoors systems also showed higher rela- tion polyunsaturated fatty acid (PUFA)/stearic fatty acid (SFA) (0.43 vs.0.29). The Subcutaneous fat from pigs of Nero Siciliano breed reared outdoors showed a higher percentage of monounsaturated fatty acids (MUFA) (53.3% vs. 47.2%), but lower percentage of polyunsaturated fatty acids (PUFA) (10.85 % vs.14.45%) (Pugliese et al. 2004). No differences were found in omega-3 content between the two groups, though the meat of animals reared outdoors 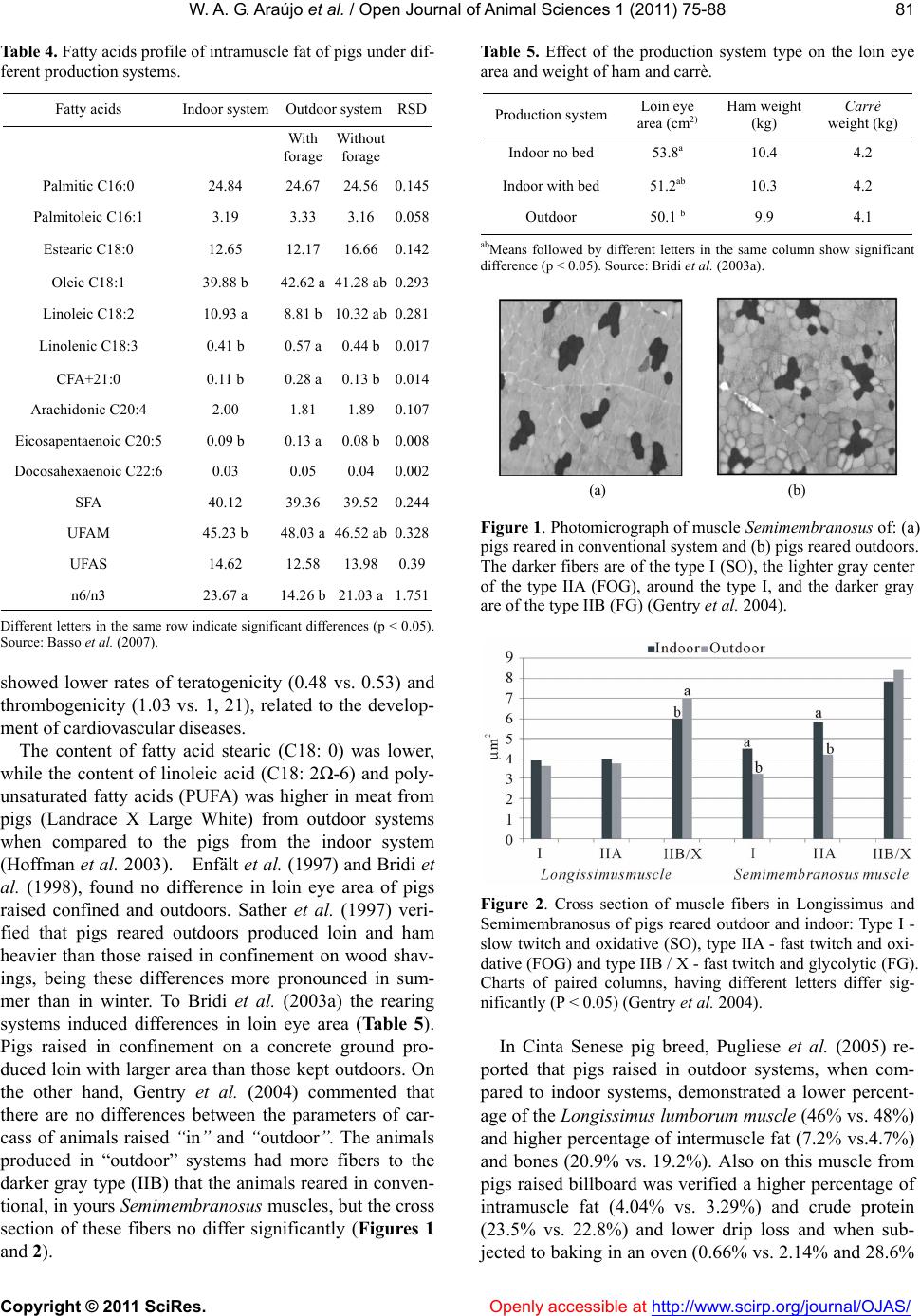 W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 81 Table 4. Fatty acids profile of intramuscle fat of pigs under dif- ferent production systems. Fatty acids Indoor system Outdoor systemRSD With forage Without forage Palmitic C16:0 24.84 24.67 24.560.145 Palmitoleic C16:1 3.19 3.33 3.16 0.058 Estearic C18:0 12.65 12.17 16.660.142 Oleic C18:1 39.88 b 42.62 a 41.28 ab0.293 Linoleic C18:2 10.93 a 8.81 b 10.32 ab0.281 Linolenic C18:3 0.41 b 0.57 a 0.44 b0.017 CFA+21:0 0.11 b 0.28 a 0.13 b0.014 Arachidonic C20:4 2.00 1.81 1.89 0.107 Eicosapentaenoic C20:5 0.09 b 0.13 a 0.08 b0.008 Docosahexaenoic C22:6 0.03 0.05 0.04 0.002 SFA 40.12 39.36 39.520.244 UFAM 45.23 b 48.03 a 46.52 ab0.328 UFAS 14.62 12.58 13.980.39 n6/n3 23.67 a 14.26 b 21.03 a1.751 Different letters in the same row indicate significant differences (p < 0.05). Source: Basso et al. (2007). showed lower rates of teratogenicity (0.48 vs. 0.53) and thrombogenicity (1.03 vs. 1, 21), related to the develop- ment of cardiovascular diseases. The content of fatty acid stearic (C18: 0) was lower, while the content of linoleic acid (C18: 2Ω-6) and poly- unsaturated fatty acids (PUFA) was higher in meat from pigs (Landrace X Large White) from outdoor systems when compared to the pigs from the indoor system (Hoffman et al. 2003). Enfält et al. (1997) and Bridi et al. (1998), found no difference in loin eye area of pigs raised confined and outdoors. Sather et al. (1997) veri- fied that pigs reared outdoors produced loin and ham heavier than those raised in confinement on wood shav- ings, being these differences more pronounced in sum- mer than in winter. To Bridi et al. (2003a) the rearing systems induced differences in loin eye area (Ta ble 5). Pigs raised in confinement on a concrete ground pro- duced loin with larger area than those kept outdoors. On the other hand, Gentry et al. (2004) commented that there are no differences between the parameters of car- cass of animals raised “in ” and “outdoor”. The animals produced in “outdoor” systems had more fibers to the darker gray type (IIB) that the animals reared in conven- tional, in yours Semimembranosus muscles, but the cross section of these fibers no differ significantly (Figures 1 and 2). Table 5. Effect of the production system type on the loin eye area and weight of ham and carrè. Production systemLoin eye area (cm2) Ham weight (kg) Carrè weight (kg) Indoor no bed 53.8a 10.4 4.2 Indoor with bed 51.2ab 10.3 4.2 Outdoor 50.1 b 9.9 4.1 abMeans followed by different letters in the same column show significant difference (p < 0.05). Source: Bridi et al. (2003a). (a) (b) Figure 1. Photomicrograph of muscle Semimembranosus of: (a) pigs reared in conventional system and (b) pigs reared outdoors. The darker fibers are of the type I (SO), the lighter gray center of the type IIA (FOG), around the type I, and the darker gray are of the type IIB (FG) (Gentry et al. 2004). Figure 2. Cross section of muscle fibers in Longissimus and Semimembranosus of pigs reared outdoor and indoor: Type I - slow twitch and oxidative (SO), type IIA - fast twitch and oxi- dative (FOG) and type IIB / X - fast twitch and glycolytic (FG). Charts of paired columns, having different letters differ sig- nificantly (P < 0.05) (Gentry et al. 2004). In Cinta Senese pig breed, Pugliese et al. (2005) re- ported that pigs raised in outdoor systems, when com- pared to indoor systems, demonstrated a lower percent- age of the Longissimus lumborum muscle (46% vs. 48%) and higher percentage of intermuscle fat (7.2% vs.4.7%) and bones (20.9% vs. 19.2%). Also on this muscle from pigs raised billboard was verified a higher percentage of intramuscle fat (4.04% vs. 3.29%) and crude protein (23.5% vs. 22.8%) and lower drip loss and when sub- jected to baking in an oven (0.66% vs. 2.14% and 28.6%  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 82 vs. 32.3% respectively) and under a high pressure in autoclave (30.3% vs. 26.6%). One of the arguments for such differences in the car- cass of these animals are the types of muscle fibers de- veloped during their lifetime. Pigs are born with a pre- dominance of type I fibers (red-SO), due to their devel- opment, these fibers change to the type IIA (FOG) and IIB / X (FOG), usually of lighter pigmentation. In gen- eral, physical activity leads to a shift of muscle fibers from the IIA, to IIB / X and from these to the type I; reduced levels of activity lead to a reversal of this path (Lefaucheur and Gerrard, 2000). According to Petersen et al. (1998) the spontaneous physical activity signify- cantly increases the proportion of fiber type IIA to IIB/X in the Longissimus muscle. In relation to muscle fiber types found in the carcass, Gentry et al. (2004) verified a higher percentage of type I fibers (SO) and lower of type IIA (FOG) in the Longis- simus muscle of pigs reared outdoors. In the same study were found lower percentage of fibers type IIB/X (FG) in Longissimus and semimembranosus of pigs reared outdoors compared to pigs raised in confinement system (indoor). However, there were negative correlations be- tween the number of fibers and the size their cross-sec- tion, being their number of greater significance for the evaluation as a whole. According Bee et al. (2004) in the Rectus femoris and Longissimus muscles of Large White pigs reared out- doors were found greater quantity of the type IIA (FOG) fibers and lower fiber content of type IIB/X (FG), when compared to traditional system confined. Moreover, other features related to consumer accep- tance are attributed to meat in general. Among these the meat color, brightness and luminosity must be cited once it may stimulate the purchase by the consumer. According to Heyer et al. (2006), pigs reared outdoors had the glycogen content and the values of L * (lightness) in Longissimus dorsi higher, while values of b * (yel- low pigmentation) were lower in the meat of pigs reared in the indoor system. In Cinta Senese pig breed, Pugliese et al. (2005), reported that in Longissimus lumborum muscle of pigs raised in outdoor systems had lower L * values (brightness) (45.8 vs. 50.1) and higher values of a * (red pigmentation) (14.9 vs. 11.8) and Chroma (15.9 vs.12.8). In Addition, according to Pugliese et al. (2004), pigs reared outdoors (Nero Siciliano breed) showed sig- nificantly higher lightness (L * = 50 vs.46.7) and yellow pigmentation (b * = 5.84 vs.4.88) in the meat. Gentry et al. (2004) commented that there were higher values of L * (lightness) and b * (yellow pigmentation) in chops from pigs born and reared outdoors. Meat from pigs crossbred (Landrace X Large White) reared outdoors showed higher a * (red pigmentation) than those raised in confinement system (Hoffman et al. 2003). A very important factor and often overlooked in re- search on meat quality is the acceptance of the final consumer. Aparicio et al. (2007) investigated these fac- tors in meat from Iberian pig breed Valdesequera raised in confined systems, semi-confined (5 m2/animal) and extensive (16,800 m2/20 animals) (Table 6). These re- searchers concluded that there was more texture and firmness in the meat of animals raised in extensive sys- tems when compared to other systems. These character- istics influenced the public’s assessment, having greater acceptance ofof meat originated from more extensive systems. Broiler Production The conventional chicken is generally known for its lack of taste and the soft and white aspect of its meat. Some consumers prefer firmer meat and with more pro- nounced flavor, features that correspond to older animals, near to the sexual maturity (Bastianelli, 2001), birds that have not suffered intense breeding (redneck strains) or the birds subjected to physical exercise (free range or semi- confined). According to Castellini et al. (2008), in Europe, alter- native systems can be a viable production method, espe- cially if suitable changes in EU Regulation. 1804/99 are made. The market opportunity for both organic and free range poultry products does not yet seem to be fully de- veloped. Age at slaughtering, genetic strains (fast- and slow- growing), physical activity, and pasture intake are key factors in determining meat quality. In conventional farm- ing, fast-growing chicks are generally used, but these are not suitable for alternative systems, since they may de- velop health and welfare problems, the most recurrent of which are leg disorders and lameness (Castellini et al. 2008). Conversely, use of slow-growing strains in alter- native systems has positive repercussions on both animal welfare and product qualitative characteristics (eating quality and appearance) perceived by consumers (Cas- tellini et al. 2008). Table 6. Indexes of consumer’s taste and preference of pork originated from different production systems. Flavor Production systemAverageStandard deviation Preference Confined 2.82 b ±0.90 9.1% Semi-confined 3.53 a ±0.96 36.4% Extensive 3.68 a ±0.94 54.5% Different letters in the same column indicate significant differences (p <0.05). Source: Aparicio et al. (2007).  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 83 In Brazil, the creation of alternative chickens then emerges as an option to consumers, in which the term alternative certificated chic ken is to designate the chicken of intensive farming raised without antibiotics, anticoc- cidial, growth promoters, chemotherapeutic agents and animal ingredients in the diet, besides a lower stocking density (birds / m²) and other requirements and standards adopted and made official in the Alternative Poultry In- dustry Association AVAL (Demattê Filho & Mendes, 2001). The redneck chicken or “brazilian colonial” is the chicken in which its feed is exclusively composed of ingredients of plant origin, being forbidden the use of growth promoters of any kind or nature. The creation is made in sheds until the 25 days old. After this age, the birds are released into the field, being now their rearing extensive, recommending 3 m 2 of pasture per bird. The slaughter is performed with a minimum age of 85 days. The strains used must be suitable for this purpose, being forbidden strains specific for commercial broiler (Circu- lar of the Ministry of Agriculture and Supply MAPA, DOI / DIPOA No 007/99 of 19.5.1999, supplemented by the Circular DOI / No 014/2000 of 11.5.2000 DIPOA). To serve this market, several colonial strains are cre- ated in Brazil, highlighting the Naked Neck Label Rouge of French origin; Embrapa 041, produced by the Na- tional Research Center of Embrapa Swine and Poultry in Concordia, SC; Paradise Pedrês, produced by the Birds Grange of Paradise in Itatiba, SP and Caipirinha, pro- duced by ESALQ / USP in Piracicaba, SP (Takahashi, 2003). According to Gessulli (1999), it should be noted that the redneck chicken does not compete in scale of pro- duction and the cost with the poultry industry, but does in the meat quality, serving a market niche that can pay more for these characteristics. 6. GENETIC FACTORS The strains of chicken produced commercially, al- though belonging to a single specie (Gallus gallus) show marked differences in conformation, size and color as its output destination. Greater peculiarities can still be de- tected by comparing these strains with their breeds of origin, once these differences are reflected in the skeletal muscles. The skeletal muscles of broiler chickens reared in in- tensive system in comparison to those created in semi-intensive (redneck chicken) presents greater mus- cle mass, composed by muscle fibers of size and length superior and with more DNA (Souza, 2005). The faster growing and the larger size of muscles of industrial chickens in relation to the rednecks are occasioned by the greater number of myofibers and their rapid hyper- trophy. But there could be influence of the production system in these results. According Fanatico et al. (2005b), fast growth geno- type birds has greatest breast yield (%) and the lowest wing yield (%), while slow growth genotype birds ex- hibit lowest breast yield (%) and the greatest leg quarter yield (%) in the same production systems. These results suggests that the genetic strain is more significant that system of rearing. Drip loss and cook loss (%) were af- fected by genotype, with the highest losses occurring with the slow growth genotype and the lowest losses occurring with the fast growth genotypes (Fanatico et al. 2005a). Selection for high growth rate led to a relative in- crease on the number and total cross-sectional area of myofibers. This may explain the greater weight of the muscles of animals of high growth rate. Madeira et al, (2006) evaluating the morphological aspects of skeletal muscle fibers types of the flexor Hallucis lon gus of four strains of broiler chickens raised in confined systems (Ross) and semi-confined (Naked Neck Label Rouge, Caipirinha and Paradise Pedrês) observed that Ross presented greater muscle mass (Ta ble 7). These results indicate that the growth rate is related to the increase in the area of the three muscle fiber types (SO, FOG and FG) in birds selected for increased body growth. In rela- tion to the rearing system no significant difference was observed between the two genetic groups. Similarly, Sartori et al. (1999) also observed a greater area of FG and SO fibers in the flexor Hallucis longus muscle of broilers when comparing strains of high growth (Hubbard and Ross) to another of slow growth (Naked Neck). According to these authors, the greater muscle mass of broilers selected can be characterized by Table 7. Mean values of area (mm²) of the glycolytic fibers type (FG), intermediate (FOG) and oxidative (SO) from the flexor hallucis longus muscle of broilers at 56 days old, ac- cording to the strain and rearing system. Area Strain FG FOG SO Ross 7104.51 a 4915.77 a 4860.84 a Paradise Pedrês 4442.22 b 4270.18 a 2735.20 b Caipirinha 3067.20 c 3308.73 b 2677.56 b Naked Neck 3062.76 c 3148.09 b 2199.20 b Production systemFG FOG SO Confined 4466,29 4014,99 3087,65 Semi-confined 4597,96 3778,25 3093,33 a,bMeans in column followed by different letters differ (P < 0.05) by Tukey test. Source: Madeira et al, (2006).  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. http://www.scirp.org/journal/OJAS/Openly accessible at 84 the increase of the muscle fibers area, principally glycol- lytic, indicating that the major selection pressure for weight gain is manifested in the hypertrophy of the mus- cle fibers. The selection for high growth rate in broilers has promoted structural and metabolic changes in muscles and changed the meat quality. High growth rates leads to injuries, larger diameter of muscle fibers, greater propor- tion of glycolytic fibers and reduced muscle proteolysis. These changes results in faster rigor mortis, which in- creases the meat pallor and diminish their capacity to fluid retention, worsening the quality of the processed products. The decrease in muscle proteolysis increases the hardness of the poultry meat (Dransfield & Sosnicki, 1999). To evaluate the smoothness can be used subjective methods like tasters in a taste test and equipment that measure the force required to shear the samples, such as methods of Allo-Kramer and Warner-Bratzler. Another indirect measure of this characteristic is the pH, which is related to the rigor mortis. To determine the rigor mortis is also made the evaluation of the glycogen level (Smith et al., 1992). Fanatico et al. (2006) working with fast, medium and slow growing strains of broilers, found that the breasts of the medium growing birds were more tender than other genotypes, however, all treatments scored “slightly to moderately tender”. The thigh meat of the medium de- velopment birds was more flavorful than that of slow growing birds, and the flavor of the slow growing broil- ers thigh meat was less liked than other genotypes. Analyzing the characteristics of meat quality in four strains of broiler chickens, being one of them commer- cial (Ross) and the other specific to the colonial produc- tion (Caipirinha, Naked Neck and Paradise Pedrês), Takahashi (2003) verified that Ross showed less weight loss due to cooking and greater shear force of breast muscle fibers compared to colonial birds (Table 8). Coelho, et al. (2007) verified that only two of eight genetic groups of broilers reared under semi-intensive system (Caipirão and Embrapa 041) presented a shear force of the Pectoralis major muscle fibers lower than the control group (Ross) reared under intensive system (Table 8). However, Rajar et al. (1999) and Lima (2005) ob- tained lower values of shear force to the fibers of the flexor Hallucis longus muscle of conventional broiler breeds (1.97 and 2.94 kgf.cm–2, respectively) in relation to redneck broiler breed (2.46 and 3.39 kgf.cm–2, re- spectively). According to Souza (2005), these different results may be related to muscle physiology, since the breast muscle glycolytic metabolism decreases the effects of the dif- ference between conventional rearing systems and semi- confined (redneck), in addition of this muscle do not be required to the birds’ movement, unlike what happens to the Flexor hallucis. Additionally, Magalhães (2004) stated that the location and function of the muscle are impor- tant factors that influence the shear force of the muscle fibers (Table 9). According to Cothenet (1998), the meat of redneck broilers Label Rouge reared under the semi-confined system when compared to commercial broilers of inten- sive farming, obtained in the taste test lower tenderness and juiciness. However, the Label Rouge broiler had higher scores for flavor and preference. Other important features related to the meat quality are determining the chemical composition, fatty acid profile, the color and the sensory analysis. According to Scheuermann (2004), with the selection pressure for high growth in broiler chickens, have been observed greater susceptibility to PSE meat (pale, soft and exudative), due to the high glycolytic potential and especially to the size of muscle fibers. These fibers de- rive energy for contraction through the glycolytic path- way and, under stress conditions with high energy de- mand (such as stirring pre-slaughter), the metabolism contributes to the rapid drop in the pH consequently raising the lactic acid production, which cannot be re- moved at post mortem. Concerning the increase in the fiber size, this may negatively affect the meat quality, leading to a greater tendency to PSE. Table 8. Effect of the broiler strain on the meat quality pa- rameters (pectoralis major muscle) at 56 days old. Shear force Strain Weight loss due to cooking (%) Thickness (cm) Width (cm) Lenght (cm) pH (Kgf/cm2) Ross 14.93 b 2.57 a 8.57 a 18.76 a 5.92 a 3.44 a P. Pedrês 18.22 a 1.42 b 7.12 b 16.10 b 5.85 ab 2.46 b Caipirinha 19.03 a 1.29 bc 6.34 c 14.85 c 5.80 b 2.79 b N. Neck 18.75 a 1.25 c 6.45 c 14.99 c 5.84 ab 2.64 b Means followed by different letters in columns differ by Tukey test (P < 0.05). Source: Takahashi (2003). 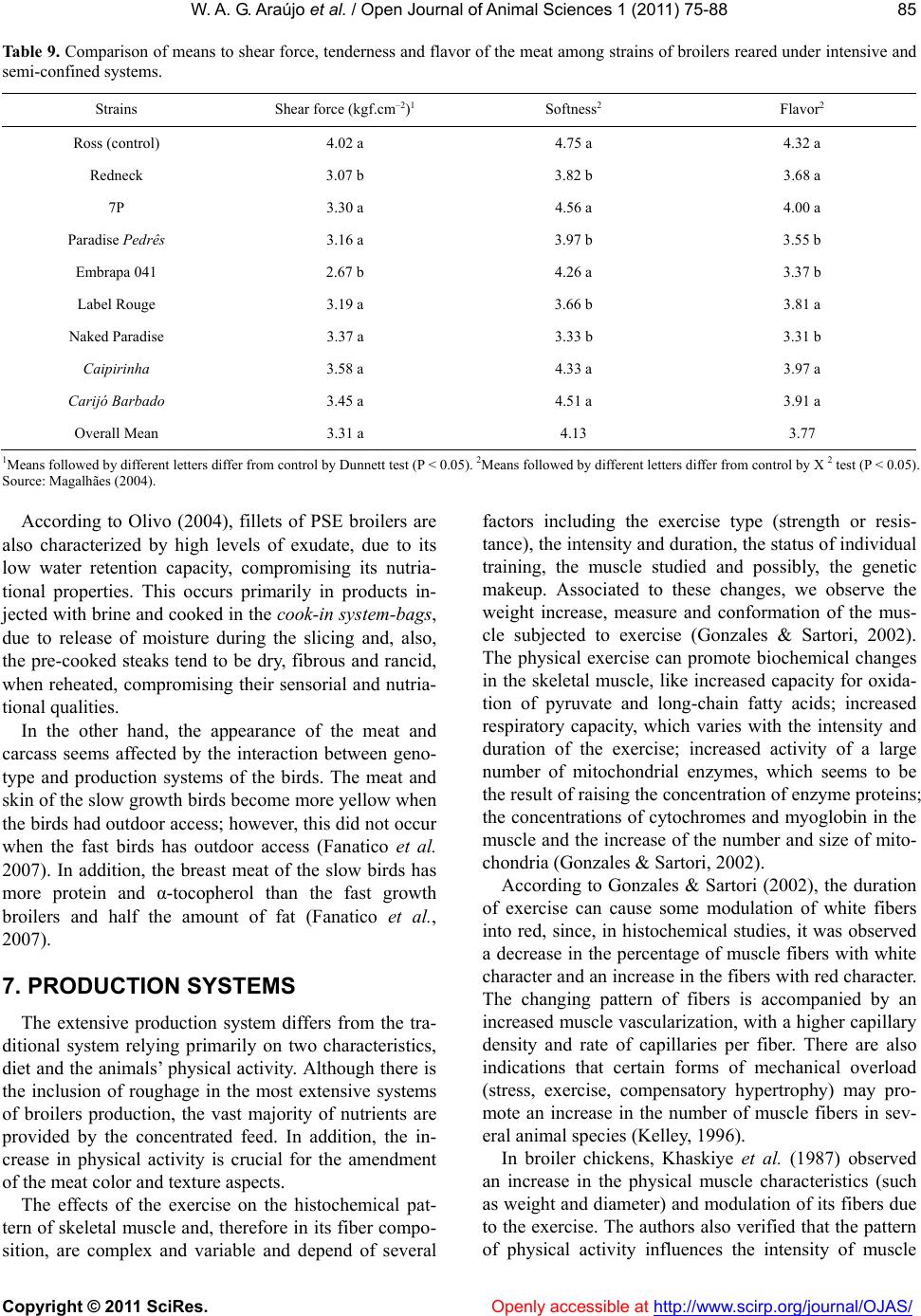 W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 85 Table 9. Comparison of means to shear force, tenderness and flavor of the meat among strains of broilers reared under intensive and semi-confined systems. Strains Shear force (kgf.cm–2)1 Softness2 Flavor2 Ross (control) 4.02 a 4.75 a 4.32 a Redneck 3.07 b 3.82 b 3.68 a 7P 3.30 a 4.56 a 4.00 a Paradise Pedrês 3.16 a 3.97 b 3.55 b Embrapa 041 2.67 b 4.26 a 3.37 b Label Rouge 3.19 a 3.66 b 3.81 a Naked Paradise 3.37 a 3.33 b 3.31 b Caipirinha 3.58 a 4.33 a 3.97 a Carijó Barbado 3.45 a 4.51 a 3.91 a Overall Mean 3.31 a 4.13 3.77 1Means followed by different letters differ from control by Dunnett test (P < 0.05). 2Means followed by different letters differ from control by X 2 test (P < 0.05). Source: Magalhães (2004). According to Olivo (2004), fillets of PSE broilers are also characterized by high levels of exudate, due to its low water retention capacity, compromising its nutria- tional properties. This occurs primarily in products in- jected with brine and cooked in the cook-in system-bags, due to release of moisture during the slicing and, also, the pre-cooked steaks tend to be dry, fibrous and rancid, when reheated, compromising their sensorial and nutria- tional qualities. In the other hand, the appearance of the meat and carcass seems affected by the interaction between geno- type and production systems of the birds. The meat and skin of the slow growth birds become more yellow when the birds had outdoor access; however, this did not occur when the fast birds has outdoor access (Fanatico et al. 2007). In addition, the breast meat of the slow birds has more protein and α-tocopherol than the fast growth broilers and half the amount of fat (Fanatico et al., 2007). 7 endment of r and size of mito- ch uscle fibers in sev- er . PRODUCTION SYSTEMS The extensive production system differs from the tra- ditional system relying primarily on two characteristics, diet and the animals’ physical activity. Although there is the inclusion of roughage in the most extensive systems of broilers production, the vast majority of nutrients are provided by the concentrated feed. In addition, the in- crease in physical activity is crucial for the am the meat color and texture aspects. The effects of the exercise on the histochemical pat- tern of skeletal muscle and, therefore in its fiber compo- sition, are complex and variable and depend of several factors including the exercise type (strength or resis- tance), the intensity and duration, the status of individual training, the muscle studied and possibly, the genetic makeup. Associated to these changes, we observe the weight increase, measure and conformation of the mus- cle subjected to exercise (Gonzales & Sartori, 2002). The physical exercise can promote biochemical changes in the skeletal muscle, like increased capacity for oxida- tion of pyruvate and long-chain fatty acids; increased respiratory capacity, which varies with the intensity and duration of the exercise; increased activity of a large number of mitochondrial enzymes, which seems to be the result of raising the concentration of enzyme proteins; the concentrations of cytochromes and myoglobin in the muscle and the increase of the numbe ondria (Gonzales & Sartori, 2002). According to Gonzales & Sartori (2002), the duration of exercise can cause some modulation of white fibers into red, since, in histochemical studies, it was observed a decrease in the percentage of muscle fibers with white character and an increase in the fibers with red character. The changing pattern of fibers is accompanied by an increased muscle vascularization, with a higher capillary density and rate of capillaries per fiber. There are also indications that certain forms of mechanical overload (stress, exercise, compensatory hypertrophy) may pro- mote an increase in the number of m al animal species (Kelley, 1996). In broiler chickens, Khaskiye et al. (1987) observed an increase in the physical muscle characteristics (such as weight and diameter) and modulation of its fibers due to the exercise. The authors also verified that the pattern of physical activity influences the intensity of muscle  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 86 m , s la rowth rates and reared with or without outdoor access. 8 ion and the fatty acid profile deposited on the carcass. ciên- n introductory text, CAL e II simpósio sobre SISCAL, Concórdia, da carne suína, ve traits. Livestock Produc- differentiation, and that both the placement of barriers and ramps in conventional boxes are crucial for obtain- ing heavier muscles, after three or four weeks of expo- sure to the new situation. The production system also in- terferes with bone formation in birds. Birds given out- door access has greater bone strength in the tibia that ani- CNP als reared in intensive system (Fanatico et al., 2005b). Rearing broilers in sheds with ramps Sandusky & Heath (1988), verified an increase in chest growth rate and greater size of the Pectoralis major, Supracora- coideo, Gastrocnemius (inside) and Femoro tibial mus- cles in birds reared in boxes with simple ramps, however in boxes with dual ramps it was found an increase in the growth of fibula long and Femorotibial muscles of broilers. Based on these considerations, it is verified that the birds’ physical activity leads to adaptive changes that elevate the oxidative capacity of skeletal musclesimi- r to those that have been observed in mammals. Studding broilers from fast and slow growing strains, reared in outdoor and indoor systems, Fanatico et al. (2005a), found that the principal effect of outdoor access was to make the meat more yellow in the case of the slow growing strain genotype, although not the fast grow- ing birds. In the same work was observed that the ten- derness was affected by production system, but only in the fast growing strain birds. The pectoralis dry matter (%), fat (%), and ash (%) were largely unaffected, both the production system and genotype (Fanatico et al., 2005a). However, the outdoor access did not impact fla- vor of the thigh and breast meat of the birds in the sen- sorial test (Fanatico et al., 2006). These data indicate that differences in sensory attributes may exist among geno- types with different g . CONCLUSIONS The production of poultry and pigs more extensively tend to get a final product with its own organoleptic characteristics, changing the meat default color and con- tent, place of fat deposit REFERENCES [1] Demattê Filho, L.C. and Mendes, C.M.I. (2001) Via- bilidade técnica e econômica na criação alternativa de frangos. Proceed ings of the Conferência APINCO de cia e tecnologia avícolas, Campinas, 650, 255-266. [2] Bastianelli, D. (2001) A produção de frangos diferen- ciados na França: mercado, aspectos organizacionais e regulamentares. Proceedings of the Conferência APINCO de ciência e tecnologia avícolas, Campinas, 650, 235-254. [3] Warriss, P.D. (2000) Meat Science: A Cabi Publishing, Wallingford, USA. [4] Edwards, S.A. (1999) Organic pig production. Proceed- ings of the II encontro do conesul de técnicos especia- listas em SIS 289, 56-68. [5] Machado Filho, L.C.P. (2000) Bem estar de suínos e qualidade da carne, uma visão brasileira. Proceedings of the I conferência virtual sobre qualidade SA – EMBRAPA, Concórdia, Brasil. [6] Dal Bosco, A., Castellini, C.Y. and Mugnani, C. (2002) Rearing rabbits on a wire net floor or straw litter: Behav- ior, growth and meat qualitati tion Science, 75, 149-156. doi:10.1016/S0301-6226(01)00307-4 [7] Lebret, B. and Mourot, J. (1998) Lipides et qualités des tissus adipeux chez le porc: Facteurs de variation non eat Quality, with Emphasis on ia da Carne. 6th Ed, Artmed, ce, 66, 177-188. généiques. INRA Production Animal, 11, 131-143. [8] Olsson, V. and Pickova, J. (2005) The Influence of Pro- duction Systems on M Pork. Ambio, 34, 4-5. [9] Lawrie, R.A. (2005) Ciênc Porto Alegre, Brasil, 384. [10] Rehfeldt, C., Fiedler, I., Dietl, G. and Ender, K. (2000) Myogenesis and postnatal skeletal muscle cell growth as influenced by seletion. Livestock Scien doi:10.1016/S0301-6226(00)00225-6 [11] Lefaucheur, L. and Gerrard, D. (2000) Muscle fiber plas- ticity in farm mammals. Proceedings of the American society of animal science, ASAS, Denver, USA, 256, t. Proceed- 55, 543-559. 58-67. [12] Dauncey, M.J. and Gilmour, R.S. (1996) Regulatory factors in the control of muscle developmen ings of the Nutrition Society, doi:10.1079/PNS19960047 [13] Gonzales, E. and Sartori, J.R. (2002) Crescimento e metabolismo muscular. In: Macari, M., Furlan, R.L., Gonzales, E., Ed., Fisiologia aviária aplicada a frangos 1st Edition, Academic Press, San Diego, os de corte. 2nd Edi- nto Animal, SBMA, Belo Horizonte, Brasil, 249, ics in the , 65-76. de corte, FUNEP/UNESP, Jaboticabal, Brasil, 279-297. [14] Pearson, A.M. and Young, R.B. (1989) Muscle and meat biochemistry. USA. 457. [15] Macari, M., Furlan, R.L. and Gonzales, E. (2002) Fisiologia aviária aplicada a frang tion, FUNEP, Jaboticabal, Brasil. [16] Sosnicki, A.A., Shellier, K.K., Wilson, E.R. and Viries, A.G. (1996) Pork quality traits: Genetic and environ- mental effects. Proceedings of the II jornada en produc- cion porcina, Ciudad del México, México, 896, 29-38. [17] Plastow, G.S. (2000) Molecular genetics in the swine industry. Proceedings of the Simpósio Nacional de Mel- horame 21-30. [18] Remignon, H., Lefaucheur, L., Blum, J.C. and Ricard, F.H. (1994) Effects of divergent selection for body weight on three skeletal muscles characterist chicken. British Poultry Science, 35 doi:10.1080/00071669408417671 [19] Soike, D. and Bergmann, V. (1997) Performance-de- pendent health disorders in poultry with special reference to differences in muscle characteristics between layer- and meat type chickens. Proceedings of the International conference on production diseases in farm animals, H. MARTINS, Berlin, Germainy, 789, 186-189. [20] Dransfield, E. and Sosnicki, A.A. (1999) Relationship  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJAS/ 87 and poultry meat quality. Poultr entation hing pig. Livestock Production between muscle growthy Science, 78, 743-746. [21] Morand-Fehr, P. and Tran, G. (2001) La fraction lipi- dique des aliments et les corps utilisés en alim animale. INRA Production Animal, 14, 285-302. [22] Lizardo, R., Van Milgen, J., Mourot, J., Noblet, J. and Bonneau, M. (2002) A nutritional model fatty composi- tion in the growing-finis Science, 75, 167-182. doi:10.1016/S0301-6226(01)00312-8 [23] Girard, J.P., Bout, J. and Salort, D. (1988) Lipides et qualités des tissus adipeux et musculaires d’porc, facteurs s fat in finishing pigs. Livestock Science, 54, 215- . Poultr de variation. Journéss Rechercher Porcine, 20, 255-278. [24] Katsumata, M., Hirose, I. and Kaji, Y. (1995) Influence of high ambient temperature and dietary fat supplemen- tation on fatty acid composition of depot fats in finishing pigs. Animal Science and Technology, 66, 225-232. [25] Wiseman, J. and Agunbiade, J.A. (1998) The influence of changes in dietary fat and oils on fatty acid profiles of carcas 225. [26] Ponte, P.I.P., Alves, S.P., Bessa, R.J.B., Ferreira, L.M.A., Gama, L.T., Bra´s, J.L.A., Fontes, C.M.G.A., and Prates, J.A.M. (2008) Influence of pasture intake on the fatty acid composition, and cholesterol, tocopherols, and toco- trienols content in meat from free-range broilersy Science, 87, 80-88. doi:10.3382/ps.2007-00148 [27] Edwards, S.A. (2005) Product quality attributes asso- ciated with outdoor pig pr Science, 94, 1-2, 5-14. oduction. Livestock Production doi:10.1016/j.livprodsci.2004.11.028 [28] Honeyman, M.S. (2005) Extensive bedded indoor and outdoor pig production systems in USA: Current trends and effects on animal care and product Production Science, 94, 15-24. quality. Livestock doi:10.1016/j.livprodsci.2004.11.029 [29] Moreira, I. (1998) Nutrição de Rebanhos (de Suínos) Geneticamente Melhorados. Proceedings of the II sim- pósio nacional de melhoramento animal, Uberaba, Brasil, de de carne cion por- a suína. 50. 892, 177-183. [30] Terra, N.N. and Fries, L.L.M. (2000) A qualidade da carne suína e sua industrialização. Proceedings of the I conferência virtual internacional sobre qualidade carne suína, CNPSA-EMBRAPA, Concórdia, Brasil. [31] Rübensam, J.M. (2000) Transformações post mortem e qualidade da carne suína. Proceedings of the I confe- rência virtual internacional sobre qualidade suína, CNPSA-EMBRAPA, Concórdia, Brasil. [32] Sosnicki, A.A, Shellier, K.K., Wilson, E.R. and Viries, A.G. (1996) Pork quality traits: Genetic and environmental effects. Proceedings of the II jornada en produc cina, Ciudad del México, 29-38. [33] Bridi, A.M., Nicolaiewsky, S., Rübensam, J.M., Both, MC. and Lobato, J.F.P. (2003) Efeito do genótipo halotano e de diferentes sistemas de produção no desempenho produtivo e na qualidade da carcaç Revista Brasileira de Zootecnia, 32, 942-9 doi:10.1590/S1516-35982003000400021 [34] Sather, A.P., Jones, S.D.M., Schaefer, A.L., Colyn, J. and Robertson, W.M. (1997) Feedlot performance, carcass composition and meat quality of free range reared Canadian Journal of Apigs. nimal Science, 77, 225-232. doi:10.4141/A96-093 [35] Heyer, A., Andersson, H.K. and Lundstrom, K. (2006) Performance, carcass and technological meat quality of pigs in indoor and outdoor production Agriculturae Scandinavica, 56, 55-64. systems. Acta doi:10.1080/09064700600670904 [36] Bridi, A.M., Nicolaiewsky, S, Rübensam, J.M., Lopes, R.F.F., Lobato, J.F.P. (2003) Efeito do genótipo halo- tano e de diferentes sistemas de produção na qualidade da carne suína. Revista Brasileira de Zootecnia, 32, 1362-1370. doi:10.1590/S1516-35982003000600011 [37] Bridi, A.M., Müller, L. and Ribeiro, J.A.R. (1998) Indoor vs. outdoor-rearing of pigs, performance, carcass and meat quality. Proceedings of the International congress of meat science and technology proceedings, Barcelona, outh African Journal of Animal Science, 33, Science, 34, 521, 114-117. [38] Hoffman, L.C., Styger, E., Muller, M. and Brand, T.S. (2003) The growth and carcass and meat characteristics of pigs raised in a free-range or conventional housing system. S 166-175. [39] Van Der Wal, P.G., Mateman, G., Vries, A.W., Vonder, G.M.A., Smulders, F.J.M., Geesink, G.H. and Engel, B. (1993) ‘Scharrel’ (free range) pigs: Carcass composition, meat quality and taste-panel studies. Meat 27-37. doi:10.1016/0309-1740(93)90016-B [40] Jones, S.D.M., Schaefer, A.L. and Dyck, R. (1993) The effects of fattening pigs in indoor pens on live perform- ance, carcass composition and meat quality. Proceedings of the International congress of meat science and tech- meat quality. Meat Sci- gri- quality traits. Journal of Animal Science, ience, nology, Montreal, Canada, 456, 129-138. [41] Enfält, A., Lundström, K., Hansson, I., Lundeheim, N. and Nyström, P. (1997) Effects of outdoor rearing and sire breed (Duroc or Yorkshire) on carcass composition and sensory and technological ence, 45, 1-15. [42] Butko, D., Senčić, D., Antunović, Z., Šperanda, M. and Steiner, Z. (2007) Pork carcass composition and the meat quality of the black slavonian pig—the endangered breeds in the indoor and outdoor keeping system a culture. Scientific and Professional Review, 13, 1-6. [43] Bee, G., Guex, G. and Herzog, W. (2004) Free-range rearing of pigs during the winter: Adaptations in muscle fiber characteristics and effects on adipose tissue compo- sition and meat 82, 1206-1218. [44] Strudsholm, K. and Hermansen, J.E. (2005) Perform- ance and carcass quality of fully or partly outdoor reared pigs in organic production. Livestock Production Sc 96, 261-268. doi:10.1016/j.livprodsci.2005.02.008 [45] Basso, L.R., Moisá, S. and Brunori, J. (2007) Calidad de carne diferencial de cerdos producidos em sistemas al aire libre. Proceedings of the IX encuentro de nutrición y producción en animales monogástricos, Montevideo, 325, n non Science, 69, 459-464. 63-67. [46] Lebret, B. And Mourot, J. (1998) Lipides et qualités des tissus adipeux chez le porc. Facteurs de variatio généiques. INRA Production Animal, 11, 131-143. [47] Pugliese, C., Bozzi, R., Campodoni, G., Acciaioli, A., Franci, O. and Gandini, G. (2005) Performance of Cinta Senese pigs reared outdoors and indoors: Meat and sub- cutaneous fat characteristics. Meat  W . A. G. Araújo et al. / Open Journal of Animal Scie nces 1 (2011) 75-88 Copyright © 2011 SciRes. http://www.scirp.org/journal/OJAS/Openly accessible at 88 doi:10.1016/j.meatsci.2004.09.001 [48] Pugliese, C., Calagna, G., Chiofalo, V., Moretti, V.M., Margiotta, S., Franci, O. and Gandini, G. (2004) Com- parison of the performances of Nero Siciliano pigs reared indoors and outdoors: Joints com traits. Meat Science, 68, 523-528. position, meat and fat doi:10.1016/j.meatsci.2004.02.020 [49] Gentry, J.G., McGlone, J.J., Miller, M.F. and Blanton Jr, J.R. (2004) Environmental effects on pig performance, meat quality, and muscle characteristics. Journal of an Soci- ión en animales monogástricos, Montevideo, 325, meat. World’s Poultry Animal Science, 82, 209-217. [50] Lefaucheur, L., Gerrard, D. (1998) Muscle fiber plastic- ity in farm mammals. Proceedings of the Americ ety of Animal Science, Ithaca, USA, 1998, 1-19. [51] Aparicio, M.A., Vargas, J.D., Prieto, L., Robledo, J., González, F., Andrada, J.A., Ladero, L. and Cava, R. (2007) Análisis del bienestar animal en diferentes siste- mas de cría del cerdo ibérico y efecto sobre la calidad de la carne. Proceedings of the IX encuentro de nutrición y producc 29-34. [52] Castellini, C., Berri, C., Le Bihan-Duval, E. and Martino, G. (2008) Qualitative attributes and consumer perception of organic and free-range poultry Science Journal, 64, 500-512. doi:10.1017/S0043933908000172 [53] Takahashi, S.E. (2003) Efeito do sistema de criação sobre o desempenho e qualidade da carne de frangos de corte tipo colonial e industrial. MSc, Dissertation, Uni- inal. 1st Edition, OPG Editores, and carcass yield. Poultry Science, oor a , 232- versidade Estadual Paulista. [54] Gessulli, O.P. (1999) Avicultura alternativa: sistema “ecologicamente correto” que busca o bem-estar animal e a qualidade do produto f Porto Feliz, Brasil, 217. [55] Souza, R.X. (2005) Características de carcaça, qualidade de carne e composição lipídica de frangos de corte criados em sistemas de produção alternativo e conven- cional. DSc Dissertation, Universidade Federal de Lavras. [56] Fanatico, A.C., Pillai, P.B., Cavitt, L. C., Owens, C.M. and Emmert, J.L. (2005) Evaluation of slower-growing broiler genotypes grown with and without outdoor access: growth performance 84, 1785-1790. [57] Fanatico, A.C., Cavitt, L. C., Pillai, P.B., Emmert, J.L. and Owens, C.M. (2005) Evaluation of slower-growing broiler genotypes grown with and without outdccess: Meat quality. Poultry Science, 84, 1321-1327. [58] Madeira, L.A., Sartori, J.R., Saldanha, E.S.P.B., Piz- zolante, C.C., Silva, M.D.P., Mendes, A.A., Takahashi, S.E. and Solarte, W.V.N. (2006) Morfologia das fibras musculares esqueléticas de frangos de corte de diferentes linhagens criados em sistemas de confinamento e semi- confinado. Revista Brasileira de Zootecnia, 35 2-2332. doi:10.1590/S1516-35982006000800018 [59] Sartori, J.R., Gonzales, E., Macari, M., Dal Pai, V. and Oliveira, H.N. (1999) Tipos de fibras do músculo flexor longo do hálux de frangos de corte machos de diferentes 181-185. linhagens. Brazilian Journal of Poultry Science, 1, [60] Smith, D.P., Fletcher, D.L. and Papa, C.M. (1992) Post mortem biochemistry of peckin ducking and broiler Pec- toralis muscle. Poultry Science, 71, 1768-1772. [61] Fanatico, A.C., Pillai, P.B., Cavitt, L. C., Emmert, J.L., Meullenet, J.F. and Owens, C.M. (2006) Evaluation of slower-growing broiler genotypes grown with and with- out outdoor access: Sensory attributes. Poultry Science, 85, 337-343. [62] Coelho, A.A.D, Savino, V.J.M., Rosário, M.F., Silva, M.A.N., Castillo, C.J.C. and Spoto, M.H.F. (2007) Características da carcaça e da carne de genótipos de frangos caipiras. Brazilian Journal of Food Technology, 10, 9-15. [63] Rajar, A., Zlender, B., Gasperlin, L., Tercic, D., Vadnejal, R. and Holcman, A. (1999) Quality parameters of ther- mally treated chickens of two provenances and free range keeping. Acta Agrária Kaposváriensis, 1, 185-194. [64] Lima, A.M.C. (2005) Avaliação de dois sistemas de produção de frango de corte: Uma visão multidisciplinar. PhD Dissertation, Faculdade de Engenharia Agrícola, Universidade Estadual de Campinas. [65] Magalhães, P.M. (2004) Efeitos da utilização de proteína de origem vegetal em substituição a proteína de origem animal sobre a absorção de água da carcaça e maciez do músculo pectoralis major em frangos de corte. MSc dissertation, Curitiba, PR:Universidade Federal do Paraná. [66] Cothenet, G.A. (1998) La production de poulets Label Rouge en France et son attractivité. Union nationale des producteurs de poulet français (sans numéro de page), Document, Saint Gilles, France. [67] Scheuermann, G.N. (2004) Alteração na quantidade e qualidade da carne de aves através da manipulação das fibras musculares. Proceedings of the Conferência APINCO de ciência e tecnologia avícolas, FACTA, Santos, Brasil, 698, 165-178. [68] Olivo, R. (2004) Atualidades na qualidade de carne de aves. Proceedings of the Conferência APINCO de ciência e tecnologia avícolas, FACTA, Santos, Brasil, 698, 165-178, [69] Fanatico, A.C., Pillai, P.B., Emmert, J.L. and Owens, C.M. (2007) Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poultry Science, 86, 2245-2255. doi.10.3382/ps.2007-00092 [70] Kelley, G. (1996) Mechanical overload and skeletal muscle fiber hyperplasia: A metaanalysis. Journal of Ap- plied Physiology, 81, 1584-1588. [71] Khaskiye, A., Renaud, D.L.E. and Douarin, G. (1987) Effects of electrical stimulation upon post-hatching de- velopment of fibre types in normally, innervated fast and slow Latissimus dorsii muscles of the chicken. Biology of the Cell, 61, 163-170. [72] Sandusky, C.L. and Heath, J.L. (1988) Growth charac- teristics of selected broiler muscles as affected by age and experimental pen design. Poultry Science, 67, 1557-1567.
|