 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 361-368 doi:10.4236/jbnb.2011.24045 Published Online October 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 361 The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides Galina Kubyshkina1, Barbara Zupančič2*, Marina Štukelj3, Dušan Grošelj4, Ljubo Marion4, Igor Emri2 1Elektromaterial Lendava d.d., Lendava, Slovenia; 2Center for Experimental Mechanics, Faculty of Mechanical Engineering, Univer- sity of Ljubljana, Ljubljana, Slovenia; 3Veterinary Faculty, University of Ljubljana, Ljubljana, Slovenia; 4Faculty of Medicine, Uni- versity of Ljubljana, Ljubljana, Slovenia. Email: *barbara.zupancic@fs.uni-lj.si Received July 18th, 2011; revised August 22nd, 2011; accepted September 27th, 2011. ABSTRACT For this investigation conventional polyamide 6 with monomodal molecular mass distribution, and the newly developed bimodal one were used. Conventional polyamide 6 was used as a reference material in order to emphasize prospects of us- ing bimodal material for medical applications from the point of view of sterilization resistance and improved creep behavior. Time-dependent mechanical properties of testing samples were characterized by torsional creep measurements in non-ster- ilized state and after sterilization with three different techniques: with autoclave, ethylene oxide, and hydrogen peroxide plasma. Results show that the two materials exhibit pronounced difference in morphology and consequently, mechanical properties. Both of them were not significantly affected by any of used sterilization techniques. However, bimodal material, originally being noticeably more time-stable in comparison to monomodal one, retains these preferences also post steriliza- tion. Keywords: Sterilization, Polyamide, Creep, Time Stability 1. Introduction Polymers are often the materials of choice for medical devices, medical packaging, and food packaging, replac- ing traditional materials such as stainless steel and glass [1]. Polymers are beside non-dental applications exten- sively used in dentistry as composite (resin-ceramic) res- torative materials, implants, dental cements, and denture based teeth. Theoretically, they can be modified for maximum bio- compatibility and acceptable mechanical properties. Al- though all three groups of polymers (thermoplasts, ther- mosets, elastomers) are used for medical applications, the thermoplastics are most widely used [2]. They offer manufacturing cost savings, lighter weight, and performance characteristics that meet and exceed the demand in many highend applications. However, the pro- cess of sterilization can strongly affect the properties of the polymers [1,3]. Sterilization is the complete elimination of microbial viability, including the vegetative forms of bacteria and spores. Sterilization of surgical equipment, implants, linens, and attire is one aspect of a series of highly regimented steps constituting aseptic technique. The methods of ster- ilization can be divided into two general groups: physi cal and chemical. Although sterility can be achieved with certain chemicals, physical methods are generally more reliable. Heat, filtration, and radiation are the most com- monly used physical methods of sterilizing medical and surgical materials. Chemical sterilization is usually ac- complished with ethylene oxide or hydrogen peroxide, although formaldehyde and β-propiolactone are also used occasionally. The most commonly used techniques in ve- terinary medicine are steam, ethylene oxide, and hydro- gen peroxide gas plasma sterilization [4]. Sterilization has increasingly been a topic of discus- sion when comes to the implantology. There are several issues to be considered when sterilization methods are being selected for implantable medical devices [5]. The number of agents capable of sterilizing product or material without adversely or deleteriously affecting pro- duct quality or material integrity is few. There is no sin- gular sterilization method that is compatible with all healthcare products including drugs, polymers, devices, and materials, because of the severity of a process to meet the sterilization criteria and definition [6].  The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides 362 In our investigation we applied the three most com- monly used sterilization techniques: autoclave, Ethylene Oxide (EtO), and H2O2 plasma. In continuation, the brief overviews of the three sterilization methods effects on polymers’ properties are presented. Steam sterilization by autoclaving has traditionally been the most widely used method for medical instru- ments [7]. The steam method is convenient, fast, and widely available. It is inexpensive and can quickly ster- ilize porous materials and accessible surfaces and spaces [8]. However, this method has some disadvantages. It is well known that heat and steam can drastically alter the mechanical and chemical properties of many synthetic biomaterials. The main disadvantage of steam steriliza- tion is the hydrophilicity of some polymers, which ren- ders the implant mechanically weaker [9]. Lastly, the steam itself may carry contaminants to implant surfaces and directly lead to a lesser degree of in vivo biocom- patibility [5]. Ethylene oxide-EtO (CH2-O-CH2) and gamma sterili- zation are the most common sterilization methods for thermoplastics used in the disposable medical device industry [10]. The gas vapor sterilant most commonly used is ethylene oxide; it is capable of killing very effi- ciently all the species and forms of micro-organisms in a short time, but in pure form is extremely toxic, irritating, water and common organic solvents soluble, and explo- sive. EtO is bactericidal, virucidal, and sporicidal. Its germicidal activity results from its action on the proteins, DNA and RNA. The flammability hazard of EtO has been reduced by mixing it with an inert gas, usually Freon chlorofluoro- carbon, and after year 1995, an alternate gas, hydro- chlorofluorocarbon is being used as a carrier for EtO sterilization [10]. A non-flammable preparation is also obtained when EtO is mixed with carbon dioxide in a ratio 1:9. Marois et al. reported on investigation of sterilization effect on poly beta-hydroxy octanoate (PHO). The phy- sical and structural properties of PHO were shown to be well preserved following EtO (ethylene oxide) steriliza- tion, whereas gamma radiation caused random chain scission and physical cross-linking, a frequent phenome- non observed with organic polymers [11]. Lucas et al. reports that EtO residues in medical de- vices and polymers depend on the type and size of the material. Some materials retain little EtO residues, such as polyurethane PU 80A and hollow catheters, whether others retain much larger amounts, such as polyurethane PU 75D and nylon 66. Presented work illustrates that EtO levels and effects are highly dependent on the type of material used. Residual EtO, the amount of EtO that is bioavailable, and the effect of EtO in vitro must be con- sidered for each type of material or device [12]. Buben et al. report on rough generalizing conclusions: acrylate polymers and copolymers frequently used as hard components of medical devices release absorbed EtO very slowly. This applies to the following materials: ABS, MBS, MMABS, SAN/ABS. In addition to acrylate polymers EtO is released also by other hard polymers e.g. parts of polycarbonate medical devices, hardened PVC or cross-linked polypropylene [13]. The study of Brown et al. investigated the effects of a range of methods on the tensile strength of latex rubber, silicone elastomer, 2 different formulations of polyure- thane, nylon, and high-density polyethylene (HDPE) spe- cimens. The methods of disinfection and sterilization used were hypochlorite bleach, peracetic acid + hydro- gen peroxide, formaldehyde gas, low-temperature per- acetic acid and gas plasma, and low-temperature hydro- gen peroxide gas plasma. The results showed that sili- cone elastomer was minimally affected, whereas the strengths of nylon, polyethylene, and latex were reduced by some of the methods [14]. Plasma-based methods a priori offer the required effi- cacy near ambient temperature to sterilize polymeric de- vices, but they inevitably also modify the exposed poly- mer surfaces. The hydrogen peroxide plasma sterilization causes surface oxidation of many types of polymers [15]. The study has shown that two commercial plasma-based sterilizers, Sterrad® and PlazlyteTM, can substantially alter the surface characteristics of all the polymers inves- tigated in this paper, namely their surface composition and morphology, and their wettability, even after a single sterilization cycle [15]. Regarding sterilization with EtO, they have generally found a lesser extent of surface modification than follow- ing plasma-based techniques, but this advantage must be weighted against the possible hazards of retained EtO or its toxic by-products in treated polymers [15]. It has already been noted that nominally identical polymer materials, for example the PVC film and tubing, or the two polyurethane catheter materials, could reveal quite different surface compositions, oxidation behav- iours, wettabilities, and surface topographies. This under- lines the important fact that the generic composition of a polymer is insufficient for judging whether a particular device can be safely resterilized; clearly, the nature and concentration of additives in the polymer formulation, the manufacturing process, and numerous other variables must also be taken into account [15]. Polymer degradation caused by sterilization is more often associated with oxidative processes rather than with hydrolysis. As known, there are some sterilization technologies, such hydrogen peroxide plasma steriliza- tion systems, which use hydrogen peroxide (H2O2)-pla- C opyright © 2011 SciRes. JBNB  The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides363 sma and peracetic acid, respectively, as an oxidative ster- ilizing agent [16]. In our study we compared the effects of steam under pressure as physical sterilant and of ethylene oxide and plasma gas as gas vapour method of sterilization on the time-dependent mechanical properties of two types of polyamide PA6 (Nylon). Nylon is biostable polymer [10]. Polyamide and co- polymers utilized for healthcare products such as cathe- ters used in cardiovascular procedures, sutures, epidural catheters, laparoscopy devices, blood sets, joints, kidney dialysis, composite materials and films, and special pack- ages might be submitted to the following sterilisation processes: steam, limited dry heat depending on steriliza- tion temperature up to 130˚C [6], EtO, limited radiation, limited H2O2. Polyamides are sensitive to crosslinking from radiation, but are suitable for a single dose of radia- tion. PA6 may be at least radiation resistant [6]. The sterilization stability of nylon and filled nylon with EtO is very good but there exist some susceptibility of material to oxidizing agents. The stability of nylon to autoclave sterilization is very good but components may swell slightly due to water absorption [17]. Sicard et al. have shown that sterilization by steam or EtO had no significant effect on the strength of the nylon fishing material. Ethylene oxide was the preferred me- thod of sterilization to preserve strength and minimize elongation of the fishing material [18]. Kirby et al. reported that there was no difference be- tween untreated and EtO sterilized bands with respect to testing to failure using a splint circular jaw mounted on a tensile testing machine, whereas bands subjected to auto- claving failed at less force [19]. Bochkova et al. investigated the uniaxial stretching of microfiltration membranes made of polyamides. Changes were found in the mechanical properties of the mem- branes after autoclaving, which had been induced by spon-taneous polymer arrangement on heating; the ar- rangement was expressed as either a great self-elongation of the membrane or its shrinkage [20]. As analyzed by Navarrete & Hermanson (1998), after exposure to HCFC 124, HCFC 124/22 and pure EtO, the tensile and Izod properties of Nylon appear relatively unaffected. The physical properties of Nylon, and Acry- lic were not significantly affected by any of the three sterilant gases [10]. Gatineau et al. compared the effects of hydrogen per- oxide gas plasma (HPGP) and ethylene oxide (EtO) ster- ilizations on the mechanical properties of nylon lines used for stabilization of the canine stifle. Sterilization with HPGP seems a good alternative to EtO for nylon lines [21]. For the purpose of our investigation two different types of polyamide material PA6 were used, conven- tional one with monomodal molecular distribution, and novel material with bimodal one. The objective of this paper was to analyze how three of the commonly used sterilization methods will affect novel bimodal material in comparison to monomodal one with respect to their time-dependent (long-term) mechanical properties. Cha- racterization of the investigated materials was performed by means of torsional creep tests. 2. Experimental Part 2.1. Materials We have tested the effect of sterilization on the newly developed I-PA6 (intelligent polyamide) described by Emri et al. [22], which was compared to commercially available PA6 material. Conventional PA6 has monomo- dal molecular mass distribution, whereas the new I-PA6 has bimodal one. Multimodality of the material was achieved by proper modification of its molecular mass distribution to put it more precisely, by varying the av- erage chain length of the PA6 polymer chains during polymerization process starting from the same PA6 mo- nomer units. The average molecular weight of both ma- terials is roughly the same. Structure of both materials, observed by means of op- tical microscopy, is shown in the Figure 1. By compari- son one may infer significant difference in morphology of non-sterilized standard material and modified one, prepared by the same technology. In the case of mono- modal PA6 obtained image indicates formation of coarse spherulites that can be observed as Maltese cross patterns (Figure 1(a)), while bimodal I-PA6 forms homogeneous fine grain structure when exposed to the same processing conditions (Figure 1(b)). From both types of polyamide materials samples were prepared to test their time-dependent mechanical proper- ties after exposure to three different sterilization tech- niques: autoclave sterilization, EtO sterilization, and H2O2 plasma sterilization. Standard monomodal PA6 was used as a control material in this study. 2.2. Sample Preparation In order to perform creep measurements PA cylindrical Figure 1. (a) Morphology of non-sterilized monomodal PA6, and (b) bimodal I-PA6; Axioskop 2 MAT (Carl Zeiss), po- larized transmitted light, 200×. Copyright © 2011 SciRes. JBNB  The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides 364 specimens with diameter D = 3.0 mm and length L = 60.0 mm were made from both materials by gravimetric casting procedure, which is briefly described below. This method was developed to avoid orientation of molecules caused by melt flow when using injection molding or extrusion in sample preparation. After casting all sam- ples are annealed to minimize the effect of physical age- ing to the level that is negligible. The annealing proce- dure was thoroughly studied in our laboratory in the past. Based on these studies we determined the temperature profile which brings sample sufficiently close to ther- modynamic equilibrium. The specimen preparation equipment is illustrated in Figures 2(a) and 2(b). The casting procedure starts with filling up a glass tube with an inner diameter of 3 mm with PA pellets (Figure 2(a)). Following this, the heater is placed on the bottom of the tube to start the melting process, and a pressure of about 1MPa is applied from above to overcome friction forces between the material and the tube inner surface. The heater temperature is maintained at 270˚C and it is moved upwards the glass tube at slow rate (2.25 mm/min), giving the pellets enough time to melt (Figure 2(b)). The applied pressure pushes upper non-molten pellets into the melt, thus re- moving any possible trapped air. This procedure is fol- lowed until the spiral heater slides over the whole surface of the tube. Then the heater is removed from the glass tube and the specimen is left to cool down slowly to the room temperature. Finally, received cylindrical rod is extracted from the glass tube, cut down to the length of 60mm, and then polished and glued to the special grips as shown in Figure 3. 2.3. Methods of Sterilization In this investigation we have applied three commonly Figure 2. Schematic representation of cylindrical rods pre- paration for creep measurements. Figure 3. Cylindrical rod sample glued to the special grips. used sterilization techniques: a) autoclave sterilization, b) EtO sterilization, and c) H2O2 plasma sterilization. Con- ditions of each method are briefly explained below: 2.3.1. Autoclave or Steam Sterilization Investigated samples were sterilized by the autoclave type Euroklav 23 V-S, manufactured by Melag, Germany. Before sterilization was applied, thin slices were placed inside the sterilization bags and sealed. The cylindrical rod specimens were put and sealed directly into steriliza- tion bags. Steam sterilization was applied for 75 min at 121˚C and 0.2 MPa. After the procedure the specimens were allowed to dry for 2 hours. 2.3.2. Ethylene Oxide In our study sterilizer De Lama S. Martino Sicc. (Pavia It.) of Blood Transfusion Centre of Slovenia has been utilized. The characteristics of the gas sterilizer are: EtO pressure 0.2 MPa, EtO/CO2 mixing ratio of 10%/90%, sterilization temperature 50˚C, relative humidity 60%, exposure time 8 hours. After the sterilization cycle 10 repeated air flushing cycles per hour are applied to re- move the EtO retained gas. The aeration procedure is validated with regard to sterilization cycle and nature of the material designed to be fumigated. For sterilization of our polyamide samples the chamber DLOG, manufac- tured by De-Lama was used. The samples were sterilized for 1 hour, and then they were aerated for 2 hours inside the chamber. Then, the sterilized samples in the sealed bags were taken out of the chamber and allowed to aerate for 10 days before tests were performed. 2.3.3. Hydrogen Peroxide Plasma Sterilization of our samples was done by using the Ster- rad® 100S system manufactured by Johnson & Johnson, USA. Both materials were sterilized at 50˚C for 1 hour. 2.4. Methods of Characterization Since properties of polymers may considerably change with time, which means that functionality and stability of polymeric products may be affected over time of their exploitation [23,24], we investigated how the steriliza- tion affects mono- and bi-modal polyamide time-depen- dent (long-term) mechanical properties. In order to char- acterize the effect of different sterilization techniques on materials’ long-term behavior, shear creep compliance measurements were performed according to the procedure described below. C opyright © 2011 SciRes. JBNB  The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides365 Shear creep measurements of investigated PA materi- als were performed with the Apparatus for Torsional Creep Measurements that has been designed and manu- factured at the Centre for Experimental Mechanics at the University of Ljubljana, Slovenia [25,26]. Initial part of the creep measuring procedure started with the annealing phase at higher temperature (110˚C) that should erase mechanical stress-strain history of the material. The thermal history to which specimens were exposed during the creep experiment is schematically shown in Figure 4. After the annealing phase the torsional creep meas- urements were performed in segmental form at 8 differ- ent elevated temperatures, nominally at 30˚C, 40˚C, 50˚C, 60˚C, 70˚C, 80˚C, 90˚C, and 100˚C in the time interval of 3 hours, under loading in shear by torque, t , of the constant magnitude ranging from 0.007 - 0.07 MPa, de- pending on the temperature at which the measurements were performed. In all cases the applied torque resulted in shear strain, not exceeding 0.05%. The response of the material was measured in terms of rotational angle of the specimen, ()t as a function of time and then recalcu- lated to the material creep compliance: 0 ttrl (1) where denotes radius of the circular cross section of the sample and -length of the sample.0 r l is the magni- tude of constant shear stress loading, calculated as: 0tp rI (2) with polar momentum for circular cross section geometry of cylindrical samples. The magnitudes of ap- plied shear stress loading were sufficiently low to ensure that the entire measurement procedure remained within the scope of the theory of linear viscoelasticity. Following the time-temperature superposition princi- ple, measured segments were shifted along the time-scale in relation to the segment, measured at the nominal ref- erence temperature, Tref = 50˚C. Shifting was executed 3715 182139 24 27 30 333642 45 485154 5760 Time, t (h) Temperature, T (°C) 110 100 90 80 70 60 50 40 30 3715 182139 24 27 30 333642 45 485154 5760 Time, t (h) Temperature, T (°C) 110 100 90 80 70 60 50 40 30 Figure 4. Schematic representation of the temperature- loading profile of the shear creep measurements on PA samples. by using the recently developed closed-form shifting (CFS) procedure [27]. CFS shifting methodology brings in error that is more than 10-times smaller as the under- lying experimental error of data being shifted. Obtained shift factors were then modeled by the WLF equation in order to obtain material constants, 1 and 2, which are presented in Table 1. By knowing these constants we calculated shift factors that corresponded to the shifting of master curves in order to represent the creep behavior at the reference temperature of 37˚C, which is a relevant temperature for medical applications. C C 3. Results and Discussion 3.1. Non-Sterilized Monomodal and Bimodal Materials Comparison Performing creep tests according to the procedure de- scribed above we firstly investigated the difference in time-dependent mechanical behavior of non-sterilized monomodal and bimodal PA6. In the concerned case it is appropriate to present the long-term creep behavior at the reference temperature close to the temperature of a hu- man body with respect to the proposed conditions of use. Figure 5 shows comparison of time-dependent creep compliances for both non-sterilized PA6 materials for the reference temperature 37˚C. Comparison of creep compliances may be interpreted in terms of long-time stability for the products made of such materials. In the diagram it is clearly seen that creep compliance of both materials increase with time, but, nevertheless, creep compliance of bimodal material is lower than such of monomodal along the complete ex- amined time scale. From the point of view of desired life-span of dental implants, which is more than 20 years [28], it seems to be reasonable to analyze mechanical properties and their change during this period of time. In our research the time period of 30 years was considered. In 30 years ≈ 109 s, it can be observed that creep com- pliance of bimodal material is approximately 25% lower Table 1. Constants C1 and C2, calculated from WLF mod- eling of shift factors for non-sterilized and sterilized mono- and bimodal PA6. Type of materialType of sterilization C1 (/) C2 (˚C) Non-sterilized 17.88 69.25 Ethylene Oxide (EtO) 10.41 31.40 Steam (autoclave) 13.84 58.92 Monomodal PA6 H2O2 plasma 10.96 36.52 Non-sterilized 17.50 81.72 Ethylene Oxide (EtO) 7.86 26.48 Steam (autoclave) 13.95 63.97 Bimodal PA6 H2O2 plasma 17.98 84.44 Copyright © 2011 SciRes. JBNB 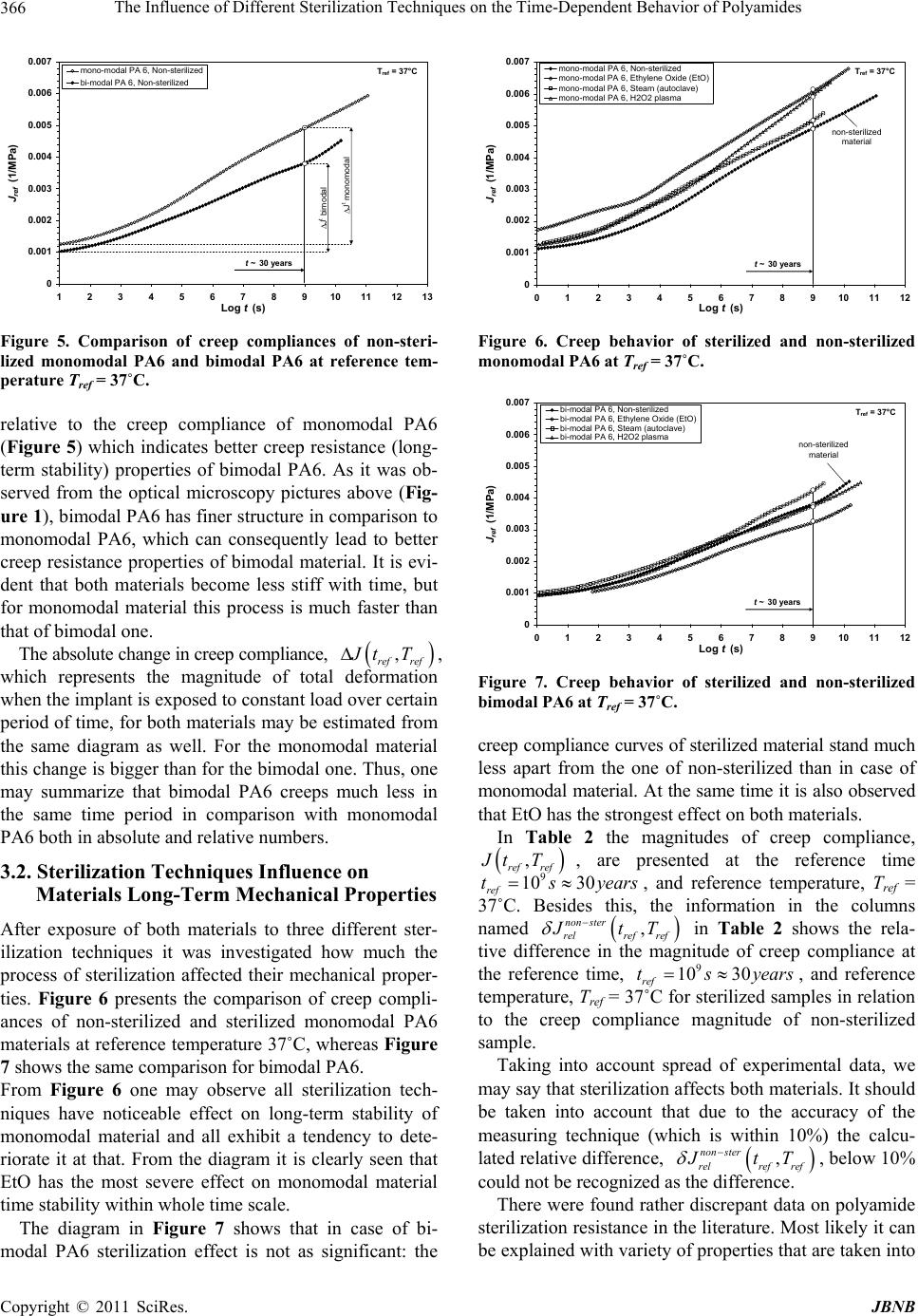 The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides 366 0 0.001 0.002 0.003 0.004 0.005 0.006 0.007 1234567891011121 Log t (s) J ref (1/MPa) 3 mono-modal PA 6, Non-sterilized bi-modal PA 6, Non-sterilizedT ref = 37°C t ~ 30 years J t bimodal J t monomodal Figure 5. Comparison of creep compliances of non-steri- lized monomodal PA6 and bimodal PA6 at reference tem- perature Tref = 37˚C. relative to the creep compliance of monomodal PA6 (Figure 5) which indicates better creep resistance (long- term stability) properties of bimodal PA6. As it was ob- served from the optical microscopy pictures above (Fig- ure 1), bimodal PA6 has finer structure in comparison to monomodal PA6, which can consequently lead to better creep resistance properties of bimodal material. It is evi- dent that both materials become less stiff with time, but for monomodal material this process is much faster than that of bimodal one. The absolute change in creep compliance, , ref ref tT, which represents the magnitude of total deformation when the implant is exposed to constant load over certain period of time, for both materials may be estimated from the same diagram as well. For the monomodal material this change is bigger than for the bimodal one. Thus, one may summarize that bimodal PA6 creeps much less in the same time period in comparison with monomodal PA6 both in absolute and relative numbers. 3.2. Sterilization Techniques Influence on Materials Long-Term Mechanical Properties After exposure of both materials to three different ster- ilization techniques it was investigated how much the process of sterilization affected their mechanical proper- ties. Figure 6 presents the comparison of creep compli- ances of non-sterilized and sterilized monomodal PA6 materials at reference temperature 37˚C, whereas Figure 7 shows the same comparison for bimodal PA6. From Figure 6 one may observe all sterilization tech- niques have noticeable effect on long-term stability of monomodal material and all exhibit a tendency to dete- riorate it at that. From the diagram it is clearly seen that EtO has the most severe effect on monomodal material time stability within whole time scale. The diagram in Figure 7 shows that in case of bi- modal PA6 sterilization effect is not as significant: the 0 0.001 0.002 0.003 0.004 0.005 0.006 0.007 012345678910111 Log t (s) J ref (1/MPa) 2 mono-modal PA 6, Non-sterilized mono-modal PA 6, Ethylene Oxide (EtO) mono-modal PA 6, Steam (autoclave) mono-modal PA 6, H2O2 plasma T ref = 37°C t ~ 30 years non-sterilized material Figure 6. Creep behavior of sterilized and non-sterilized monomodal PA6 at Tref = 37˚C. 0 0.001 0.002 0.003 0.004 0.005 0.006 0.007 012345678910111 Log t (s) J ref (1/MPa) 2 bi-modal PA 6, Non-sterilize d bi-modal PA 6, Ethylene Oxide (EtO) bi-modal PA 6, Steam (autoclave) bi-modal PA 6, H2O2 plasma T ref = 37°C t ~ 30 years non-sterilized material Figure 7. Creep behavior of sterilized and non-sterilized bimodal PA6 at Tref = 37˚C. creep compliance curves of sterilized material stand much less apart from the one of non-sterilized than in case of monomodal material. At the same time it is also observed that EtO has the strongest effect on both materials. In Table 2 the magnitudes of creep compliance, , ref ref Jt T 9 10 ref t s , are presented at the reference time , and reference temperature, Tref = 37˚C. Besides this, the information in the columns named 30years T 10 , ster Jt ref t non rel refref in Table 2 shows the rela- tive difference in the magnitude of creep compliance at the reference time, , and reference temperature, Tref = 37˚C for sterilized samples in relation to the creep compliance magnitude of non-sterilized sample. 930s years Taking into account spread of experimental data, we may say that sterilization affects both materials. It should be taken into account that due to the accuracy of the measuring technique (which is within 10%) the calcu- lated relative difference, , below 10% could not be recognized as the difference. , non ster relref ref JtT There were found rather discrepant data on polyamide sterilization resistance in the literature. Most likely it can be explained with variety of properties that are taken into C opyright © 2011 SciRes. JBNB 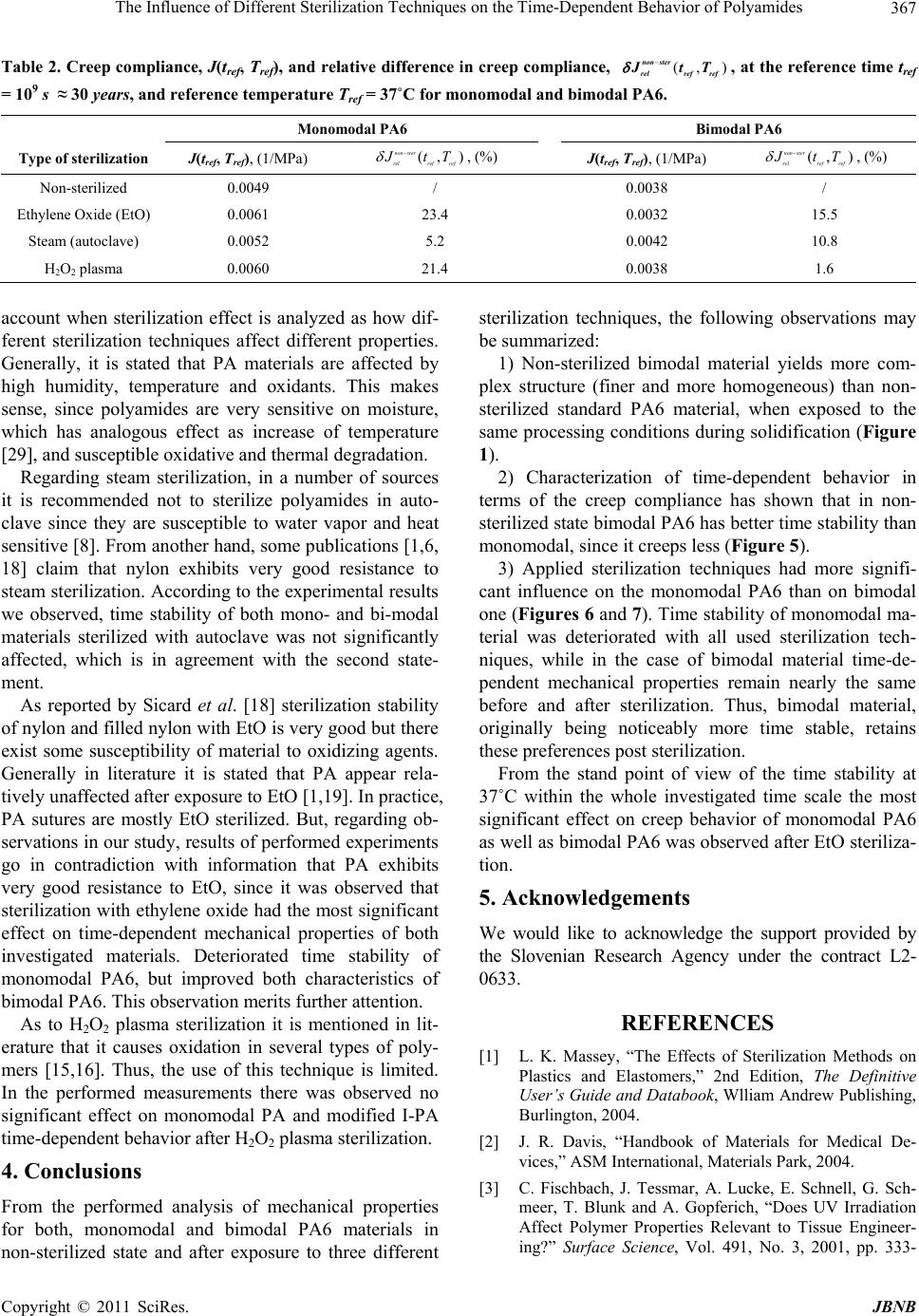 The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides Copyright © 2011 SciRes. JBNB 367 Table 2. Creep compliance, J(tref, Tref), and relative difference in creep compliance, (, ) non ster relref ref tT , at the reference time tref = 109 s ≈ 30 years, and reference temperature Tref = 37˚C for monomodal and bimodal PA6. Monomodal PA6 Bimodal PA6 Type of sterilization J(tref, Tref), (1/MPa) (, ) non ster relref ref tT , (%) J(tref, Tref), (1/MPa) (, ) non ster relref ref tT , (%) Non-sterilized 0.0049 / 0.0038 / Ethylene Oxide (EtO) 0.0061 23.4 0.0032 15.5 Steam (autoclave) 0.0052 5.2 0.0042 10.8 H2O2 plasma 0.0060 21.4 0.0038 1.6 account when sterilization effect is analyzed as how dif- ferent sterilization techniques affect different properties. Generally, it is stated that PA materials are affected by high humidity, temperature and oxidants. This makes sense, since polyamides are very sensitive on moisture, which has analogous effect as increase of temperature [29], and susceptible oxidative and thermal degradation. Regarding steam sterilization, in a number of sources it is recommended not to sterilize polyamides in auto- clave since they are susceptible to water vapor and heat sensitive [8]. From another hand, some publications [1,6, 18] claim that nylon exhibits very good resistance to steam sterilization. According to the experimental results we observed, time stability of both mono- and bi-modal materials sterilized with autoclave was not significantly affected, which is in agreement with the second state- ment. As reported by Sicard et al. [18] sterilization stability of nylon and filled nylon with EtO is very good but there exist some susceptibility of material to oxidizing agents. Generally in literature it is stated that PA appear rela- tively unaffected after exposure to EtO [1,19]. In practice, PA sutures are mostly EtO sterilized. But, regarding ob- servations in our study, results of performed experiments go in contradiction with information that PA exhibits very good resistance to EtO, since it was observed that sterilization with ethylene oxide had the most significant effect on time-dependent mechanical properties of both investigated materials. Deteriorated time stability of monomodal PA6, but improved both characteristics of bimodal PA6. This observation merits further attention. As to H2O2 plasma sterilization it is mentioned in lit- erature that it causes oxidation in several types of poly- mers [15,16]. Thus, the use of this technique is limited. In the performed measurements there was observed no significant effect on monomodal PA and modified I-PA time-dependent behavior after H2O2 plasma sterilization. 4. Conclusions From the performed analysis of mechanical properties for both, monomodal and bimodal PA6 materials in sterilization techniques, the following observations may be summarized: 1) Non-sterilized non-sterilized state and after exposure to three different bimodal material yields more com- pl ) Characterization of time-dependent behavior in te ore signifi- ca the time stability at 37 knowledgements he support provided by REFERENCES [1] L. K. Masseyzation Methods on dbook of Materials for Medical De- G. Sch- ex structure (finer and more homogeneous) than non- sterilized standard PA6 material, when exposed to the same processing conditions during solidification (Figure 1). 2 rms of the creep compliance has shown that in non- sterilized state bimodal PA6 has better time stability than monomodal, since it creeps less (Figure 5). 3) Applied sterilization techniques had m nt influence on the monomodal PA6 than on bimodal one (Figures 6 and 7). Time stability of monomodal ma- terial was deteriorated with all used sterilization tech- niques, while in the case of bimodal material time-de- pendent mechanical properties remain nearly the same before and after sterilization. Thus, bimodal material, originally being noticeably more time stable, retains these preferences post sterilization. From the stand point of view of ˚C within the whole investigated time scale the most significant effect on creep behavior of monomodal PA6 as well as bimodal PA6 was observed after EtO steriliza- tion. 5. Ac We would like to acknowledge t the Slovenian Research Agency under the contract L2- 0633. , “The Effects of Sterili Plastics and Elastomers,” 2nd Edition, The Definitive User’s Guide and Databook, Wlliam Andrew Publishing, Burlington, 2004. [2] J. R. Davis, “Han vices,” ASM International, Materials Park, 2004. [3] C. Fischbach, J. Tessmar, A. Lucke, E. Schnell, meer, T. Blunk and A. Gopferich, “Does UV Irradiation Affect Polymer Properties Relevant to Tissue Engineer- ing?” Surface Science, Vol. 491, No. 3, 2001, pp. 333-  The Influence of Different Sterilization Techniques on the Time-Dependent Behavior of Polyamides 368 345. doi:10.1016/S0039-6028(01)01297-3 [4] D. Slatter, “Textbook of small animal surgery,” 3rd Edi- M. J. Drews, J. are Prod- nce tion, Elsevier Science, Amsterdam, 2003. [5] Y. H. An, F. I. Alvi, Q. Kang, M. Laberge, Zhang, M. A. Matthews and C. R. Arciola, “Effects of Sterilization on Implant Mechanical Property and Bio- compatibility,” The International Journal of Artificial Organs, Vol. 28, No. 11, 2005, pp. 1126-1137. [6] W. Rogers, “Sterilisation of Polymer Healthc ucts,” Rapra Technology limited, Shrewsbury, 2005. [7] P. D. Nair, P. J. Doherty and D. F. Williams, “Influe of Steam Sterilization Induced Surface Changes of Poly- ester Materials on Its Biocompatibility,” Bulletin of Ma- terial Science, Vol. 20, No. 7, 1997, pp. 9-991. doi:10.1007/BF02744887 [8] J. H. Young, “Steam Sterilization: Scientific Principles,” . H. Samuel, “Sterilisa- )90082-5 In: M. Reichert and J. H. Young, Eds., Sterilization Tech- nology for the Health Care Facility, Aspen Publishers, Gaithersburg, 1997, pp. 33-124. [9] I. P. Matthews, C. Gibson and A tion of Implantable Devices,” Clinical Materials, Vol. 15, No. 3, 1994, pp. 191-215. doi:10.1016/0267-6605(94 gradation Resistance d R. 6 [10] R. C. Portnoy, “Medical Plastics-De & Failure Analysis. Plastics Design Library,” A Division of William Andrew Inc., Norwich, New York, 1998. [11] Y. Marois, Z. Zhang, M. Vert, X. Deng, R. Lenz an Guidoin, “Effect of Sterilization on the Physical and Structural Characteristics of Polyhydroxyoctanoate (PHO),” Journal of Biomaterials Science, Polymer Edition, Vol. 10, No. 4, 1999, pp. 469-482. doi:10.1163/156856299X0021 itchins, T. O. Woods, S. Novotna and R. Svi- K. Merritt, T. O. Woods, S. G. McNamee .CO;2 [12] A. D. Lucas, K. Merritt, V. M. H G. McNamee, D. B. Lyle and S. A. Brown, “Residual Ethylene Oxide in Medical Devices and Device Mate- rial,” Journal of Biomedical Materials Research, Part B, Vol. 66, No. 2, 2003, pp. 52-548. [13] I. Buben, V. Melichercikova, N. takova, “Problems Associated with Sterilization using Ethylene Oxide. Residues in Treated Materials,” Central European Journal of Public Health, Vol. 7, No. 4, 1999, pp. 197-202. [14] S. A. Brown, and V. M. Hitchins, “Effects of Different Disinfection and sterilization methods on tensile strength of materials used for single-use devices,” Biomedical Instrumentation and Technology, Vol. 36, No. 1, 2002, pp. 23-27. doi:10.2345/0899-8205(2002)36[23:EODDAS]2.0 [15] S. Lerouge, M. Tabrizian, M. R. Wertheimer, R. Mar- chand, L. H. Yahia, “Safety of Plasma-Based Sterilization: Surface Modifications of Polymeric Medical Devices In- duced by Sterrad® and PlazlyteTM Processes,” Bio- Medical Materials and Engineering, Vol. 12, No. 1, 2002, pp. 3-13. [16] N. Ma, A. Petit, O. L. Huk, L. Yahia and M. Tabrizian, “Safety Issue of Re-sterilization of Polyurethane Elec- trophysiology Catheters: A Cytotoxicity Study,” Journal of Biomaterials Science, Polymer Edition, Vol. 14, No. 3, 2003, pp. 213-226. doi:10.1163/156856203763572671 [17] http://www.eldonjames.com/frames/sterilize.html (last access on the 15th of July, 2011). [18] G. K. Sicard, K. Hayashi and P. A. Manley, “Evaluation of 5 Types of Fishing Material, 2 Sterilization Methods, and a Crimp-Clamp System for Extra-Articular Stabiliza- tion of the Canine Stifle Joint,” Veterinary Surgery, Vol. 31, No. 1, 2002, pp. 78-84. doi:10.1053/jvet.2002.30539 [19] B. M. Kirby and J. W. Wilson, “Knot Strength of Ny- lon-Band Cerclage,” Acta Orthopaedica Scandinavica, Vol. 60, No. 6, 1989, pp. 8-696. [20] G. B. Bochkova and A. S. Yushin, “Impact of Some Liq- uids and Steam Sterilization on the Strength of Polyamide Membranes,” Khimiko-Farmatsevticheskii Zhurnal, Vol. 25, No. 5, 1991, pp. 78-81. [21] M. Gatineau, L. Huneault, B. Lussier and J. Lefevre- Lavoie, “Mechanical Evaluation of Hydrogen Peroxide Gas Plasma Sterilization of Nylon Lines Used for Ex- tra-Articular Stabilization of the Canine Stifle Joint,” Ve- terinary Surgery, Vol. 39, No. 1, 2010, pp. 48-53. doi:10.1111/j.1532-950X.2009.00616.x [22] I. Emri and B. S. von Bernstorff, “The Effect of Molecu- lar Mass Distribution on Time-Dependent Behavior of Polyamides,” Journal of Applied Mechanics, Vol. 73, No. 5, 2006, pp. 1-6. doi:10.1115/1.2173008 [23] N. W. Tschoegl, “The Phenomenological Theory of Lin- ear Viscoelastic Behavior: An Introduction,” Springer- Verlag, Berlin, 1989. [24] J. J. Aklonis., W. J. Macknight and M. Shen, “Introduc- tion to Polymer Viscoelasticity,” 2nd Edition, John Wiley & Sons, New York, 1983. [25] P. Metlikovič and I. Emri, “Analiza Procesa Lezenja Viskoelastičnih Materialov Pod Vplivom Strižne Obre- menitve,” Kovine, Zlitine, Tehnologije, Vol. 28, No. 1-2, 1994, pp. 407-409. [26] R. Cvelbar and I. Emri, “Analiza Prehodnega Pojava Pri merjenju Lezenja Vviskoelastičnih Materialov,” Kovine, zlitine, tehnologije, Vol. 28, No. 1-2, 1994, pp. 359-362. [27] M. Gergesova, B. Zupančič, I. Saprunov and I. Emri, “The Closed Form T-T-P Shifting (CFS) Algorithm,” Journal of Rheology, Vol. 55, No. 1, 2011, pp. 1-16. doi:10.1122/1.3503529 [28] M. Ayson, J. Leichter and K. Lyons, “Dental Implants: Patient Selection Factors. Evidence-Based Review,” University of Otago, Dunedin, 2009. [29] V. Pavšek and I. Emri, “On the Influence of Moisture on the Mechanical-Properties of Polymers,” Journal of Me- chanical Engineering, Vol. 41, No. 1-2, 1995, pp. 39-45. C opyright © 2011 SciRes. JBNB
|