 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 454-460 doi:10.4236/jbnb.2011.24055 Published Online October 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and a Sensitive Biosensor Platform Yong Gao1, Yu Cao1, Guiling Song1, Yiming Tang1, Huaming Li1,2* 1College of Chemistry, Xiangtan University, Xiangtan, China; 2Key Laboratory of Polymeric Materials and Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymeric Materials of College of Hunan Province, and Key Lab of Environment-Friendly Chemistry and Application in Ministry of Education, Xiangtan University, Xiangtan, China. Email: *lihuaming@xtu.edu.cn Received July 30th, 2011; revised August 18th, 2011; accepted September 1st, 2011. ABSTRACT In this study, we presented the preparation of β-cyclodextrin (β-CD) covalently functionalized single-walled carbon nano- tubes (SWCNTs) and its application in modifying the solid glass carbon electrode (GCE). Cyclic voltammetry (CV) method was employed to evaluate the performance of the modified GCE. Solubility experiment indicated the conjugation of SWCNTs and β-CD, SWCNTs-β-CD with 8 wt% β-CD content could be well dispersed in water. High-resolution transmis- sion electron microscopy (HRTEM) demonstrated that the aggregated SWCNTs bundle were effectively exfoliated to small bundle, even individual tube. The β-CD component was grafted on the side walls as well as tips of SWCNTs, and the grafted β-CD component was not uniformly coated on the surface of SWCNTs. The CV measurements indicated the performance of the GCE modified by SWCNTs-β-CD was better than that of the GCE modified by the hybrid of SWCNTs/β-CD, where ascorbic acid (AA) and uric acid (UA) were selected as a prelimiltary substrate to evaluate it. The enhanced performance of the modified GCE should be ascribed to the integration of the excellent electrocatalytic property of SWCNTs with the inclu- sion ability of β-CD to analyte molecule. Keywords: Single-Walled Carbon Nanotubes (SWCNTs), β-Cyclodextrin (β-CD), Electrochemical Sensor, Covalently 1. Introduction Since the landmark paper on carbon nanotubes (CNTs) in 1991 by Iijima [1], they have attracted great interdis- ciplinary interest due to the extraordinarily electrical, optical, and mechanical properties that CNTs possess [2,3]. Nowadays, CNTs have the potential of being trans- formed into new materials that will traverse a wide range of applications, such as sensors, nanoelectronics, bio- medical devices, and high-strength fibers [4-7]. However, aggregated large nanotube bundles were insolubility in routine solvent, which makes it difficult to manipulate and utilize CNTs [8,9]. Two strategies, including noncovalent and covalent modification, have been developed to modify the CNTs to overcome the solubility limitations [10-16]. In the cased of the noncovalent modification strategy, a rather limited surfactants and compounds with benzene ring or condensed aromatic ring are usually used to disperse the CNTs bundle. The virtues of noncovalent modification lie in the maintaining of the nanotube’s electronic struc- ture and its relatively simple process [17]. With respect to noncovalent modification, covalent functionalization of CNTs provides a broad space in singling out the spe- cies of compound, for a broad range of functional groups could be incorporated onto the surface of CNTs by more versatile modification approaches. Covalently function- alized CNTs demonstrated wide applications in many fields, such as in catalytic and biological applications etc [18-22]. For example, Neelgund, et al. [18] reported the synthesis of poly(lactic acid) functionalized CNTs-Pd nanocatalyst by covalent grafting of poly(lactic acid) onto CNTs and subsequent deposition of Pd nano- parti- cles. The nanocatalyst demonstrated more effective activity in the promotion of Heck cross-coupling reaction between aryl halides and n-butyl acrylate. Karousis, et al.  β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and 455 a Sensitive Biosensor Platform [22] reported that the preparation of water-soluble pep- tidomimetics covalently grafted carbon nanotubes. The prepared peptidomimetic functionalized CNT conjugates showed extremely advantageous in the inhibition of in- flammation or malignancy and potential future biological applications in the area of drug delivery systems. Cyclodextrins (CDs) are cyclic oligosaccharides with D-(+)-glucose as the repeating unit coupled by 1,4-a- linkages. α-, β-, and γ-CDs are commonly available forms which consist of 6, 7, and 8 glucose units, respec- tively. The most characteristic property of CDs is their remarkable ability to form inclusion complexes with a wide variety of guest molecules due to their different cavity sizes. This property endowed CDs many wide- spread applications not only in pharmaceutical chemistry, food technology, analytical chemistry, chemical synthe- sis, and catalysis [23-27], but also for constructing mo- lecular devices and machines [28-32]. Therefore, the combination of β-CD and CNTs is sure to generate sig- nificant and interesting object for supramolecular chem- istry, biomedicine and nanodevice construction. How- ever, most studies were focused on the hybrids of β-CD/CNTs due to its easy operation, and the studies concerning the preparation of β-CD covalently function- alized CNTs and its application were rare except for sev- eral reports [17,33-37]. Herein, we presented the preparation of β-CD cova- lently functionalized SWCNTs by a reaction sequence involving oxidation and amidation reaction. The obtained conjugation of SWCNTs and β-CD, SWCNTs-β-CD showed an excellent stability in pure water. And then, SWCNTs- β-CD was applied to modify the bare GCE. The performance of modified GCE was investigated by cyclic voltammetry (CV). Interestingly, the performance of GCE modified by SWCNTs-β-CD conjugation was better than that of GCE modified by the hybrids of β-CD/SWCNTs under identical conditions. FTIR, TGA as well as HRTEM were employed to characterize the titled product. To the best of our knowledge, there is no report on the modification of GCE with β-CD covalently functionalized SWCNTs. 2. Experimental 2.1. Materials SWCNTs with a purity of 60 wt% were obtained from Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China), which were prepared by the chemical vapor deposition (CVD) method. β-CD was purchased from Sigma-Al- drich. p-Toluenesulfonyl (p-TsCl), 1,6-Hexanediamine (HDA) were chemical grade. Freshly prepared AA and UA solutions prior to measurements were used. All other reagents and solvents were purchased from commercial suppliers and used as received. 2.2. Oxidation of SWCNTs In a typical experiment, a round bottom flask was char- ged with 200 mg of SWCNTs and cooled in an ice bath. Then, 20 mL of concentrated H2SO4/HNO3 (3:1 by vol- ume) was slowly added. The mixture was subjected to sonication at 0˚C for 30 min. The flask was stirred for 20 h at 20˚C. The dispersion was then carefully poured into 1000 mL of deionized water. The black slurry was va- cuum filtered on a 0.22 µm Teflon membrane, resulting in the formation of a black filter cake. The black filter cake was then subjected to dispersion by sonication and rinsed with deionized water until the pH value of the filtrate was neutral. The product was dried under vac- uum. 2.3. Preparation of Mono-6-(hexanediamino)-β-cy-clodextrin (HDA-β-CD) HDA-β-CD was prepared by two successive chemical processes. Firstly, Mono-6-OTs-β-CD was synthesized according to the method reported in the literature [38]. In a typical experiment, β-CD (30.0 g, 26.4 mmol) was suspended in 250 mL of water, and 10 mL NaOH aque- ous solution (8.2 mol·L–1) was added dropwise over 1 h. When the suspension became homogeneous slightly yel- low solution, 15 mL of p-TsCl acetonitrile solution (13.2 mol·L –1) was added dropwise over 2 h. This process re- sulted in the formation of a white precipitate. After 2 h of stirring at 25˚C, the precipitate was removed by filtration under reduced press and the filtrate was refrigerated overnight at 4˚C. The resulting white precipitate was recrystallized from deionized water and dried under va- cuum. 1H NMR (400 MHz, CDCl3): δ = 7.42 - 7.45 (2H, =CHmeta), 7.74 - 7.77(2H,=CHortho), 2.42(3H,CH3), 3.83 - 3.68 (28H; C(3)–H, C(6)–H, C(5)–H), 3.51 - 3.25 (14H; C(2)–H, C(4)–H); IR (KBr, cm-1): 3382, 2927, 1029, 1320. The preparation of HDA-β-CD was performed as de- scribed by Liu, et al. [39]. In a typical experiment, 5.0 g of Mono-6-OTs-β-CD (3.8 mmol) and 10.0 g (0.17 mol) of HAD were charged into a bottom flask equipped with a condenser. The mixture was stirred at 75˚C for 4 h, and then the mixture was allowed to cool to room tempera- ture. Subsequently, 300 mL of cold acetone was added, resulting in the formation of a precipitate. The obtained precipitate was dissolved in 10 mL of water-methanol (v/v 3:1) mixture, and then poured into 300 mL of ace- tone. This operation was repeated several times for the complete removal of unreacted HAD. The obtained sam- Copyright © 2011 SciRes. JBNB 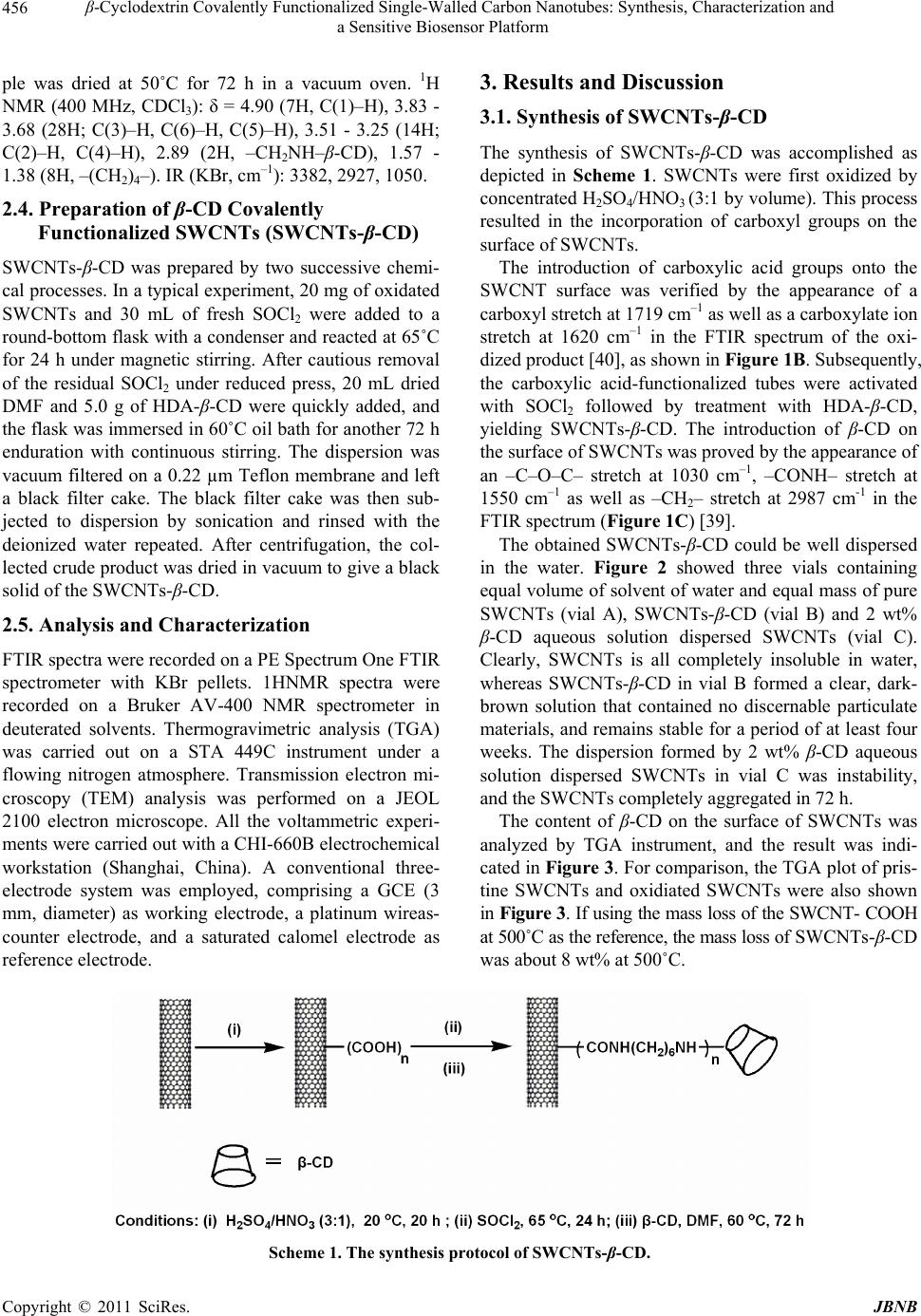 β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and a Sensitive Biosensor Platform Copyright © 2011 SciRes. JBNB 456 3. Results and Discussion ple was dried at 50˚C for 72 h in a vacuum oven. 1H NMR (400 MHz, CDCl3): δ = 4.90 (7H, C(1)–H), 3.83 - 3.68 (28H; C(3)–H, C(6)–H, C(5)–H), 3.51 - 3.25 (14H; C(2)–H, C(4)–H), 2.89 (2H, –CH2NH–β-CD), 1.57 - 1.38 (8H, –(CH2)4–). IR (KBr, cm–1): 3382, 2927, 1050. 3.1. Synthesis of SWCNTs-β-CD The synthesis of SWCNTs-β-CD was accomplished as depicted in Scheme 1. SWCNTs were first oxidized by concentrated H2SO4/HNO3 (3:1 by volume). This process resulted in the incorporation of carboxyl groups on the surface of SWCNTs. 2.4. Preparation of β-CD Covalently Functionalized SWCNTs (SWCNTs-β-CD) SWCNTs- β-CD was prepared by two successive chemi- cal processes. In a typical experiment, 20 mg of oxidated SWCNTs and 30 mL of fresh SOCl2 were added to a round-bottom flask with a condenser and reacted at 65˚C for 24 h under magnetic stirring. After cautious removal of the residual SOCl2 under reduced press, 20 mL dried DMF and 5.0 g of HDA-β-CD were quickly added, and the flask was immersed in 60˚C oil bath for another 72 h enduration with continuous stirring. The dispersion was vacuum filtered on a 0.22 µm Teflon membrane and left a black filter cake. The black filter cake was then sub- jected to dispersion by sonication and rinsed with the deionized water repeated. After centrifugation, the col- lected crude product was dried in vacuum to give a black solid of the SWCNTs-β-CD. The introduction of carboxylic acid groups onto the SWCNT surface was verified by the appearance of a carboxyl stretch at 1719 cm–1 as well as a carboxylate ion stretch at 1620 cm–1 in the FTIR spectrum of the oxi- dized product [40], as shown in Figure 1B. Subsequently, the carboxylic acid-functionalized tubes were activated with SOCl2 followed by treatment with HDA-β-CD, yielding SWCNTs-β-CD. The introduction of β-CD on the surface of SWCNTs was proved by the appearance of an –C–O–C– stretch at 1030 cm–1, –CONH– stretch at 1550 cm–1 as well as –CH2– stretch at 2987 cm-1 in the FTIR spectrum (Figure 1C) [39]. The obtained SWCNTs-β-CD could be well dispersed in the water. Figure 2 showed three vials containing equal volume of solvent of water and equal mass of pure SWCNTs (vial A), SWCNTs-β-CD (vial B) and 2 wt% β-CD aqueous solution dispersed SWCNTs (vial C). Clearly, SWCNTs is all completely insoluble in water, whereas SWCNTs-β-CD in vial B formed a clear, dark- brown solution that contained no discernable particulate materials, and remains stable for a period of at least four weeks. The dispersion formed by 2 wt% β-CD aqueous solution dispersed SWCNTs in vial C was instability, and the SWCNTs completely aggregated in 72 h. 2.5. Analysis and Characterization FTIR spectra were recorded on a PE Spectrum One FTIR spectrometer with KBr pellets. 1HNMR spectra were recorded on a Bruker AV-400 NMR spectrometer in deuterated solvents. Thermogravimetric analysis (TGA) was carried out on a STA 449C instrument under a flowing nitrogen atmosphere. Transmission electron mi- croscopy (TEM) analysis was performed on a JEOL 2100 electron microscope. All the voltammetric experi- ments were carried out with a CHI-660B electrochemical workstation (Shanghai, China). A conventional three- electrode system was employed, comprising a GCE (3 mm, diameter) as working electrode, a platinum wireas- counter electrode, and a saturated calomel electrode as reference electrode. The content of β-CD on the surface of SWCNTs was analyzed by TGA instrument, and the result was indi- cated in Figure 3. For comparison, the TGA plot of pris- tine SWCNTs and oxidiated SWCNTs were also shown in Figure 3. If using the mass loss of the SWCNT- COOH at 500˚C as the reference, the mass loss of SWCNTs-β-CD was about 8 wt% at 500˚C. Scheme 1. The synthesis protocol of SWCNTs-β-CD. 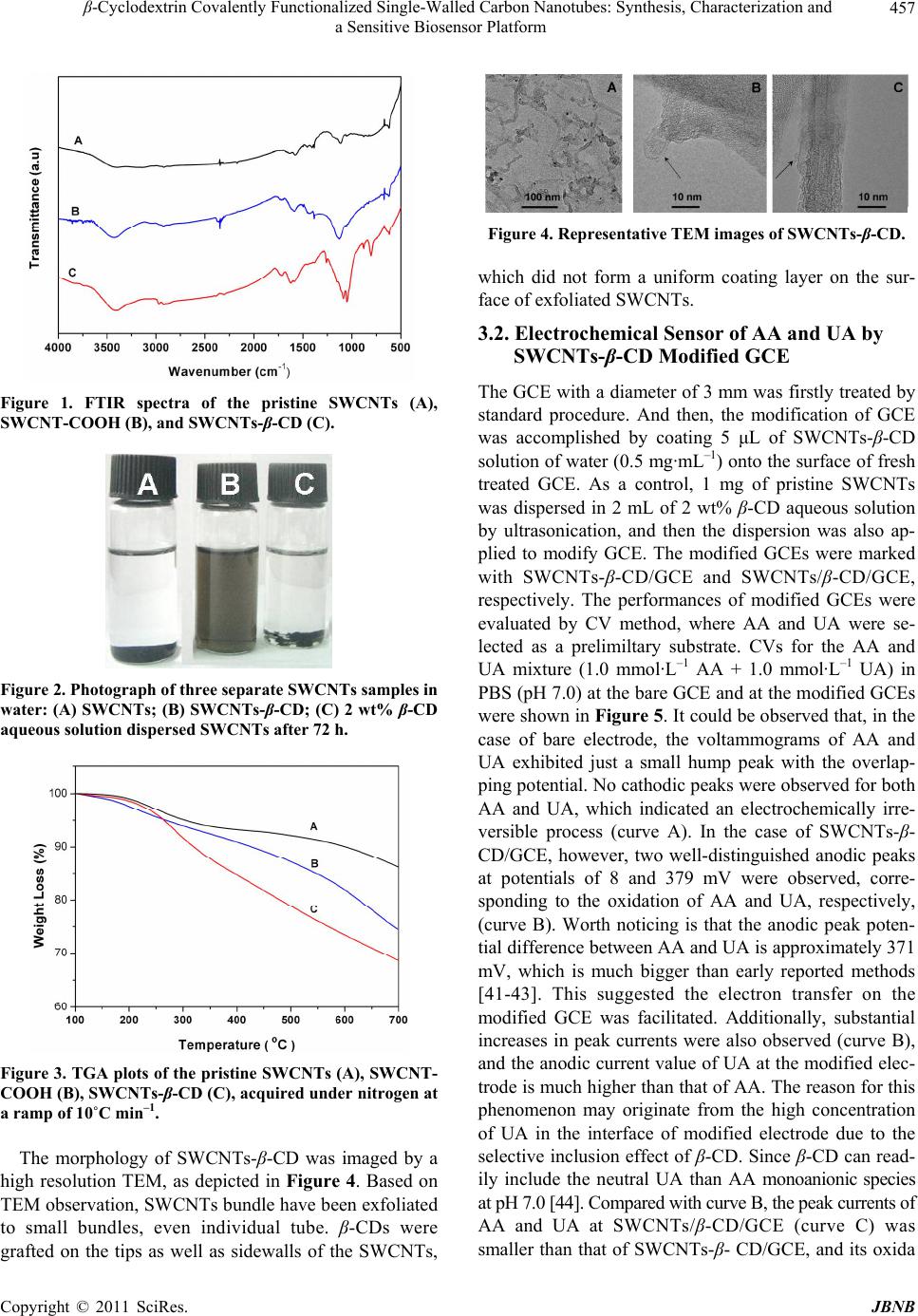 β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and 457 a Sensitive Biosensor Platform Figure 1. FTIR spectra of the pristine SWCNTs (A), SWCNT-COOH (B), and SWCNTs-β-CD (C). Figure 2. Photograph of three separate SWCNTs samples in water: (A) SWCNTs; (B) SWCNTs-β-CD; (C) 2 wt% β-CD aqueous solution dispersed SWCNTs after 72 h. Figure 3. TGA plots of the pristine SWCNTs (A), SWCNT- COOH (B), SWCNTs-β-CD (C), acquired under nitrogen at a ramp of 10˚C min–1. The morphology of SWCNTs-β-CD was imaged by a high resolution TEM, as depicted in Figure 4. Based on TEM observation, SWCNTs bundle have been exfoliated to small bundles, even individual tube. β-CDs were grafted on the tips as well as sidewalls of the SWCNTs, Figure 4. Representative TEM images of SWCNTs-β-CD. which did not form a uniform coating layer on the sur- face of exfoliated SWCNTs. 3.2. Electrochemical Sensor of AA and UA by SWCNTs-β-CD Modified GCE The GCE with a diameter of 3 mm was firstly treated by standard procedure. And then, the modification of GCE was accomplished by coating 5 μL of SWCNTs-β-CD solution of water (0.5 mg·mL–1) onto the surface of fresh treated GCE. As a control, 1 mg of pristine SWCNTs was dispersed in 2 mL of 2 wt% β-CD aqueous solution by ultrasonication, and then the dispersion was also ap- plied to modify GCE. The modified GCEs were marked with SWCNTs-β-CD/GCE and SWCNTs/β-CD/GCE, respectively. The performances of modified GCEs were evaluated by CV method, where AA and UA were se- lected as a prelimiltary substrate. CVs for the AA and UA mixture (1.0 mmol·L–1 AA + 1.0 mmol·L–1 UA) in PBS (pH 7.0) at the bare GCE and at the modified GCEs were shown in Figure 5. It could be observed that, in the case of bare electrode, the voltammograms of AA and UA exhibited just a small hump peak with the overlap- ping potential. No cathodic peaks were observed for both AA and UA, which indicated an electrochemically irre- versible process (curve A). In the case of SWCNTs-β- CD/GCE, however, two well-distinguished anodic peaks at potentials of 8 and 379 mV were observed, corre- sponding to the oxidation of AA and UA, respectively, (curve B). Worth noticing is that the anodic peak poten- tial difference between AA and UA is approximately 371 mV, which is much bigger than early reported methods [41-43]. This suggested the electron transfer on the modified GCE was facilitated. Additionally, substantial increases in peak currents were also observed (curve B), and the anodic current value of UA at the modified elec- trode is much higher than that of AA. The reason for this phenomenon may originate from the high concentration of UA in the interface of modified electrode due to the selective inclusion effect of β-CD. Since β-CD can read- ily include the neutral UA than AA monoanionic species at pH 7.0 [44]. Compared with curve B, the peak currents of AA and UA at SWCNTs/β-CD/GCE (curve C) was smaller than that of SWCNTs-β- CD/GCE, and its oxida Copyright © 2011 SciRes. JBNB 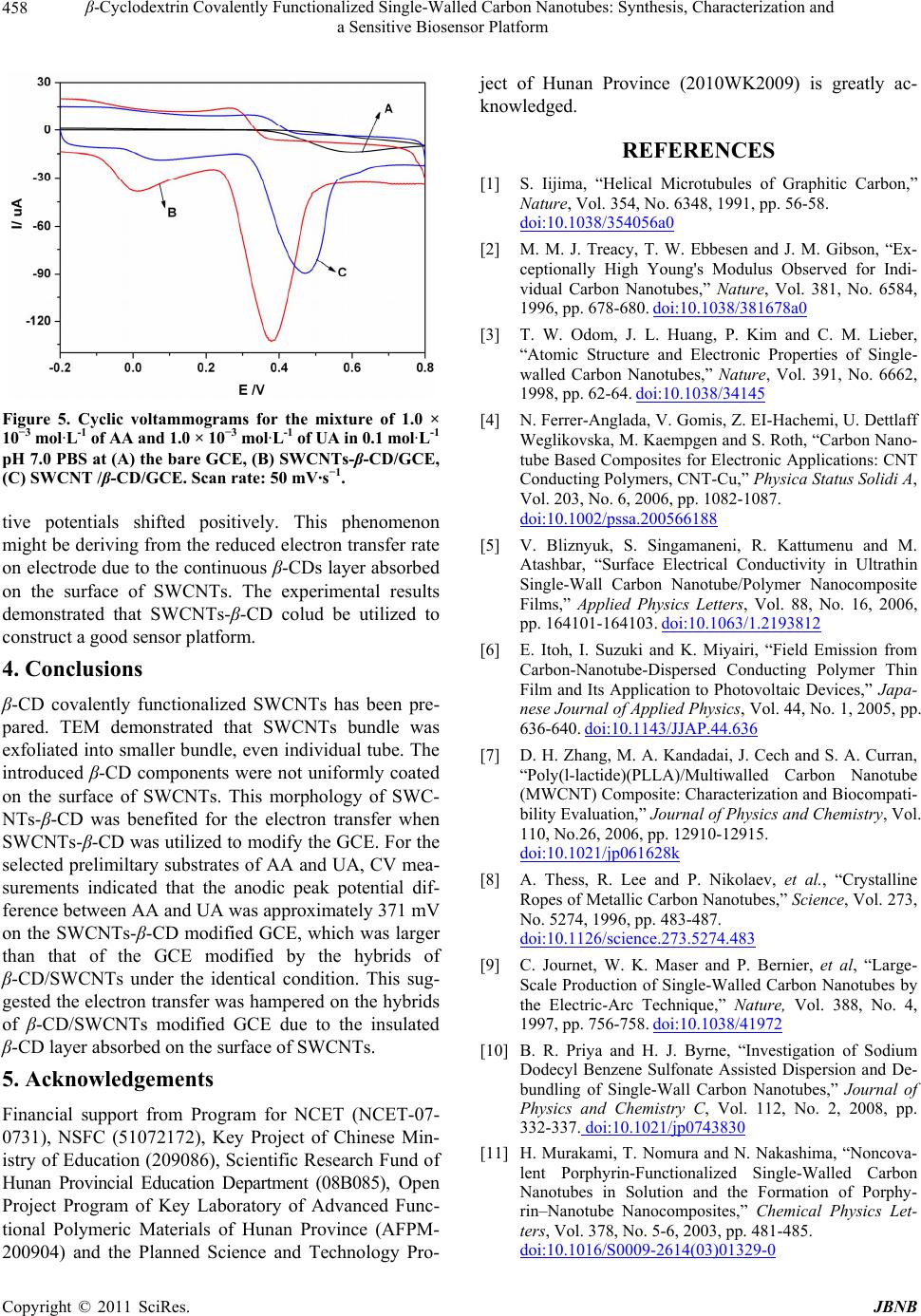 β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and 458 a Sensitive Biosensor Platform Figure 5. Cyclic voltammograms for the mixture of 1.0 × 10−3 mol·L-1 of AA and 1.0 × 10−3 mol·L-1 of UA in 0.1 mol·L-1 pH 7.0 PBS at (A) the bare GCE, (B) SWCNTs-β-CD/GCE, (C) SWCNT /β-CD/GCE. Scan rate: 50 mV·s−1. tive potentials shifted positively. This phenomenon might be deriving from the reduced electron transfer rate on electrode due to the continuous β-CDs layer absorbed on the surface of SWCNTs. The experimental results demonstrated that SWCNTs-β-CD colud be utilized to construct a good sensor platform. 4. Conclusions β-CD covalently functionalized SWCNTs has been pre- pared. TEM demonstrated that SWCNTs bundle was exfoliated into smaller bundle, even individual tube. The introduced β-CD components were not uniformly coated on the surface of SWCNTs. This morphology of SWC- NTs-β-CD was benefited for the electron transfer when SWCNTs- β-CD was utilized to modify the GCE. For the selected prelimiltary substrates of AA and UA, CV mea- surements indicated that the anodic peak potential dif- ference between AA and UA was approximately 371 mV on the SWCNTs-β-CD modified GCE, which was larger than that of the GCE modified by the hybrids of β-CD/SWCNTs under the identical condition. This sug- gested the electron transfer was hampered on the hybrids of β-CD/SWCNTs modified GCE due to the insulated β-CD layer absorbed on the surface of SWCNTs. 5. Acknowledgements Financial support from Program for NCET (NCET-07- 0731), NSFC (51072172), Key Project of Chinese Min- istry of Education (209086), Scientific Research Fund of Hunan Provincial Education Department (08B085), Open Project Program of Key Laboratory of Advanced Func- tional Polymeric Materials of Hunan Province (AFPM- 200904) and the Planned Science and Technology Pro- ject of Hunan Province (2010WK2009) is greatly ac- knowledged. REFERENCES [1] S. Iijima, “Helical Microtubules of Graphitic Carbon,” Nature, Vol. 354, No. 6348, 1991, pp. 56-58. doi:10.1038/354056a0 [2] M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, “Ex- ceptionally High Young's Modulus Observed for Indi- vidual Carbon Nanotubes,” Nature, Vol. 381, No. 6584, 1996, pp. 678-680. doi:10.1038/381678a0 [3] T. W. Odom, J. L. Huang, P. Kim and C. M. Lieber, “Atomic Structure and Electronic Properties of Single- walled Carbon Nanotubes,” Nature, Vol. 391, No. 6662, 1998, pp. 62-64. doi:10.1038/34145 [4] N. Ferrer-Anglada, V. Gomis, Z. EI-Hachemi, U. Dettlaff Weglikovska, M. Kaempgen and S. Roth, “Carbon Nano- tube Based Composites for Electronic Applications: CNT Conducting Polymers, CNT-Cu,” Physica Status Solidi A, Vol. 203, No. 6, 2006, pp. 1082-1087. doi:10.1002/pssa.200566188 [5] V. Bliznyuk, S. Singamaneni, R. Kattumenu and M. Atashbar, “Surface Electrical Conductivity in Ultrathin Single-Wall Carbon Nanotube/Polymer Nanocomposite Films,” Applied Physics Letters, Vol. 88, No. 16, 2006, pp. 164101-164103. doi:10.1063/1.2193812 [6] E. Itoh, I. Suzuki and K. Miyairi, “Field Emission from Carbon-Nanotube-Dispersed Conducting Polymer Thin Film and Its Application to Photovoltaic Devices,” Japa- nese Journal of Applied Physics, Vol. 44, No. 1, 2005, pp. 636-640. doi:10.1143/JJAP.44.636 [7] D. H. Zhang, M. A. Kandadai, J. Cech and S. A. Curran, “Poly(l-lactide)(PLLA)/Multiwalled Carbon Nanotube (MWCNT) Composite: Characterization and Biocompati- bility Evaluation,” Journal of Physics and Chemistry, Vol. 110, No.26, 2006, pp. 12910-12915. doi:10.1021/jp061628k [8] A. Thess, R. Lee and P. Nikolaev, et al., “Crystalline Ropes of Metallic Carbon Nanotubes,” Science, Vol. 273, No. 5274, 1996, pp. 483-487. doi:10.1126/science.273.5274.483 [9] C. Journet, W. K. Maser and P. Bernier, et al, “Large- Scale Production of Single-Walled Carbon Nanotubes by the Electric-Arc Technique,” Nature, Vol. 388, No. 4, 1997, pp. 756-758. doi:10.1038/41972 [10] B. R. Priya and H. J. Byrne, “Investigation of Sodium Dodecyl Benzene Sulfonate Assisted Dispersion and De- bundling of Single-Wall Carbon Nanotubes,” Journal of Physics and Chemistry C, Vol. 112, No. 2, 2008, pp. 332-337. doi:10.1021/jp0743830 [11] H. Murakami, T. Nomura and N. Nakashima, “Noncova- lent Porphyrin-Functionalized Single-Walled Carbon Nanotubes in Solution and the Formation of Porphy- rin–Nanotube Nanocomposites,” Chemical Physics Let- ters, Vol. 378, No. 5-6, 2003, pp. 481-485. doi:10.1016/S0009-2614(03)01329-0 C opyright © 2011 SciRes. JBNB  β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and 459 a Sensitive Biosensor Platform [12] H. Kong, C. Gao and D. Yan, “Controlled Functionaliza- tion of Multiwalled Carbon Nanotubes by in Situ Atom Transfer Radical Polymerization,” Journal of the Ameri- can Chemical Society, Vol. 126, No. 2, 2004, pp 412-413. doi:10.1021/ja0380493 [13] T. Morishita, M. Matsushita, Y. Katagiri and K. Fuku- mori, “Synthesis and Properties of Macromer-Grafted Polymers for Noncovalent Functionalization of Multi- Walled Carbon Nanotubes,” Carbon, Vol. 47, No. 11, 2009, pp. 2716-2726. doi:10.1016/j.carbon.2009.05.032 [14] Y. Zhang, H. K. He, C. Gao and J. Y. Wu, “Covalent Layer-by-Layer Functionalization of Multiwalled Carbon Nanotubes by Click Chemistry,” Langmuir, Vol. 25, No. 10, 2009, pp. 5814-5824. doi:10.1021/la803906s [15] M. Yang, Y. Gao, H. Li and A. Adronov, “Functionaliza- tion of Multiwalled Carbon Nanotubes with Polyamide 6 by Anionic Ring-Opening Polymerization,” Carbon, Vol. 45, No. 12, 2007, pp. 2327-2333. doi:10.1016/j.carbon.2007.07.021 [16] Y. Gao, G. Song, A. Alex and H. M. Li, “Functionaliza- tion of Single-Walled Carbon Nanotubes with Poly- (methyl methacrylate) by Emulsion Polymerization,” Journal of Physics and Chemistry C, Vol. 114, No. 39, 2010, pp. 16242-16249. doi:10.1021/jp104894a [17] Z. Guo, L. Liang and J. J. Liang, et al, “Covalently β-Cyclodextrin Modified Single-Walled Carbon Nano- tubes: A Novel Artificial Receptor Synthesized by ‘Click’ Chemistry,” Journal of Nanoparticle Research, Vol. 10, No. 6, 2008, pp. 1077-1083. doi:10.1007/s11051-007-9338-z [18] G. Neelgund and A. Oki, “Pd Nanoparticles Deposited on Poly(lactic acid) Grafted Carbon Nanotubes: Synthesis, Characterization and Application in Heck C–C Coupling Reaction,” Applied Catalysis A: General, Vol. 399, No.1-2, 2011, pp.154-160. doi:10.1016/j.apcata.2011.03.050 [19] D. Pantarotto, C. D. Partidos and J. Hoebeke, et al., “Im- munization with Peptide-Functionalized Carbon Nano- tubes Enhances Virusspecific Neutralizing Antibody Re- sponses,” Chemistry and Biology, Vol. 10, No. 10, 2003, pp. 961-966. doi:10.1016/j.chembiol.2003.09.011 [20] K. Shi, T. C. Jessop, P. A. Wender and H. Dai, “Nano- tube Molecular Transporters: Internalization of Carbon Nanotube-Protein Conjugates into Mammalian Cells,” Journal of the American Chemical Society, Vol. 126, No. 22, 2004, pp. 6850-6851. doi:10.1021/ja0486059 [21] D. Pantarotto, R. Singh and D. McCarthy, et al., “Func- tionalised Carbon Nanotubes for Plasmid DNA Gene De- livery,” Angewandte Chemie, Vol. 116, No. 39, 2004, pp.5354-5358. doi:10.1002/ange.200460437 [22] K. Nikolaos, P. Rigini M and S. Argiris, et al., “Pepti- domimetic-Functionalized Carbon Nanotubes with Anti- trypsin Activity,” Carbon, Vol. 47, No. 15, 2009, pp. 3550-3558. doi:10.1016/j.carbon.2009.08.025 [23] T. Loftsson and M. E. Brewster, “Pharmaceutical appli- cations of cyclodextrins.1. Drug solubilization and stabi- lization,” Journal of Pharmaceutical Sciences, Vol. 85, No. 10, 1996, pp. 1017-1025. doi:10.1021/js950534b [24] R. A. Rajewskix and V. J. Stella, “Pharmaceutical Appli- cations of Cyclodextrins 2. in Vivo Drug Delivery,” Journal of Pharmaceutical Sciences, Vol. 85, No. 11, 1996, pp. 1142-1169. doi:10.1021/js960075u [25] Y. Liu and Y. Chen, “Cooperative Binding and Multiple Recognition by Bridged Bis(b-cyclodextrin)s with Func- tional Linkers”, Accounts of Chemical Research, Vol. 39, No. 10, 2006, pp. 681-691. doi:10.1021/ar0502275 [26] R. Villalonga, R. Cao and A. Fragoso, “Supramolecular Chemistry of Cyclodextrins in Enzyme Technology,” Chemical Reviews, Vol. 107, No. 7, 2007, pp. 3088-3116. doi:10.1021/cr050253g [27] Y. Chen and Y. Liu, “Cyclodextrin-Based Bioactive Su- pramolecular Assemblies,” Chemical Society Reviews, Vol. 39, No. 2, 2010, pp. 495-505. doi:10.1039/b816354p [28] S. A. Nepogodiev and J. F. Stoddart, “Cyclodextrin- Based Catenanes and Rotaxanes,” Chemical Reviews, Vol. 98, No. 5, 1998, pp. 1959-1976. doi:10.1021/cr970049w [29] A. Harada, “Cyclodextrin-Based Molecular Machines,” Accounts of Chemical Research, Vol. 34, No. 6, 2001, pp. 456-464. doi:10.1021/ar000174l [30] G. Wenz, B. H. Han and A. Müller, “Cyclodextrin Ro- taxanes and Polyrotaxanes,” Chemical Reviews, Vol. 106, No. 3, 2006, pp. 782-817. doi:10.1021/cr970027+ [31] H. Tian and Q. C. Wang, “Recent Progress on Switchable Rotaxanes,” Chemical Society Reviews, Vol. 35, No. 4, 2006, pp. 361-374. doi:10.1039/b512178g [32] A. Harada, Y. Takashima and H. Yamaguchi, “Cyclodex- trin-Based Supramolecular Polymers,” Chemical Society Reviews, Vol. 38, No. 4, 2009, pp. 875-882. doi:10.1039/b705458k [33] K. Michael and R. Helmut, “Supramolecular Gels Based on Multi-Walled Carbon Nanotubes Bearing Covalently Attached Cyclodextrin and Water-Soluble Guest Poly- mers,” Macromol. Rapid Commun, Vol. 29, No. 14, 2008, pp. 1208-1211. doi:10.1002/marc.200800142 [34] S. Kang, Z. Cui and L. Liu, et al., “Sensitizing Effect of Oxazine on the Photoluminescence of Cyclodextrin-Mo- dified Carbon Nanotubes,” Journal of Dispersion Science and Technology, Vol. 27, No. 1, 2006, pp. 45-47. doi:10.1081/DIS-200066711 [35] Q. Jiang, H. Zhang and Y. liu, “Solvent-Controlled Photoinduced Electron Transfer between Porphyrin and Carbon Nanotubes,” Journal of Organic Chemistry, Vol. 73, No 6. 2008, pp. 2163-2168. doi:10.1021/jo702400k [36] P. Liang, H. Y. Zhang, Z. L. Yu and Y. Liu, “Preparation and Characterization of Soluble Methyl-B-Cyclodextrin Functionalized Single-Walled Carbon Nanotubes,” Physica E, Vol. 40, No. 3, 2008, pp. 689-692. doi:10.1016/j.physe.2007.09.082 [37] Y. Yang, C. Tsui and C.Tang, et al., “Functionalization of Carbon Nanotubes with Biodegradable Supramolecular Copyright © 2011 SciRes. JBNB  β-Cyclodextrin Covalently Functionalized Single-Walled Carbon Nanotubes: Synthesis, Characterization and a Sensitive Biosensor Platform Copyright © 2011 SciRes. JBNB 460 Polypseudorotaxanes from Grafted-Poly(ε-caprolactone) and α-cyclodextrins”, European Polymer Journal, Vol. 46, No. 2, 2010, pp.145-155. doi:10.1016/j.eurpolymj.2009.10.020 [38] R. C. Petter, J. S. Salek, C. T. Sikorski, R. C. Petter, J. S. Salek, C. T. Sikorski, G. Kumaravel, and F. T. Lin, “Co- operative Binding by Aggregated Mono-6-(alky1amino)- β-Cyclodextrins,” Journal of the American Chemical So- ciety, Vol. 112, No. 10, 1990, pp. 3860-3868. doi:10.1021/ja00166a021 [39] Y. Y. Liu, X. D. Fan and L. Gao, “Synthesis and Charac- terization of β-Cyclodextrin Based Functional Monomers and Its Copolymers with N-isopropylacrylamide,” Mac- romolecular Bioscience, Vol. 3, No. 12, 2003, pp. 715- 719. doi:10.1002/mabi.200300052 [40] J. Liu, A. G. Rinzler and H. Dai, et al., “Fullerene Pipes,” Science, Vol. 280, No. 5367, 1998, pp. 1253-1256. doi:10.1126/science.280.5367.1253 [41] M. C. Rodriguez, J. Sandoval, L. Galicia, S. Gutierrez and G. A. Rivas, “Highly Selective Determination of Uric Acid in the Presence of Ascorbic Acid at Glassy Carbon Electrodes Modified with Carbon Nanotubes Dispersed in Polylysine,” Sensors and Actuators B, Vol. 134, No. 2, 2008, pp. 559-565. doi:10.1016/j.snb.2008.05.035 [42] Y. X. Li and X. Q. Lin, “Simultaneous Electroanalysis of Dopamine, Ascorbic Acid and Uric Acid by Poly(vinyl alcohol) Covalently Modified Glassy Carbon Electrode,” Sensors and Actuators B, Vol. 115, No. 1, 2006, pp. 134-139. doi:10.1016/j.snb.2005.08.022 [43] S. G. Wu, T. L. Wang, Z. Y., H. H. Xu, B. N. Zhou and C. Wang, “Selective Detection of Uric Acid in the Presence of Ascorbic Acid at Physiological pH by Using a β-cyclo- dextrin Modified Copolymer of Sulfanilic Acid and N-Ace-Tylaniline,” Biosensors and Bioelectronics, Vol. 23, No. 12, 2008, pp. 1776-1780. doi:10.1016/j.bios.2008.02.012 [44] L. Z. Zheng, S. G. Wu, X. Q. Lin, L. Nie and L. Rui, “Se- lective Determination of Uric Acid by Using a β-Cyclo- dextrin Modified Electrode,” Electroanalysis, Vol. 13, No. 16, 2001, pp. 1351-1354. doi:10.1002/1521-4109(200111)13:16<1351::AID-ELAN 1351>3.0.CO;2-F
|