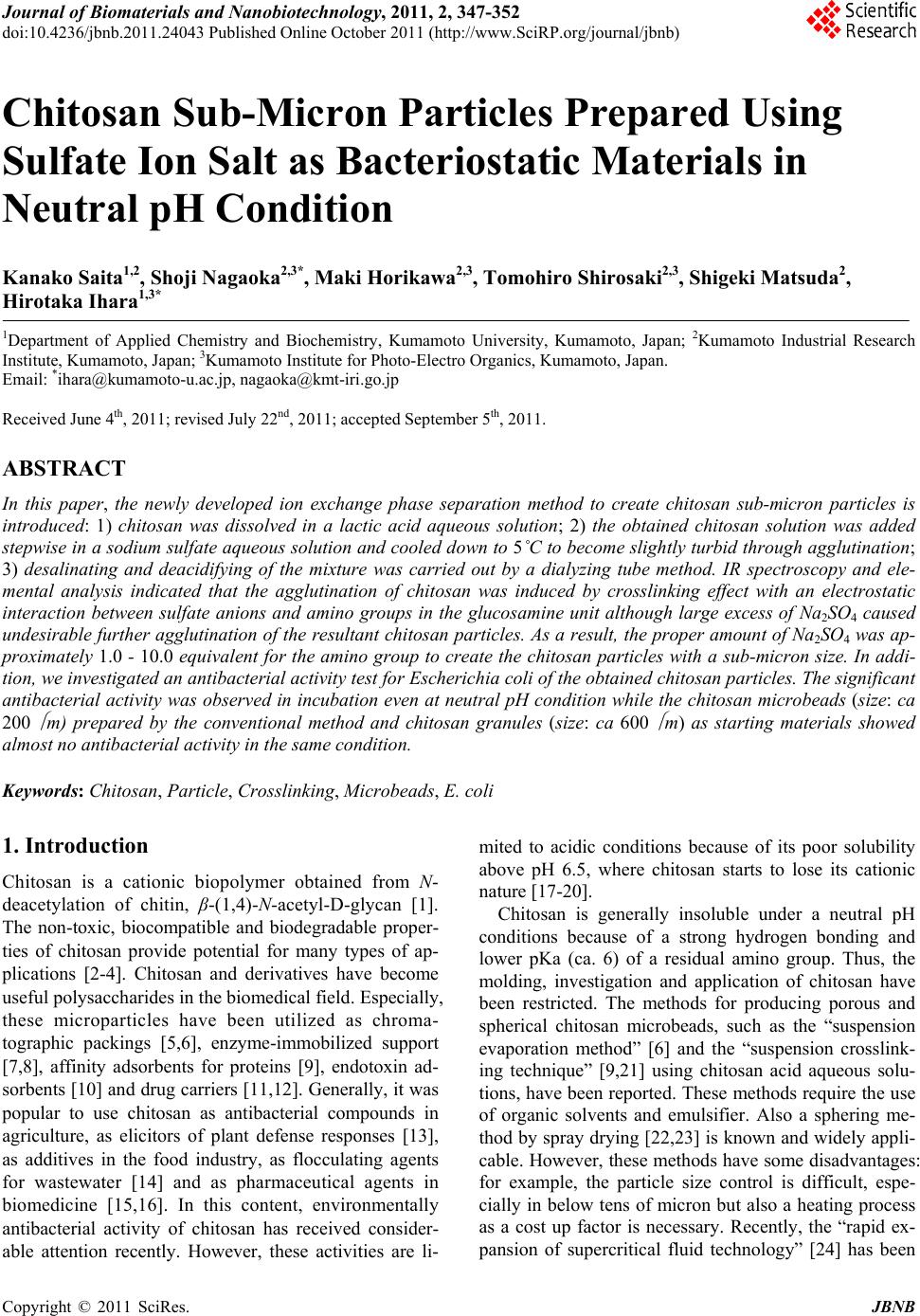
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 347-352 doi:10.4236/jbnb.2011.24043 Published Online October 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 347 Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition Kanako Saita1,2, Shoji Nagaoka2,3*, Maki Horikawa2,3, Tomohiro Shirosaki2,3, Shigeki Matsuda2, Hirotaka Ihara1,3* 1Department of Applied Chemistry and Biochemistry, Kumamoto University, Kumamoto, Japan; 2Kumamoto Industrial Research Institute, Kumamoto, Japan; 3Kumamoto Institute for Photo-Electro Organics, Kumamoto, Japan. Email: *ihara@kumamoto-u.ac.jp, nagaoka@kmt-iri.go.jp Received June 4th, 2011; revised July 22nd, 2011; accepted September 5th, 2011. ABSTRACT In this paper, the newly developed ion exchange phase separation method to create chitosan sub-micron particles is introduced: 1) chitosan was dissolved in a lactic acid aqueous solution; 2) the obtained chitosan solution was added stepwise in a sodium sulfate aqueous solution and cooled down to 5˚C to become slightly turbid through agglutination; 3) desalinating and deacidifying of the mixture was carried out by a dialyzing tube method. IR spectroscopy and ele- mental analysis indicated that the agglutination of chitosan was induced by crosslinking effect with an electrostatic interaction between sulfate anions and amino groups in the glucosamine unit although large excess of Na2SO4 caused undesirable further agglutination of the resultant chitosan particles. As a result, the proper amount of Na2SO4 was ap- proximately 1.0 - 10.0 equivalent for the amino group to create the chitosan particles with a sub-micron size. In addi- tion, we investigated an antibacterial activity test for Escherichia coli of the obtained chitosan particles. The significant antibacterial activity was observed in incubation even at neutral pH condition while the chitosan microbeads (size: ca 200 m) prepared by the conventional method and chitosan granules (size: ca 600 m) as starting materials showed almost no antibacterial activity in the same condition. Keywords: Chitosan, Particle, Crosslinking, Microbeads, E. coli 1. Introduction Chitosan is a cationic biopolymer obtained from N- deacetylation of chitin, β-(1,4)-N-acetyl-D-glycan [1]. The non-toxic, biocompatible and biodegradable proper- ties of chitosan provide potential for many types of ap- plications [2-4]. Chitosan and derivatives have become useful polysaccharides in the biomedical field. Especially, these microparticles have been utilized as chroma- tographic packings [5,6], enzyme-immobilized support [7,8], affinity adsorbents for proteins [9], endotoxin ad- sorbents [10] and dr ug carriers [11,12]. Generally, it w as popular to use chitosan as antibacterial compounds in agriculture, as elicitors of plant defense responses [13], as additives in the food industry, as flocculating agents for wastewater [14] and as pharmaceutical agents in biomedicine [15,16]. In this content, environmentally antibacterial activity of chitosan has received consider- able attention recently. However, these activities are li- mited to acidic conditions because of its poor solubility above pH 6.5, where chitosan starts to lose its cationic nature [17-20]. Chitosan is generally insoluble under a neutral pH conditions because of a strong hydrogen bonding and lower pKa (ca. 6) of a residual amino group. Thus, the molding, investigation and application of chitosan have been restricted. The methods for producing porous and spherical chitosan microbeads, such as the “suspension evaporation method” [6] and the “suspension crosslink- ing technique” [9,21] using chitosan acid aqueous solu- tions, have been reported. These methods require the use of organic solvents and emulsifier. Also a sphering me- thod by sp ray drying [22 ,23] is known and wid ely appli- cable. However, these methods have some disadvantages: for example, the particle size control is difficult, espe- cially in below tens of micron but also a heating process as a cost up factor is necessary. Recently, the “rapid ex- pansion of supercritical fluid technology” [24] has been  Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition 348 developed as a method preparing sub-micron particles. Since this technique uses supercritical CO2, the process is environmentally safe but it is also known that the co n- trol of particle size, shape and composition are difficult [25]. In this paper, we introduce a new method as the “ion exchange phase separation method” to create submicron particles from chitosan without using any organic emul- sifier and solvent as well as with no heating process. This method can be characterized by solidification with Na2SO4 and following dialysis. It is also reported that the obtained chitosan particles shows excellent antibacterial activity even at neutral pH 7 towards Escherichia coli although chitosan exhibits higher antibacterial activity only in an acidic medium [26,27]. 2. Materials and Methods Chitosan sub-micron particles were prepared as follows: two kinds of chitosan materials (CS85 and CS485) were selected. Their chitosans are 85 mol% in the deacetyla- tion degr ee, and w ere 70 - 100 kD a and 440 - 530 kD a in the molecular weight, respectively. Chitosan was dis- solved in a 1.3 wt% lactic acid aqueous solution to be 1.5 wt% and then 20 ml of the solution was added stepwise into Na2SO4 aqueous solutions (1.0, 2.0, 5.0 and 10.0 equivalents for the amino group in a glucosamine unit) at 30˚C. The mixture was cooled down to 5˚C and kept for 10 min to become turbid. The mixture was desalinated and deacidified by dialyzing with a dialysis tube (MWCO, 12000 - 14000, Spectra/por 5) in distilled wa- ter for 4 days. Th e obtained chitosan particles are abbre- viated as PCS85-sm and PCS485-sm. The other type of chitosan spherical microbeads was prepared by slight modification of the suspension evapo- ration method reported previously [6]. 25 ml of a 1.5 wt% chitosan (CS85 or CS485) lactic acid solution was added to 250 ml of decahydronaphthalene containing 5 g of polyethylene glycol mono-4-nonylphenyl ether (po- lymerization degree, 10) and suspended by stirring at 80˚C for 24 h. By gradual removal of water, the chito- san-containing suspension particles were solidified to be spherical. The obtained microbeads were deacidified with 1 M NaOH, and successively washed with ethanol and ether. The obtained chitosan particles are abbrevi- ated as PCS85-m and PCS485-m. Surface area analysis of the particles was carried out by the Brunau-Emmet Teller (BET) method using Auto- sorb-1 (Aionics Co. Ltd., Japan). Fourier transformed in- frared (FT-IR) spectroscopy was carried out with JASCO FT/IR-700. The particle size and distribution were meas- ured by dynamic light scattering (DLS) method (Zetasiz- ernano-ZS, Sysmex Corp., Japan). The particles were also observed using a field emission scanning electron microscope (FE-SEM) (S-4000, Hitachi, Co. Ltd., Japan) and stereomicroscope (KH-7700S, Hirox Co. Ltd., Ja- pan). For assay of antibacterial activity, Mueller-Hinton Broth (Becton Dickinson and Company, USA) adjusted by cation, which contained 92 mg of CaCl22H2O, 104.5 mg of MgCl26H2O and 15 g of agar for 1l of broth, re- spectively, was used as culture media. Escherichia coli NBRC 3972 (E. coli) was incubated at 37˚C for 4 - 6 h in the Mueller-Hinton Broth until logarithmic phase was reached. The assay was carried out with 0, 0.5, 1.0 and 5.0 mg/ml of the chitosan sub-micron particles (PCS85- sm and PCS485-sm), the chitosan microbeads (PCS85-m and PCS485-m) and ch itosan granules as starting materi- als (abbreviated as CS85-g and CS485-g). The chitosans were put into sterile glass-plates, 15 ml of Mueller-Hin- ton Broth was added to each sterile glass-plate contain- ing E. coli culture and then were spread on the plates to be 1.0 × 102, 1.0 × 104 and 1.0 × 106 cfu/plate, and these were incubated at 37˚C for 18 h. Antibacterial activity was observed by comparison of with the control plate without chitosan. 3. Results and Discussion 3.1. Preparation of Chitosan Sub-Micron Particles Figure 1 shows the FT-IR spectra of the product pre- pared by the ion exchange phase separation method from CS85. As shown in Figure 1(a), the product before dia- lyzing showed a distinct adsorption at 1700 cm–1 corre- sponding to C=O of a carboxyl group of lactic acid. The adsorptions of S=O at 1110 cm–1 and S=O at 620 cm-1 increased with increasing amount of Na2SO4 added in the sphering process. This indicates that the product was the mixture of Na2SO4, chitosan and lactic acid. After dia- lyzation for 4 days, the resultant product sho wed that the adsorption at 1700 cm–1 for C=O disappeared completely but also the adsorptions based on S = O at 1110 cm–1 and S = O at 620 cm–1 decreased remarkably. However, S=O at 1110 cm–1 and S = O at 620 cm–1 of the products re- mained regardless of sufficient dialyzing. Similar IR spectrum was observed for the product prepared from CS485. These results indicate that large excesses of lac- tic acid and Na2SO4 could be removed by dialysis, but that anion-exchange from a lactate ion to a sulfate ion towards an ammonium ion of chitosan occurred. The final products, PCS85-sm and PCS485-sm were insolu- ble in water. 3.2. Microscopic Observation of Chitosan Particles In the ion exchange phase separation method, a chitosan C opyright © 2011 SciRes. JBNB 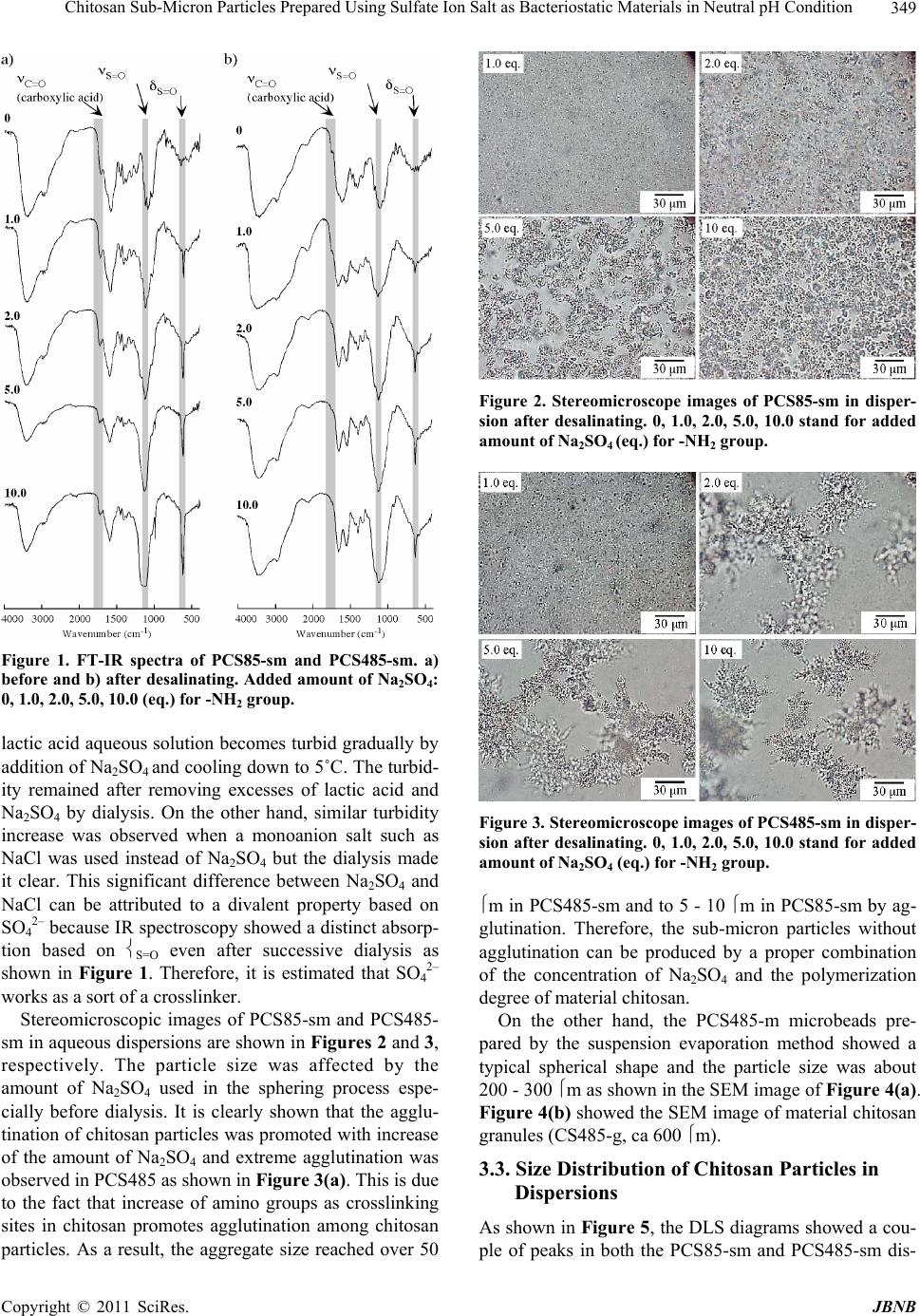 Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition349 Figure 1. FT-IR spectra of PCS85-sm and PCS485-sm. a) before and b) after desalinating. Added amou nt of Na2SO4: 0, 1.0, 2.0, 5.0, 10.0 (eq.) for -NH2 group. lactic acid aqueous solution becomes turbid gradually by addition of Na2SO4 and cooling down to 5˚C. The turbid- ity remained after removing excesses of lactic acid and Na2SO4 by dialysis. On the other hand, similar turbidity increase was observed when a monoanion salt such as NaCl was used instead of Na2SO4 but the dialysis made it clear. This significant difference between Na2SO4 and NaCl can be attributed to a divalent property based on SO42– because IR spectroscopy showed a distinct absorp- tion based on S=O even after successive dialysis as shown in Figure 1. Therefore, it is estimated that SO42– works as a sort of a crosslinker. Stereomicroscopic images of PCS85-sm and PCS485- sm in aqueous dispersions are shown in Figures 2 and 3, respectively. The particle size was affected by the amount of Na2SO4 used in the sphering process espe- cially before dialysis. It is clearly shown that the agglu- tination of chitosan particles was promoted with increase of the amount of Na2SO4 and extreme agglutination was observed in PCS485 as shown in Figure 3(a). This is due to the fact that increase of amino groups as crosslinking sites in chitosan promotes agglutination among chitosan particles. As a result, the aggregate size reached over 50 Figure 2. Stereomicroscope images of PCS85-sm in disper- sion after desalinating. 0, 1.0, 2.0, 5.0, 10.0 stand for added amount of Na2SO4 (eq.) for -NH2 group. Figure 3. Stereomicroscope images of PCS485-sm in disper- sion after desalinating. 0, 1.0, 2.0, 5.0, 10.0 stand for added amount of Na2SO4 (eq.) for -NH2 group. m in PCS485-sm and to 5 - 10 m in PCS85-sm by ag- glutination. Therefore, the sub-micron particles without agglutination can be produced by a proper combination of the concentration of Na2SO4 and the polymerization degree of material chitosan. On the other hand, the PCS485-m microbeads pre- pared by the suspension evaporation method showed a typical spherical shape and the particle size was about 200 - 300 m as shown in the SEM image of Figure 4(a). Figure 4(b) showed the SEM image of material chitosan granules (CS485-g, ca 600 m). 3.3. Size Distribution of Chitosan Particles in Dispersions As shown in Figure 5, the DLS dia grams show ed a cou- ple of peaks in both the PCS85-sm and PCS485-sm dis- Copyright © 2011 SciRes. JBNB 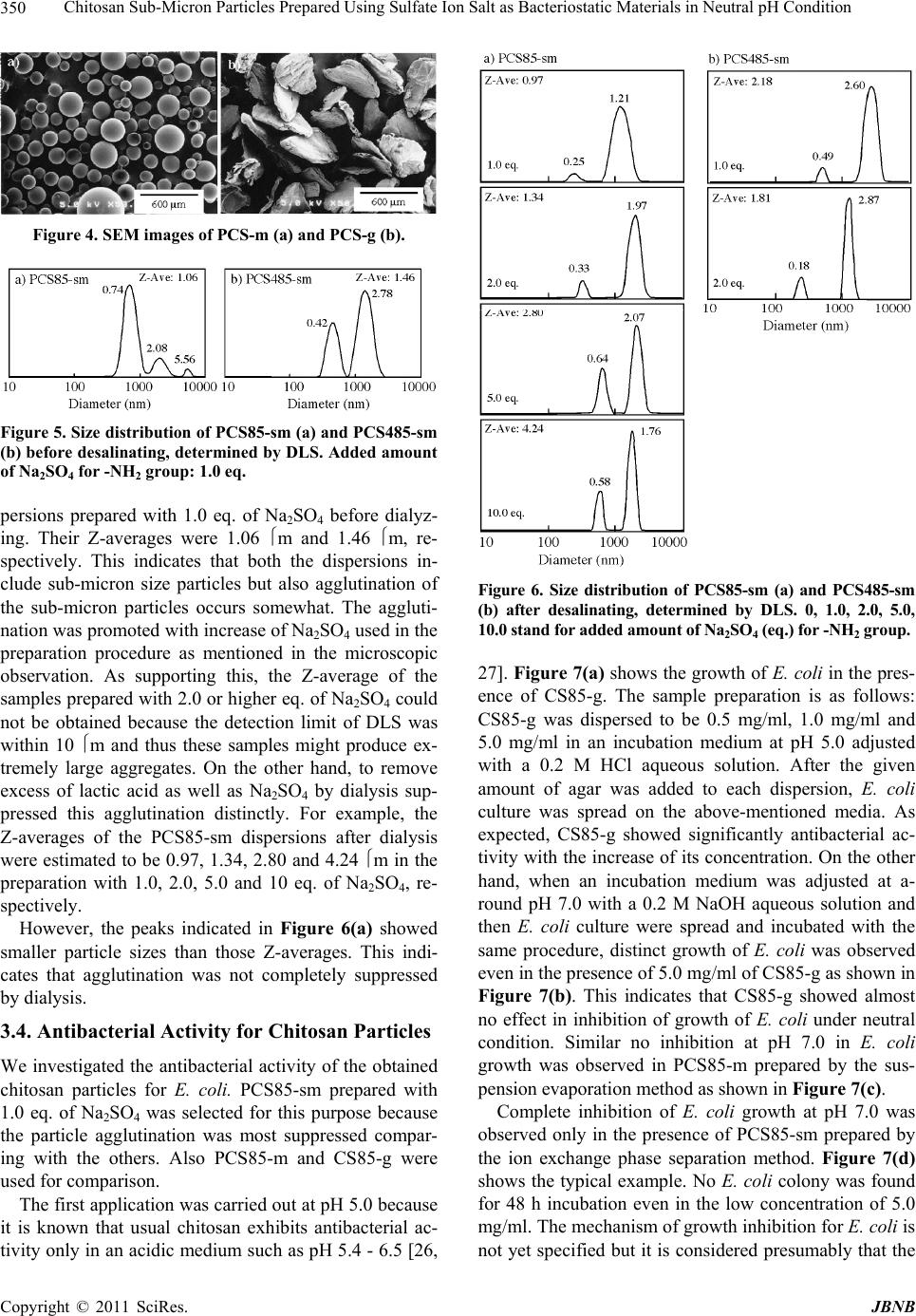 Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition 350 Figure 4. SEM images of PCS-m (a) and PCS-g (b). Figure 5. Size distribution of PCS85-sm (a) and PCS485-sm (b) before desalinating, determined by DLS. Added amoun t of Na2SO4 for -NH2 group: 1.0 eq. persions prepared with 1.0 eq. of Na2SO4 before dialyz- ing. Their Z-averages were 1.06 m and 1.46 m, re- spectively. This indicates that both the dispersions in- clude sub-micron size particles but also agglutination of the sub-micron particles occurs somewhat. The aggluti- nation was promoted with increase of Na2SO4 used in the preparation procedure as mentioned in the microscopic observation. As supporting this, the Z-average of the samples prepared with 2.0 or higher eq . of Na2SO4 could not be obtained because the detection limit of DLS was within 10 m and thus these samples might produce ex- tremely large aggregates. On the other hand, to remove excess of lactic acid as well as Na2SO4 by dialysis sup- pressed this agglutination distinctly. For example, the Z-averages of the PCS85-sm dispersions after dialysis were estimated to be 0.97, 1.34, 2.80 and 4.24 m in the preparation with 1.0, 2.0, 5.0 and 10 eq. of Na2SO4, re- spectively. However, the peaks indicated in Figure 6(a) showed smaller particle sizes than those Z-averages. This indi- cates that agglutination was not completely suppressed by dialysis. 3.4. Antibacterial Activity for Chitosan Particles We investigated the antibacterial activity of the obtained chitosan particles for E. coli. PCS85-sm prepared with 1.0 eq. of Na2SO4 was selected for this purpose because the particle agglutination was most suppressed compar- ing with the others. Also PCS85-m and CS85-g were used for comparison. The first application was carried out at pH 5.0 because it is known that usual chitosan exhibits antibacterial ac- tivity only in an acidic medium such as pH 5.4 - 6.5 [26, Figure 6. Size distribution of PCS85-sm (a) and PCS485-sm (b) after desalinating, determined by DLS. 0, 1.0, 2.0, 5.0, 10.0 stand for added amount of Na2SO4 (eq.) for -NH2 group. 27]. Figure 7(a ) shows the growth of E. coli in the pres- ence of CS85-g. The sample preparation is as follows: CS85-g was dispersed to be 0.5 mg/ml, 1.0 mg/ml and 5.0 mg/ml in an incubation medium at pH 5.0 adjusted with a 0.2 M HCl aqueous solution. After the given amount of agar was added to each dispersion, E. coli culture was spread on the above-mentioned media. As expected, CS85-g showed significantly antibacterial ac- tivity with the increase of its concentration. On the other hand, when an incubation medium was adjusted at a- round pH 7.0 with a 0.2 M NaOH aqueous solution and then E. coli culture were spread and incubated with the same procedure, distinct growth of E. coli was observed even in the presence of 5.0 mg/ml of CS85-g as shown in Figure 7(b). This indicates that CS85-g showed almost no effect in inhibition of growth of E. coli under neutral condition. Similar no inhibition at pH 7.0 in E. coli growth was observed in PCS85-m prepared by the sus- pension evaporation method as shown in Figure 7(c). Complete inhibition of E. coli growth at pH 7.0 was observed only in the presence of PCS85-sm prepared by the ion exchange phase separation method. Figure 7(d) shows the typical example. No E. coli colony was found for 48 h incubation even in the low concentration of 5.0 mg/ml. The mechanism of growth inhibition fo r E. coli is not yet specified but it is consid ered presumably that the C opyright © 2011 SciRes. JBNB  Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition351 Figure 7. Growth of E. coli in culture containing PCS-g in pH5.0 (a) PCS-g in neutral condition (b) PCS85-m in neu- tral condition (c) and PCS-sm in neutral condition (d). size effect may be included but also the specific surface area of the particles can play an important role. For ex- ample, the specific surface areas were determined by BET method to be 127 m2/g, 15.19 m2/g, and 2.82 m2/g in PCS85-sm, PCS85-m and CS85-g, respectively. It is reasonable to consider that increase of the surface area promotes the adsorption of E. coli onto the chitosan par- ticles and thus effective antibacterial activity can be de- rived from amino groups of chitosan. 4. Conclusions The chitosan submicron particles have been prepared by the newly developed ion exchange phase separation method which is characterized by the facts that chitosan is dissolved in a lactic acid aqueous solution and solidi- fication can be realized by addition of Na2SO4 and cool- ing down to 5 ˚C. By removal of excess of lactic acid and Na2SO4, particle agglutination can be sufficiently sup- pressed. Probably a stable dispersion state in water can be attributed to crosslinking effect between amino groups of chitosan and sulfate anion from Na2SO4. In addition, antibacterial activity for E. coli has been investigated. It was conf irmed that only the chitosan sub- micron particles prepared by the present method showed distinct inhibition of E. coli growth at neutral pH al- though the material chitosan granules showed effective antibacterial activity only in acidic condition as it has been known [26,27]. 5. Acknowledgements This work was partially supported by a grant-in-aid for Industrial Technology Center. REFERENCES [1] R. N. Tharanathan and F. S. Kittur, “Chitin-The Undis- puted Biomolecule of Great Potential,” Critical Reviews in Food Science and Nutrition, Vol. 43, No. 1, 2003, pp. 61-87. doi:10.1080/10408690390826455 [2] R. Bodmeier, K. H. Oh and Y. Pramar, “Preparation and Evaluation of Drug-containing Chitosan Beads,” Drug Development and Industrial Pharmacy, Vol. 15, No. 9, 1989, pp. 1475-1494. doi:10.3109/03639048909062758 [3] Y. Nishioka, S. Kyotani, H. Masui, M. Okamura, M. Miyazaki, K. Okazaki, S. Ohnishi, Y. Yamamoto and K. Ito, “Preparation and Release Characteristics of Cisplatin Albumin Microspheres Containing Chitin and Treated with Chitosan,” Chemical & Pharmaceutical Bulletin, Vol. 37, No. 11, 1989, pp. 3074-3077. [4] Y. Ohya, M. Shiratani, H. Kobayashi and T. Ouchi, “Re- lease Behavior of 5-Fluorouracil from Chitosan-Gel Na- nospheres Immobilizing 5-Fluorouracil Coated with Poly- saccharides and Their Cell Specific Cytotoxicity,” Jour- nal of Macromolecular Science, Part A: Pure and Ap- plied Chemistry, Vol. 31, No. 5, 1994, pp. 629-642. doi:10.1080/10601329409349743 [5] Y. C. Shi, Y. M Jiang, D. X. Sui, Y. L. Li, T. Chen, L. Ma and Z. T. Ding, “Affinity Chromatography of Trypsin Using Chitosan as Ligand Support,” Journal of Chroma- tography A, Vol. 742, No. 1-2, 1996, pp. 107-112. doi:10.1016/0021-9673(96)00260-9 [6] T. Adachi, J. Ida, M. Wa kita, M. Hashimoto, H. Iha ra and C. Hirayama, “Preparation of Spherical and Porous Chi- tosan Particles by Suspension Evaporation with O/W/O Multiple Emulsions,” Polymer Journal, Vol. 31, No. 4, 1999, pp. 319-323. doi:10.1295/polymj.31.319 [7] T. Hayashi and Y. Ikada, “Protease Immobilization onto Porous Chitosan Bead,” Journal of Applied Polymer Sci- ence, Vol. 42, No. 1, 1991, pp. 85-92. doi:10.1002/app.1991.070420110 [8] T. Feng, Y. Du, J. Yang, J. Li and X. Shi, “Immobiliza- tion of a Nonspecific Chitosan Hydrolytic Enzyme for Application in Preparation of Water-soluble Low-mo- lecular weight Chitosan,” Journal of Applied Polymer Science, Vol. 101, No. 3, 2006, pp. 1334-1339. doi:10.1002/app.22959 [9] E. B. Denkbas, M. Odabasi, E. Kiliçay and N. Özdemir, “Human Serum Albumin (HSA) Adsorption With Chito- san Microspheres,” Journal of Applied Polymer Science, Vol. 86, No. 12, 2002, pp. 3035-3039. doi:10.1002/app.11318 [10] T. Adachi, J. Ida, M. Wakita an d M. Hashimoto, Japa nese Patent No. 3569927. [11] H. Li, G. Yan, S. Wu, Z. Wang and K. Y. Lam, “Nu- merical Simulation of Controlled Nifedipine Release from Chitosan Microgels,” Journal of Applied Polymer Science, Vol. 93, No. 4, 2004, pp. 1928-1937. doi:10.1002/app.20652 [12] X. Yuan, H. Li and Y. Yuan, “Preparation of Choles- terol-modified Chitosan Self-aggregated Nanoparticles for Delivery of Drugs to Ocular Surface,” Carbohydrate Polymers, Vol. 65, No. 3, 2006, pp. 337-345. doi:10.1016/j.carbpol.2006.01.020 Copyright © 2011 SciRes. JBNB  Chitosan Sub-Micron Particles Prepared Using Sulfate Ion Salt as Bacteriostatic Materials in Neutral pH Condition Copyright © 2011 SciRes. JBNB 352 [13] S. Lee, H. Choi, S. Suh, I. Doo, K. Oh, E. J. Choi, A. T. S. Taylor, P. S. Low and Y. Lee, “Oligogalacturonic Acid and Chitosan Reduce Stomatal Aperture by Inducing the Evolution of Reactive Oxygen Species from Guard Cells of Tomato and Commelina communis,” Plant Physiology, Vol. 121, No. 1, 1999, pp. 147-152. doi:10.1104/pp.121.1.147 [14] S. P. Strand, K. M. Vårum a nd K. Østgaard, “Interactions between Chitosans and Bacterial Suspensions: Adsorp- tion and Flocculation,” Colloids and Surfaces B: Bio- interfaces, Vol. 27, No. 1, 2003, pp. 71-81. doi:10.1016/S0927-7765(02)00043-7 [15] G. Ikinci, S. Senel, H. Akincibay, S. Kas, S. Ercis, C. G. Wilson and A. A. Hincal, “Effect of Chit osan on a Peri o- dontal Pathogen Porphyromonas Gingivalis,” Interna- tional Journal of Pharmaceutics, Vol. 235, No. 1-2, 2002, pp. 121-127. doi:10.1016/S0378-5173(01)00974-7 [16] K. Aiedeh and M. O. Taha, “Synthesis of Iron-cross- linked Chitosan Succinate and Iron-crosslinked Hydrox- amated Chitosan Succinate and Their in Vitro Evaluation as Potential Matrix Materials for Oral Theophylline Sus- tained- release Beads,” European Journal of Pharmaceu- tical Sciences, Vol. 13, No. 2, 2001, pp. 159-168. doi:10.1016/S0928-0987(00)00217-7 [17] J. H. Park, Y. W. Cho, H. Chung, I. C. Kwon and S. Y. Jeong, “Synthesis and Characterization of Sugar-Bearing Chitosan Derivatives: Aqueous Solubility and Biode- gradability,” Biomacromolecules , Vol. 4, No. 4, 2003, pp. 1087-1091. doi:10.1021/bm034094r [18] G. J. Tsai and W. H. Su, “Antibacterial Activity of Sh ri mp Chitosan against Escherichia coli,” Journal of Food Pro- tection, Vol. 62, No. 3, 1999, pp. 239-243. [19] I. M. Helander, E. L. Nurmiaho-Lassila, R. Ahvenainen, J. Rhoades and S. Roller, “Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-negative Bacteria,” International Journal of Food Microbiology, Vol. 71, No. 2-3, 2001, pp. 235-244. doi:10.1016/S0168-1605(01)00609-2 [20] X. F. Liu, Y. L. Guan, D. Z. Yang, Z. Li and K. D. Yao, “Antibacterial Action of Chitosan and Carboxymethy- lated Chitosan,” Journal of Applied Polymer Science, Vol. 79, No. 7, 2001, pp. 1324-1335. [21] E. B. Denkba and M. Odabasi, “Chitosan Microspheres and Sponges: Preparation and Characterization,” Journal of Applied Polymer Science, Vol. 76, No. 11, 2000, pp. 1637-1643. doi:10.1002/(SICI)1097-4628(20000613)76:11<1637::AI D-APP4>3.0.CO;2-Q [22] F. L. Mi, T. B. Wong, S. S. Shyu and S. F. Chang, “Chi- tosan Microspheres: Modification of Polymeric Chem- physical Properties of Spray-dried Microspheres to Con- trol the Release of Antibiotic Drug,” Journal of Applied Polymer Science, Vol. 71, No. 5, 1999, pp.747-759. [23] H. Takahashi, R. Chen, H. Okamoto and K. Danjo, “Acetaminophen Particle Design Using Chitosan and a Spray- Drying Technique,” Chemical & Pharmaceutical Bulletin, Vol. 53, No. 1, 2005, pp. 37-41. doi:10.1248/cpb.53.37 [24] M. Gimeno, N. Ventosa, Y. Boumghar, J. Fournier, I. Boucher and J. Veciana, “Micronization of the Chitosan Derivatives D-Glucosamine Hydrochloride and D-Gluco- samine Sulphate Salts by Dense Gas Anti-solvent Pre- cipitation Techniques,” The Journal of Supercritical Flu- ids, Vol. 38, No. 1, 2006, pp. 94-102. doi:10.1016/j.supflu.2005.11.009 [25] V. J. Mohanraj and Y. Chen, “Nanoparticles-A Review,” Tropical Journal of Pharmaceutical Research, Vol. 5, No. 1, 2006, pp. 561-573. [26] E. I. Rabea, M. T. B. Badawy, C. V. Stevens, G. Smagg- he and W. Steurbaut, “Chitosan as Antimicrobial Agent: Applications and Mode of Action,” Biomacromolecules, Vol. 4, No. 6, 2003, pp. 1457-1465. doi:10.1021/bm034130m [27] Z. Li, X. P. Zhyang, X. F. Liu, Y. L. Guan and K. D. Yao, “Study on Antibacterial O-carboxymethylated Chito- san/cellulose Blend Film from LiCl/N, N-dimethylace- tamide Solution,” Polymer, Vol. 43, No. 4, 2001, pp. 1541-1547. doi:10.1016/S0032-3861(01)00699-1
|