 American Journal of Anal yt ical Chemistry, 2011, 2, 731-738 doi:10.4236/ajac.2011.26084 Published Online October 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Thiocyanate Ion Selective Solid Contact Electrode Based on Mn Complex of N,N'-BIs-(4-Phenylazosalicylidene)-O-Phenylene Diamine Ionophore Won-Sik Han1, Tae-Kee Hong2, Young-Hoon Lee2* 1The Research Center of Conservation Science for Cultural Heritage, Hanseo University, Seosan, Choong-Nam, Korea 2Depsrtment of Chemistry, Hanseo University, Seosan, Choong-Nam, Ko rea E-mail: *wondo@unitel.co.kr Received May 3, 2011; revised June 18, 2011; accepted June 30, 2011 Abstract A thiocyanate ion selective poly(aniline) solid contact electrode based on manganese complex of N,N’-bis-(4-phenylazosalicylidene)-o-phenylene diamine ionophore was successfully developed. The elec- trode exhibits a good linear response of 58.1 mV/decade (at 20˚C ± 0.2˚C, r2 = 0.998) with in the concentra- tion range of 1 × 10–1.0 ~ 1 × 10–5.8 M thiocyanate solution. The composition of this electrode was: ionophore 0.040, polyvinylchloride 0.300, dibutylphthalate 0.660 (mass). This dibutylphthalate plasticizer provides the best response characteristics. The electrode shows good selectivity for thiocyanate ion in comparison with any other anions and is suitable for use with aqueous solutions of pH 4.0 ~ 6.0. The standard deviations of the measured emf difference were ±1.70 and ±2.01 mV for thiocyanate sample solutions of 1.0 × 10–2 M and 1.0 × 10–3 M, respectively. The stabilization time was less than 170 sec. and response time was less than 17 sec. Keywords: Thiocyanate Ion, SCEs, ISEs, Mn Complexed Ionophore, Schiff Base, Ion Sensor 1. Introduction The thiocyanate ion is usually present in low concentra- tions in human serum, saliva, and urine as a result of the digestion of some vegetables of the genus Brassica con- taining glucosinolates (cabbage, turnip, kale) or by intake of thiocyanate-containing foods such as milk and cheese. Higher concentration of this ion, which is a metabolic product of cyanide, arises from tobacco smoke [1,2]. In this respect, the concentration level of thiocyanate is considered to be a good probe to distinguish between smokers and non-smokers. It has been found that there is a correlation among the blood cyanide, the plasma thio- cyanate, and the salivary thiocyanate [3]. Therefore, an accurate, simple, and rapid method for the determination of thiocyanate is significant in medicine and in the life sciences [4]. Several methods for the determination of thiocyanate ion such as spectrophotometry [5-9], chro- matography [10-15], polarography [16], capillary zone electrophoresis [17], amperometry [18], potentiometric methods [19-38] etc., have been reported previously. Among the various methods, the thiocyanate ion selec- tive electrodes (ISEs) are useful since it provides high sensitivity and a wide dynamic range. Especially, solid contact type electrode (SCEs) have a electro-conductive polymer layer, such as poly(aniline), to conduct ions and electrons, and which are reported to strengthen the bonds between metal substrates and reaction membranes and to have stable and highly selective electrodes as they move electrons to the metal substrate and the ions to the outer PVC layer that provides new advantages. Simplicity of design, lower costs, mechanical flexibility and the possi- bility of miniaturization and micro-fabrication have widened the applications for these type electrodes, espe- cially in the fields of medicine and biotechnology. In this work, we report the anion response behavior of manganese chelate of a new Schiff base, N,N’-bis-(4- phenylazosalicylidene)-o-phenylene diamine. The results  W.-S. HAN ET AL. 732 show that the Mn(II) chelate-based electrodes demon- strate a highly specific response towards thiocyanate ion. This paper describes the construction, potentiometric characterization, and analytical application of a thiocy- anate ion selective poly(aniline) solid contact electrode based on the use of a manganese chelated N,N’-bis-(4- phenylazo salicylidene)-o-phenylene diamine ionophore. 2. Experimental 2.1. Reagents Aniline and tetrahydrofuran (THF) from Aldrich were purified by vacuum distillation. salicylaldehyde, ethylene diamine and manganese acetate (Mn(OAc)2) from Al- drich was used without further purification. For all ex- periments, analytical grade chemicals were used and all aqueous solutions were prepared in doubly distilled wa- ter obtained from a Millipore Milli-Q water purification system. The membrane matrix high molecular weight poly(vinylchloride) (PVC, n = 1,100), the plasticizers 2-nitrophenyloctylether (o-NPOE), tris(ethylhexyl)pho- sphate (TEHP), bis(2-ethylhexyl)adipate (DOA), dioc- tylphthalate (DOP), bis(2-ethylhexyl)sebacate (DOS), dibutylphthalate (DBP) were from Aldrich. 2.2. Synthesis of N,N’-Bis-(4-Phenylazosalicylidene)- O-Phenylene Diamine (Mn-PASPD) Ionophore To a solution of 4.43 mL of freshly distilled aniline (0.05 mol) in 18.0 mL of concentrated hydrochloride and 20.0 mL of water, was added 4.00 g of sodium nitrite (0.10 mol) in 20 mL of water, the reaction was carried out for 1 h. below 5˚C. The reaction mixture was then added to a solution of 18.0 g of sodium carbonate (0.17 mol) and 5.24 mL of salicylaldehyde in 150 mL of water, the reac- tion temperature was remained within 0˚C ~ 5˚C. The yellow precipitate was filtered off after 1 h. and recrys- tallized from ethanol. The above product (2.26 g, 0.01 mol) refuxed with ethylene diamine (0.30 g, 5.0 mmol) in 50 mL of absolute alcohol for 2 h. with stirring to give a golden yellow solid which was purified by recrystalli- zation from alcohol. Melting point is about 225˚C. 5.0 mmol of this product was mixed with Mn(OAc)2 (5.5 mmol) in absolute ethanol. The reaction mixture was refluxed for 2 h. After cooled to the room temperature and stand overnight, the manganese chelates obtained were filtered, washed with absolute alcohol and dried in vacuum. Melting point of this final product is about 345˚C. 2.3. Polymerization Electrochemical experiments were performed in a con- ventional cell with three electrodes. A saturated calomel electrode was used as the reference electrode and all po- tentials were recorded and reported with respect to this electrode. Platinum wires (1 mm in diameter, 50 mm in length) were used as the working and counter electrodes. Electro-polymerization was carried out at the one end of a platinum wire by cyclic voltammetry in 3.0 × 10–2 M aniline and 6.0 × 10–2 M HCl solution. Cyclic voltam- mograms were recorded using a potentiostat (EG & G 273A). For electrochemical polymerization of aniline, the potential was swept between 0.0 V and 1.0 V at a scan rate of 100 mV/s. The potential cycling was re- peated up to 30 cycles and stopped at 1.0 V. After elec- trodeposition, the poly(aniline) was washed with distilled water and then dried for 24 h. in an 80˚C oven. Then the metal part of the Pt-poly(aniline) electrode was covered with a thermo-contractive insulation tube. 2.4. Preparation of Cocktail Solutions and Fabrication of Solid Contact Electrode Typical cocktail solution consists of ionophore 2.0% - 5.0% : PVC 30.0% : plasticizer 65.0% - 68.0%. All com- ponents were dissolved in THF. The solid contact elec- trodes were produced by dipping the Pt-poly(aniline) electrode directly into the cocktail solution to coat it with a thin film. The resulting solid contact electrode contains three layers of Pt/electro-conductive polymer/PVC film with an ionophore with a thickness of 2.5 ± 0.1 mm. 2.5. Measurements of Stabilization Time and Response Time The stabilization time was obtained as follows. First, the dry electrode was deposited in a 1.0 × 10–3 M pH 5.5 Tris buffer NaSCN solution. Then the time until the po- tential stabilized to lower than ±0.1 mV/min was meas- ured. The response time was obtained as follows. After the potential of the electrode had stabilized, 10.00 mL of 1.0 × 10–2 M NaSCN - pH 5.5 Tris buffer solution was added to the 1.0 × 10–3 M pH 5.5 tris buffer NaSCN so- lution while they were vigorously stirred. Then the time until the potential stabilized to lower than ±0.1 mV/min was measured. 2.6. Standard Thiocyanate Sample Solution A stock solution of NaSCN (1.0 × 10–1 M) was prepared using pH 5.5 Tris buffer solution. Dilute solutions (1.0 × 10–1 to 1.0 × 10–7 M) of NaSCN were freshly prepared by Copyright © 2011 SciRes. AJAC  W.-S. HAN ET AL. Copyright © 2011 SciRes. AJAC 733 2.8. E.M.f. Measurements diluting the stock solution with doubly distilled water and Tris buffer solution of pH 5.5. The emf values were measured at 20˚C ± 0.2˚C using a model 355 Ion-analyzer (Mettler-Toledo Ltd., England). In all experiments, the pH measurements of the sample solutions were determined with a Mettler-Toledo InLab 412 glass electrode. The external reference electrode was a double-junction calomel electrode Orion 90-20-00 (Orion Research, USA.). The standard deviation arising from this equipment was <0.1 mV for a single determi- nation. Before use, the electrodes were conditioned in distilled water for at least 1 h. 2.7. Selectivity of the Developed Sensors The thiocyanate sensor was immersed in the 1.0 × 10–3.0 M NaSCN solution that had been adjusted to pH 5.5 with Tris buffer and the potential was measured. The poten- tials of solution adjusted to pH 5.5 with an interferent concentration of 1.0 × 10–3.0 M were measured. The se- lectivity coefficients , were determined by em- ploying the separate solution method (SSM) with the following generalized equation: pot SCN X K 3. Result and Discussion ,12 log1 1log pot SCN X EEZ a For six solid contact electrodes with different plasticizers based on Mn-PASPD ionophore (Figure 1), there was no tendency in the dynamic range and response slope as dielectric constant of plasticizers was increased (Table 1). The electrodes based on Mn-PASPD ionophore where E1 is the potential measured in 1.0 × 10–3.0 M NaSCN, E2 the potential measured in 1.0 × 10–3.0 M of the interfering compound, z is the charges of interfering species X respectively, and S is slope of the electrode calibration plot. N Mn N O CH HC O N NN N Figure 1. The scheme of Mn complex of N,N’-bis-(4-phenylazosalicylidene)-o-phenylene diamine ionophore. Table 1. The response characteristics of thiocyanate ion selective solid contact electrode based on Mn-PASPD ionophore with various plasticizers and composition. Mn-PASPD PVC Plasticizers Response slope Dynamic range r2 3.0 30.0 DBP 67.0 56.4 mV/decade 1×10–1.0 M ~ 1×10–5.0 M 0.996 4.0 30.0 DBP 66.0 58.1 mV/decade 1×10–1.0 M ~ 1×10–5.8 M 0.998 3.0 30.0 DOS 67.0 50.2 mV/decade 1×10–1.0 M ~ 1×10–5.0 M 0.997 4.0 30.0 DOS 66.0 49.8 mV/decade 1×10–1.0 M ~ 1×10–5.2 M 0.977 3.0 30.0 DOA 67.0 50.4 mV/decade 1×10–1.0 M ~ 1×10–4.8 M 0.996 4.0 30.0 DOA 66.0 52.1 mV/decade 1×10–1.0 M ~ 1×10–4.7 M 0.977 3.0 30.0 TEHP 67.0 40.2 mV/decade 1×10–1.0 M ~ 1×10–4.6 M 0.976 4.0 30.0 TEHP 66.0 40.2 mV/decade 1×10–1.0 M ~ 1×10–4.7 M 0.995 3.0 30.0 NPOE 67.0 40.2 mV/decade 1×10–1.0 M ~ 1×10–4.9 M 0.989 4.0 30.0 NPOE 66.0 40.5 mV/decade 1×10–1.0 M ~ 1×10–4.8 M 0.978 3.0 30.0 DOP 67.0 58.0 mV/decade 1×10–1.0 M ~ 1x10–5.5 M 0.997 4.0 30.0 DOP 66.0 54.9 mV/decade 1×10–1.0 M ~ 1×10–5.2 M 0.997 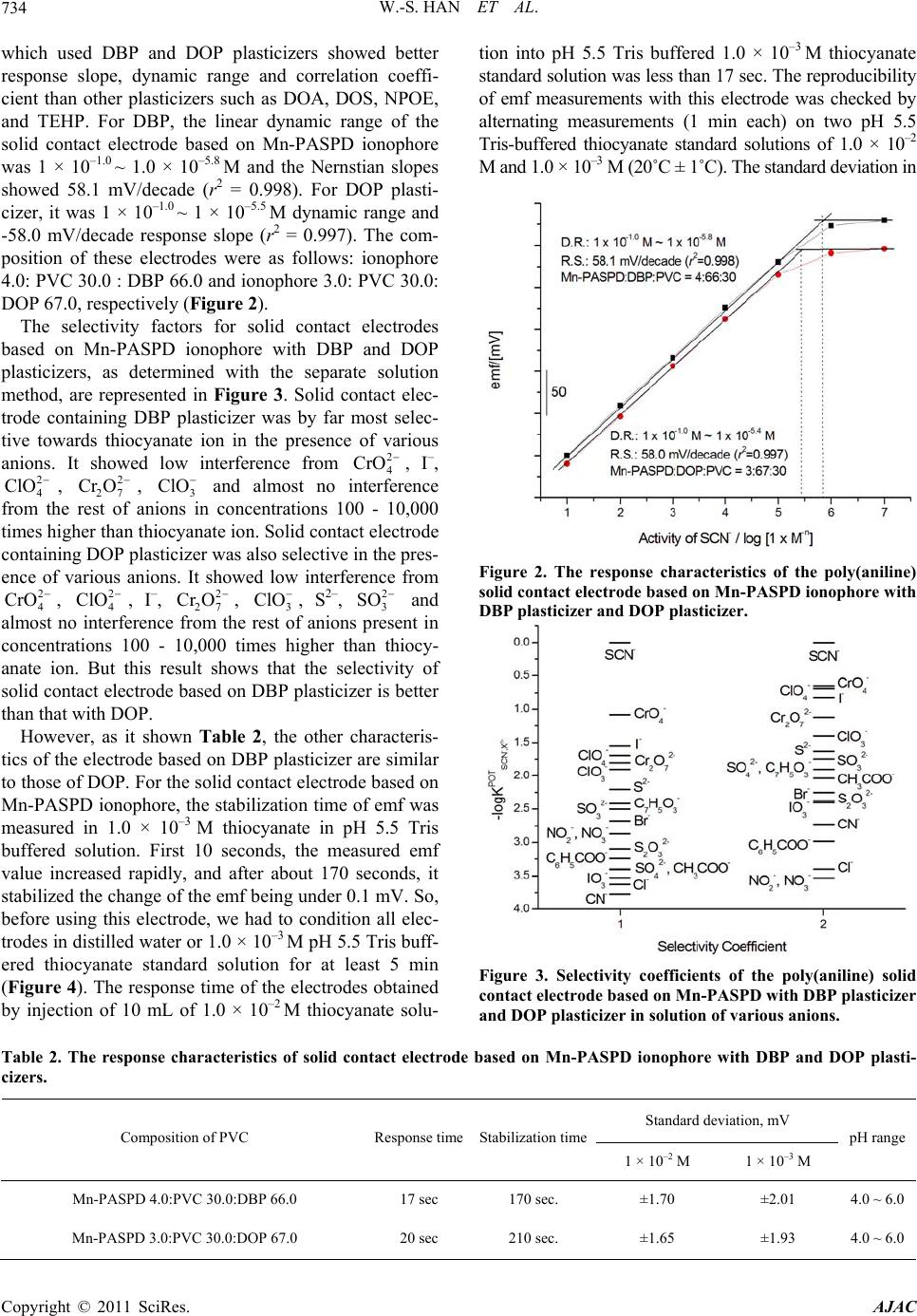 W.-S. HAN ET AL. Copyright © 2011 SciRes. AJAC 734 which used DBP and DOP plasticizers showed better response slope, dynamic range and correlation coeffi- cient than other plasticizers such as DOA, DOS, NPOE, and TEHP. For DBP, the linear dynamic range of the solid contact electrode based on Mn-PASPD ionophore was 1 × 10–1.0 ~ 1.0 × 10–5.8 M and the Nernstian slopes showed 58.1 mV/decade (r2 = 0.998). For DOP plasti- cizer, it was 1 × 10–1.0 ~ 1 × 10–5.5 M dynamic range and -58.0 mV/decade response slope (r2 = 0.997). The com- position of these electrodes were as follows: ionophore 4.0: PVC 30.0 : DBP 66.0 and ionophore 3.0: PVC 30.0: DOP 67.0, respectively (Figure 2). The selectivity factors for solid contact electrodes based on Mn-PASPD ionophore with DBP and DOP plasticizers, as determined with the separate solution method, are represented in Figure 3. Solid contact elec- trode containing DBP plasticizer was by far most selec- tive towards thiocyanate ion in the presence of various anions. It showed low interference from 2 4 CrO , I–, , , 3 and almost no interference from the rest of anions in concentrations 100 - 10,000 times higher than thiocyanate ion. Solid contact electrode containing DOP plasticizer was also selective in the pres- ence of various anions. It showed low interference from , , I–, , 3, S2–, 2 4 ClO 2 4 CrO 2 27 Cr O 2 4 ClO ClO Cr 2 27 OClO2 3 SO and almost no interference from the rest of anions present in concentrations 100 - 10,000 times higher than thiocy- anate ion. But this result shows that the selectivity of solid contact electrode based on DBP plasticizer is better than that with DOP. However, as it shown Table 2, the other characteris- tics of the electrode based on DBP plasticizer are similar to those of DOP. For the solid contact electrode based on Mn-PASPD ionophore, the stabilization time of emf was measured in 1.0 × 10–3 M thiocyanate in pH 5.5 Tris buffered solution. First 10 seconds, the measured emf value increased rapidly, and after about 170 seconds, it stabilized the change of the emf being under 0.1 mV. So, before using this electrode, we had to condition all elec- trodes in distilled water or 1.0 × 10–3 M pH 5.5 Tris buff- ered thiocyanate standard solution for at least 5 min (Figure 4). The response time of the electrodes obtained by injection of 10 mL of 1.0 × 10–2 M thiocyanate solu- tion into pH 5.5 Tris buffered 1.0 × 10–3 M thiocyanate standard solution was less than 17 sec. The reproducibility of emf measurements with this electrode was checked by alternating measurements (1 min each) on two pH 5.5 Tris-buffered thiocyanate standard solutions of 1.0 × 10–2 M and 1.0 × 10–3 M (20˚C ± 1˚C). The standard deviation in Figure 2. The response characteristics of the poly(aniline) solid contact electrode based on M n-PASPD ionophor e w ith DBP plasticizer and DOP plasticizer. Figure 3. Selectivity coefficients of the poly(aniline) solid contact electrode based on Mn-PASPD with DBP plasticizer and DOP plasticizer in solution of various anions. Table 2. The response characteristics of solid contact electrode based on Mn-PASPD ionophore with DBP and DOP plasti- cizers. Standard deviation, mV Composition of PVC Response timeStabilization time 1 × 10–2 M 1 × 10–3 M pH range Mn-PASPD 4.0:PVC 30.0:DBP 66.0 17 sec 170 sec. ±1.70 ±2.01 4.0 ~ 6.0 Mn-PASPD 3.0:PVC 30.0:DOP 67.0 20 sec 210 sec. ±1.65 ±1.93 4.0 ~ 6.0 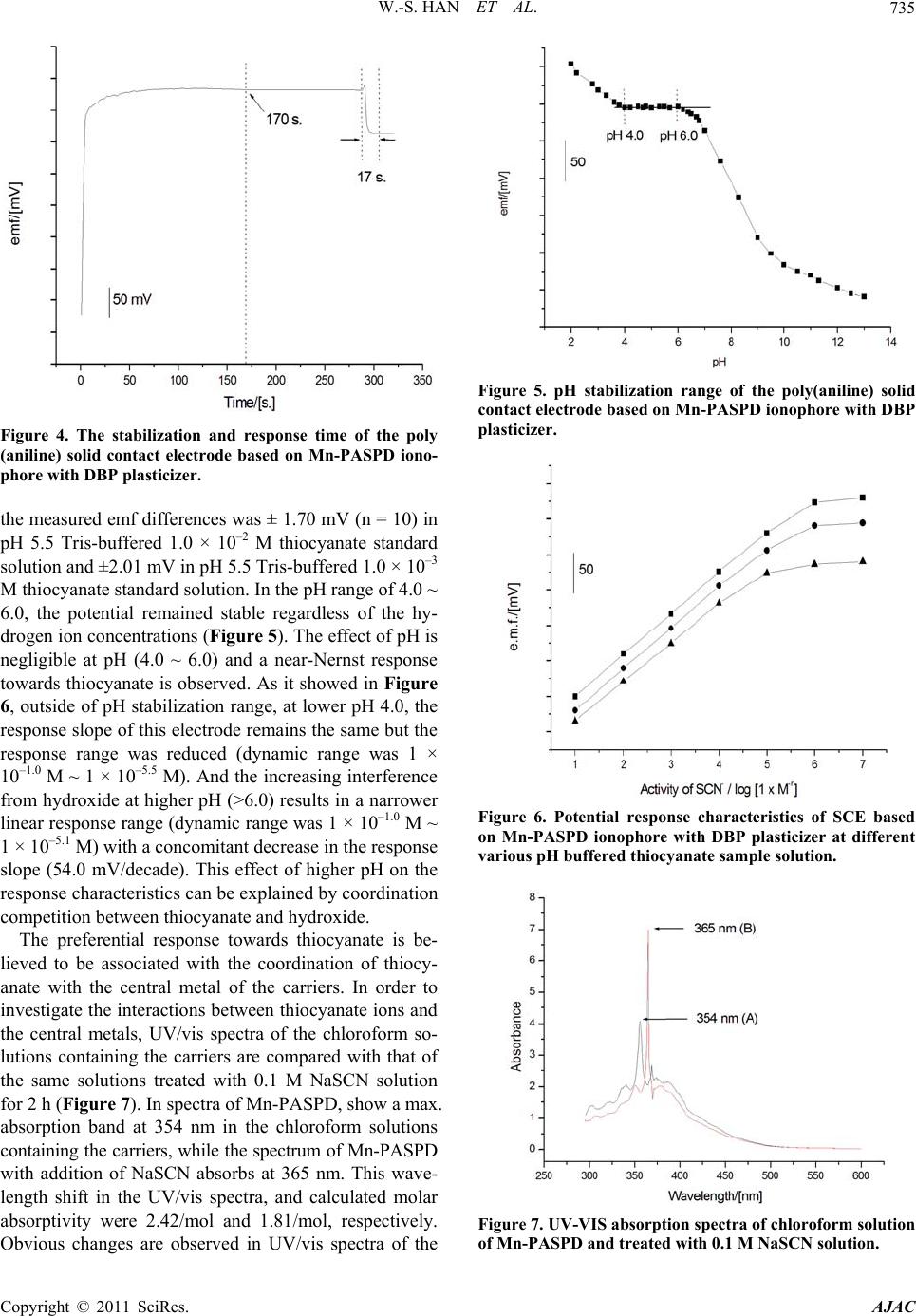 W.-S. HAN ET AL. Copyright © 2011 SciRes. AJAC 735 Figure 4. The stabilization and response time of the poly (aniline) solid contact electrode based on Mn-PASPD iono- phore with DBP plasticizer. the measured emf differences was ± 1.70 mV (n = 10) in pH 5.5 Tris-buffered 1.0 × 10–2 M thiocyanate standard solution and ±2.01 mV in pH 5.5 Tris-buffered 1.0 × 10–3 M thiocyanate standard solution. In the pH range of 4.0 ~ 6.0, the potential remained stable regardless of the hy- drogen ion concentrations (Figure 5). The effect of pH is negligible at pH (4.0 ~ 6.0) and a near-Nernst response towards thiocyanate is observed. As it showed in Figure 6, outside of pH stabilization range, at lower pH 4.0, the response slope of this electrode remains the same but the response range was reduced (dynamic range was 1 × 10–1.0 M ~ 1 × 10–5.5 M). And the increasing interference from hydroxide at higher pH (>6.0) results in a narrower linear response range (dynamic range was 1 × 10–1.0 M ~ 1 × 10–5.1 M) with a concomitant decrease in the response slope (54.0 mV/decade). This effect of higher pH on the response characteristics can be explained by coordination competition between thiocyanate and hydroxide. The preferential response towards thiocyanate is be- lieved to be associated with the coordination of thiocy- anate with the central metal of the carriers. In order to investigate the interactions between thiocyanate ions and the central metals, UV/vis spectra of the chloroform so- lutions containing the carriers are compared with that of the same solutions treated with 0.1 M NaSCN solution for 2 h (Figure 7). In spectra of Mn-PASPD, show a max. absorption band at 354 nm in the chloroform solutions containing the carriers, while the spectrum of Mn-PASPD with addition of NaSCN absorbs at 365 nm. This wave- length shift in the UV/vis spectra, and calculated molar absorptivity were 2.42/mol and 1.81/mol, respectively. Obvious changes are observed in UV/vis spectra of the Figure 5. pH stabilization range of the poly(aniline) solid contact electrode based on Mn-PASPD ionophore with DBP plasticizer. Figure 6. Potential response characteristics of SCE based on Mn-PASPD ionophore with DBP plasticizer at different various pH buffered thiocyanate sample solution. Figure 7. UV-VIS absorption spectra of chloroform solution of Mn-PASPD and treated with 0.1 M NaSCN solution.  W.-S. HAN ET AL. 736 chloroform solution containing Mn-PASPD after treated with 0.1 M NaSCN solution, it can be due to the unique interactions between the central metal manganese in Mn-PASPD and thiocyanate ion. As it is to be expected from the selectivity data given above, there is no interference from electrolytes in artifi- cial serum in the physiologically relevant various ions. (Figure 8) In artificial serum, their Nernstian slope of these electrodes showed 57.9 mV/decade (20˚C ± 0.5˚C) but their dynamic range of these electrodes showed mi- nor decrease to ~ 1.0 × 10–5.1 M. Measured results in artificial serum are the almost same values as the one’s which were measured in acetate buffered (57.9 mV/ decade of Nernstian slope, ~1 × 10–5.8 M of dynamic range) and tris buffered thiocyanide sample solution. The result of decreasing Nernstian slope is considered to be the lower interference affect of inorganic cations as like Ca2+ or Mg2+. Thus it doesn’t seem to be affected by abundant interference ions existing in human whole blood. Solid contact electrode continuously contacted with Tris 5.5 buffered thiocyanate solutions and distilled wa- ter for one month did not lose its performance. Also, these electrodes, dried daily after daily measurements in the Tris buffered thiocyanate solution, maintained a sta- ble electrode potential for more than 6 months. Therefore it seems that the electrodes can be used for over 6 months. 4. Conclusions A highly selective electrode for thiocyanate ion based on Mn-PASPD as an excellent ionophore is described. The poly(aniline) solid contact electrode based on Mn-PASPD Figure 8. The response characteristics of solid contact elec- trode based on Mn-PASPD ionophore in acetate buffer solution and artificial serum. ionophore may provide an alternative for the direct de- termination of thiocyanate. This electrode and ionophore are very easy to prepare, show high selectivity and sensi- tivity, wide dynamic range, low detection limit and very fast response time. These properties make this electrode suitable for measuring the concentration of thiocyanate in a wide variety of samples without the need for pre- concentration or pre-treatment steps, and without sig- nificant interferences from other anionic species present in the sample. 5. Acknowledgements This work was supported by the research fund of Hanseo University made in program year 2011 6. References [1] W. Weuffen, C. Franzke and B. Turkow, “Fortschritts- bericht Zur Alimentären Aufnahme, Analytik und biolo- gischen Bedeutung des Thiocyanats,” Nahrung, Vol 28, No. 4, 1984, pp. 341-355. doi:10.1002/food.19840280403 [2] R. E. Bliss, K. A., “Problems with Thiocyanate as an Index of Smoking Status: A Critical Review with Sug- gestions with Improving the Usefulness of Biochemical Measures in Smoking Cessation Research,” Health Psy- chology, Vol. 3, No. 6, 1984, p. 563-581. doi:10.1037/0278-6133.3.6.563 [3] K. Tsuge, M. Kataoka and Y. Seto, “Rapid Determination of Azide and Cyanide in Beverages Using Micro Diffu- sion Method Rapid Determination of Azide and Cyanide in Beverages Using Micro Diffusion Method,” Journal of Health Science, Vol. 46, 2000, p. 343. [4] Q. Li, W. Wei and Q. Liu, “Indirect Determination of Thiocyanate with Ammonium Sulfate and Ethanol by Extraction-Flotation of Copper,” Analyst, Vol. 125, No. 10, 2000, pp. 1885-1888. doi:10.1039/b004497k [5] R. G. Bowler, “The Determination of Thiocyanate in Blood Serum,” Biochemical Journal, Vol. 38, 1944, pp. 385-388. [6] A. R. Pettigrew and G. S. Fell, “Microdiffusion Method for Estimation of Cyanide in Whole Blood and Its Appli- cation to the Study of Conversion of Cyanide to Thiocy- anate,” Cl in ic al Chemistry, Vol. 18, 1972, pp. 996. [7] W. C. Butts, M. Kuehneman and G. M. Widdowson, “Automated Method for Determining Serum Thiocyanate, to Distinguish Smokers from Nonsmokers,” Clinical Chemistry, Vol. 20, No. 10, 1974, pp. 1344-1348. [8] S. Nagashima, “Simultaneous Reaction Rate Spectro- photometric Determination of Cyanide and Thiocyanate by Use of the Pyridine-Barbituric Acid Method,” Ana- lytical Chemistry, Vol. 56, No. 11, 1984, pp. 1944-1947. doi:10.1021/ac00275a042 [9] Y. K. Agrawal and P. N. Bhatt, “Spectrophotometric Determination of Thiocyanate Following Complexation Copyright © 2011 SciRes. AJAC  W.-S. HAN ET AL.737 with Mercury(II) and N-Phenylbenzohydroxamic Acid,” Analyst, Vol. 112, No. 12, 1987, pp. 1767-1769. doi:10.1039/an9871201767 [10] T. Chikamoto and T. Maitani, “Gas Chromatographic Determination of Thiocyanate Ion in Biological Fluids Using Immobilized Phase-Transfer Catalyst for Derivati- zation,” Analytical Science, Vol. 2, No. 2, 1986, pp. 161- 164. doi:10.2116/analsci.2.161 [11] S. Tanabe, M. Kitahara, N. Nawata and K. Kawanabe, “Determination of Oxidizable Inorganic Anions by High-Performance Liquid Chromatography with Fluo- rescence Detection and Application to the Determination of Salivary Nitrite and Thiocyanate and Serum Thiocy- anat,” Journal of Chromatography, Vol. 424, 1988, p. 29. [12] Y. Michigami, T. Takahashi, F. He, Y. Yamamoto and K. Ueda, “Determination of Thiocyanate in Human Serum by Ion Chromatography,” Analyst, Vol. 113, No. 3, 1988, pp. 389-392. doi:10.1039/an9881300389 [13] Y. Muira and T. Koh, “Determination of Thiocyanate in Human Urine Samples by Suppressed Ion Chromatogra- phy,” Analytical Science, Vol. 7, 1991, pp. 167-170. doi:10.2116/analsci.7.Supple_167 [14] C. Bjergegaard, P. Moller and H. Sorensen, “Determina- tion of Thiocyanate, Iodide, Nitrate and Nitrite in Bio- logical Samples by Micellar Electrokinetic Capillary Chromatography,” Journal of Chromatography A, Vol. 717, No. 1-2, 1995, pp. 409-414. doi:10.1016/0021-9673(95)00554-1 [15] S. H. Chen, Z. Y. Yang, H. L. Wu, H.S. Kou and S. J. Lin, “Determination of Thiocyanate Anion by High-Perfor- mance Liquid Chromatography with Fluorimetric Detec- tion,” Journal of Analytical Toxicology, Vol. 20, No. 1, 1996, pp. 38-42. [16] X. Cai and Z. Zhao, “Determination of Trace Thiocyanate by Linear Sweep Polarography,” Analytica Chimica Acta, Vol. 212, 1988, pp. 43-48. doi:10.1016/S0003-2670(00)84127-6 [17] Z. Glatz, S. Nov’akov’a and H. Sterbova, “Analysis of Thiocyanate in Biological Fluids by Capillary Zone Elec- trophoresis,” Journal of Chromatography A, Vol. 916, No. 1-2, 2001, pp. 273-277. doi:10.1016/S0021-9673(00)01238-3 [18] J. A. Cox, T. Gray and K. R. Kulkarni, “Stable Modified Electrodes for Flow-Injection Amperometry: Application to the Determination of Thiocyanate,” Analytical Chem- istry, Vol. 60, No. 17, 1988, pp. 1710-1713. doi:10.1021/ac00168a015 [19] A. Hodinar and A. Jyo, “Thiocyanate Solvent Polymeric Membrane Ion-Selective Electrode Based on Cobalt(III) α, β, γ, δ-Tetraphenylporphyrin Anion Carrier,” Chemis- try Letters, Vol. 17, No. 6, 1988, pp. 993-996. doi:10.1246/cl.1988.993 [20] D. Gao, J. Z. Li and R. Q. Yu, “Metalloporphyrin Deriva- tives as Neutral Carriers for PVC Membrane Electrodes,” Analytical Chemistry, Vol. 66, No. 12, 1994, pp. 2245- 2249. doi:10.1021/ac00086a008 [21] D. Gao, J. Gu, R. Q. Yu and G.-D. Zheng, “Substituted Metalloporphyrin Derivatives as Anion Carrier for PVC Membrane Electrodes,” Analytica Chimica Acta, Vol. 302, No. 2-3, 1995, pp. 263-268. doi:10.1016/0003-2670(94)00447-T [22] D. V. Brown, N. A. Chaniotakis, I. H. Lee, S. C. Ma, S. B. Park, M. E. Meyerhoff, R. J. Nick and J. T. Groves, “Mn(III)—Porphyrin-Based Thiocyanate-Selective Mem- brane Electrodes: Characterization and Application in Flow Injection Determination of Thiocyanate in Saliva,” Electroanalysis, Vol. 1, No. 6, 1989, pp. 477-484. doi:10.1002/elan.1140010602 [23] J. H. Khorasani, M. K. Amini, H. Motaghi, S. Tanges- taninejad and M. Moghadam, “Manganese Porphyrin De- rivatives as Ionophores for Thiocyanate-Selective Elec- trodes: The Influence of Porphyrin Substituents and Ad- ditives on the Response Properties,” Sensors and Actua- tors B, Vol. 87, No. 3, 2002, pp. 448-456. doi:10.1016/S0925-4005(02)00294-0 [24] M. Shamsipur, G. Khayatian and S. Tangestaninejad, “Thiocyanate-Selective Membrane Electrode Based on (Octabromotetraphenylporphyrinato) Manganese(III) Chlo- ride,” Electroanalysis, Vol. 11, No. 18, 1999, pp. 1340-1344. doi:10.1002/(SICI)1521-4109(199912)11:18<1340::AID- ELAN1340>3.0.CO;2-M [25] M. K. Amini, S. Shahrokhian and S. Tangestaninejad, “Thiocyanate-Selective Electrodes Based on Nickel and Iron Phthalocyanines,” Analytica Chimica Acta, Vol. 402, No. 2-3, 1999, pp. 137-143. doi:10.1016/S0003-2670(99)00549-8 [26] M. K. Amini, S. Shahrokhian and S. Tangestaninejad, “PVC-Based Cobalt and Manganese Phthalocyanine Coated Graphite Electrodes for Determination of Thio- cyanate,” Analytical Letters, Vol. 32, No. 14, 1999, pp. 2737-2750. doi:10.1080/00032719908543002 [27] A. Florido, L. G. Bachas, M. Valiente and I. Villaescusa, “Anion-Selective Electrodes Based on a Gold(III)-Triiso- butylphosphine Sulfide Complex,” Analyst, Vol. 119, No. 11, 1994, pp. 2421-2425. doi:10.1039/an9941902421 [28] Z. Q. Li, Z. Y. Wu, R. Yuan, M. Ying, G. L. Shen and R.Q. Yu, “Thiocyanate-Selective PVC Membrane Elec- trodes Based on Mn(II) Complex of N,N’-Bis-(4- Phenylazosalicylidene) O-Phenylene Diamine as a Neu- tral Carrier,” Electrochimica Acta, Vol. 44, No. 15, 1999, pp. 2543-2548. doi:10.1016/S0013-4686(98)00361-2 [29] M. R. Ganjali, T. Poursaberi, F. Basiripour, M. Salavati- Niassari, M. Yousefi and M. Shamsipur, “Highly Selec- tive Thiocyanate Poly(Vinyl Chloride) Membrane Elec- trode Based on a Cadmium-Schiff's Base Complex,” Fresenius’ Journal of Analytical Chemistry, Vol. 370, No. 8, 2001, pp. 1091-1095. [30] A. Abbaspour, M. A. Kamyabi, A. R. Esmaeilbeig and R. Kia, “Thiocyanate-Selective Electrode Based on Un- symmetrical Benzon4 Nickel(II) Macrocyclic Com- plexes,” Talanta, Vol. 57, No. 5, 2002, pp. 859-867. doi:10.1016/S0039-9140(02)00129-7 [31] S. M. Lim, H. J. Jung, H. Won, N. Myung and K.-J. Paeng, “Potentiometric Behavior of the Membrane Elec- Copyright © 2011 SciRes. AJAC  W.-S. HAN ET AL. Copyright © 2011 SciRes. AJAC 738 trodes Based on the Model Compound of a Ni-Por-phin- oid,” Analytical Science, Vol. 17, 2001, p. 1701. [32] M. Ying, R. Yuan, Z. Q. Li, Y. Q. Song, W. X. Li, H. G. Lin, G. L. Shen and R. Q. Yu, “Thiocyanate-Selective Electrode Based on Cobalt(II) Complexes of Pyrazolone Heterocyclic Schiff Bases,” Fresenius’ Journal of Ana- lytical Chemistry, Vol. 361, No. 5, 1998, pp. 437-441. [33] M. M. Ardakani, A. A. Ensafi, M. S. Niasari and S. C. Mirhoseini, “Selective Thiocyanate Poly(Vinyl Chloride) Membrane Based on a 1,8-Dibenzyl-1,3,6,8,10, 13-Hexaa- zacyclotetradecane-Ni(II) Perchlorate,” Analytica Chimica Acta, Vol. 462, No. 1, 2002, pp. 25-30. doi:10.1016/S0003-2670(02)00314-8 [34] M. K. Amini, A. Rafi, M. Ghaedi, M. H. Habibi and M. M. Zohory, “Bis(2-Mercaptobenzoxazolato)Mercury(II) and Bis(2-Pyridinethiolato)Mercury(II) Complexes as Carriers for Thiocyanate Selective Electrodes,” Micro- chemical Journal, Vol. 73, No. 3, 2003, pp. 143-150. doi:10.1016/S0026-265X(03)00091-2 [35] M. M. Ardakani, M. Salavati-Niassari and A. Sadegui, “Novel Selective Thiocyanate PVC Membrane Electrode Based on New Schiff Base Complex of 2.2-[(1,3-Di- methyl-1,3-Propanediylidene)Dinitrilo]Bis-Benzenethiola to Cadmium(II),” New Journal of Chemistry, Vol. 28, No. 5, 2004, pp. 595-599. doi:10.1039/b400681j [36] M. Shamsipur, S. Ershad, N. Samadi, A. R. Rezvani and H. Haddadzadeh, “The First Use of a Rh(III) Complex as a Novel Ionophore for Thiocyanate-Selective Polymeric Membrane Electrodes,” Talanta, Vol. 65, No. 4, 2005, pp. 991-997. doi:10.1016/j.talanta.2004.08.032 [37] M. Shamsipur, T. Poursaberi, M. Rezapour, M. R. Gan- gali, M. F. Mousavi, V. Lippolis and D. R. Montesu, “[Cu(L)](NO3)2(L=4,7-Bis(3-aminopropyl)-1-thia-4,7-dia zacyclononane) as a Suitable Ionophore for Construction of Thiocyanate-Selective Electrodes and Their Use in Determination of Urinary and Salivary Thiocyanate Concentration,” Electroanalysis, Vol. 16, No. 16, 2004, pp. 1336-1342. doi:10.1002/elan.200302957 [38] S. S. M. Hassan, M. H. Abou Ghalia, A. G. E. Amr and A. H. K. Mohamed, “Novel Thiocyanate-Selective Mem- brane Sensors Based on Di-, Tetra-, and Hexa-Imidepyri- dine Ionophores,” Analytica Chimica Acta, Vol. 482, No. 1, 2003, pp. 9-18. doi:10.1016/S0003-2670(03)00172-7
|