 American Journal of Anal yt ical Chemistry, 2011, 2, 665-674 doi:10.4236/ajac.2011.26076 Published Online October 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Chemical Profiling and Quantification of Isoflavone Phytoestrogens in Kudzu Using LC/UV/MSD Qing-Li Wu1*, Yong-Hong Yang2, Jim Simon1 1New Use Agriculture and Natural Plant Products Program Department of Plant Biology and Pathology, Rutgers University, New Brunswick, USA 2Key Laboratory, Department of Plant Pathology, Yunnan Agricultural University, Kunmin, China E-mail: *qlwu@aesop.rutgers.edu Received February 21, 2011; revised May 3, 2011; accepted May 20, 2011 Abstract Method development for determination of isoflavones in kudzu was achieved by HPLC/UV/ESI-MSD. Us- ing three kudzu species of Pueraria lobata, P. thomsonii and P. edulis, and analyzing the isoflavones sepa- rately by species and from different plant tissues (roots, stems, leaves, flowers and fruits) in each species, a total of 25 isoflavones were identified by their molecular ions and characteristic fragment ion peaks using LC/MSD under MS and MS/MS mode, and in comparison with standard isoflavones. Two main chemical groups were identified: 1) 8-C-glycosyl isoflavone of puerarin and the analogues of 5-OH puerarin, 3’-OH puerarin, 3’-OMe puerarin, and their glycosides; and 2) daidzein, genistein, glycitein and their glycosyl and malonyl derivatives, which are similar to those known in soy. To accurately quantitate total isoflavones, acidic hydrolysis during extraction of kudzu samples was applied to convert the oxygen glycosides into their respective isoflavone aglycones of daidzein, genistein and glycitein, or non-hydrolyzed carbon glycosides of puerarin, 5-OH puerarin, 3’-OH puerarin and 3’-OMe puerarin. Under the multiple optimized conditions, all seven isoflavones in acidic hydrolyzed kudzu extracts were successfully separated within 30 min and quanti- fied individually with calycosin used as internal standard by both UV and MS detectors. For the quantitative study, several standards e.g. 5-OH puerarin, 3’-OH puerarin and 3’-OMe puerarin are not commercially available. Using polyamide, sephdex-LH20 chromatography and Prep-HPLC, we purified these three stan- dards from kudzu extracts and then elucidated their structures by UV, MS and NMR spectrometric methods. This is the first method to simultaneously quantitate all the isoflavones in kudzu. Keywords: Kudzu, Pueraria, Isoflavones, LC/UV/MSD 1. Introduction The kudzu root, called “Gegen” in Chinese medicine which is obtained mainly from Pueraria lobata (lobed kudzuvine), P. thomsonii (Thomson kudzuvine) and P. edulis (edible kudzuvine), has been primarily used for the treatment of common cold, influenza, and wrist and shoulder stiffness, or as antidipsotropic agent [1]. Kudzu was reported to contain high amounts of phytoestrogenic isoflavones, such as daidzein, genistein, puerarin and their derivatives [2-4]. These compounds based on the structural similarity to internal estrogen have received much interest for the prevention of menopausal symp- toms, osteoporosis, high cholesterol, heart disease and cancer [5-10]. Several laboratories have provided evi- dence that the major isoflavones isolated from kudzu are effective in reducing alcohol intake [11-15]. Therefore, it is important to better understand the biology of the plant, and the tissues and sites of isoflavone accumulation. Several methods for determining isoflavones in kudzu and the derived products using high performance liquid chromatography combined with ultraviolet and/or mass spectrometric detector have been reported [14-21]. Qua- litative studies on kudzu have only led to the identifyca- tion of numerous isoflavones. The quantitative studies focused on the original or acidic hydrolyzed fractions kudzu extracts. However, only part of major components, puerarin, and soy-like isoflavones daidzein, genistein and the glycosides were quantified, and the researchers were not able to determine accurate levels of the total isofla- vones in kudzu and the derived products. The purpose of this research was to chemically profile the isoflavones, 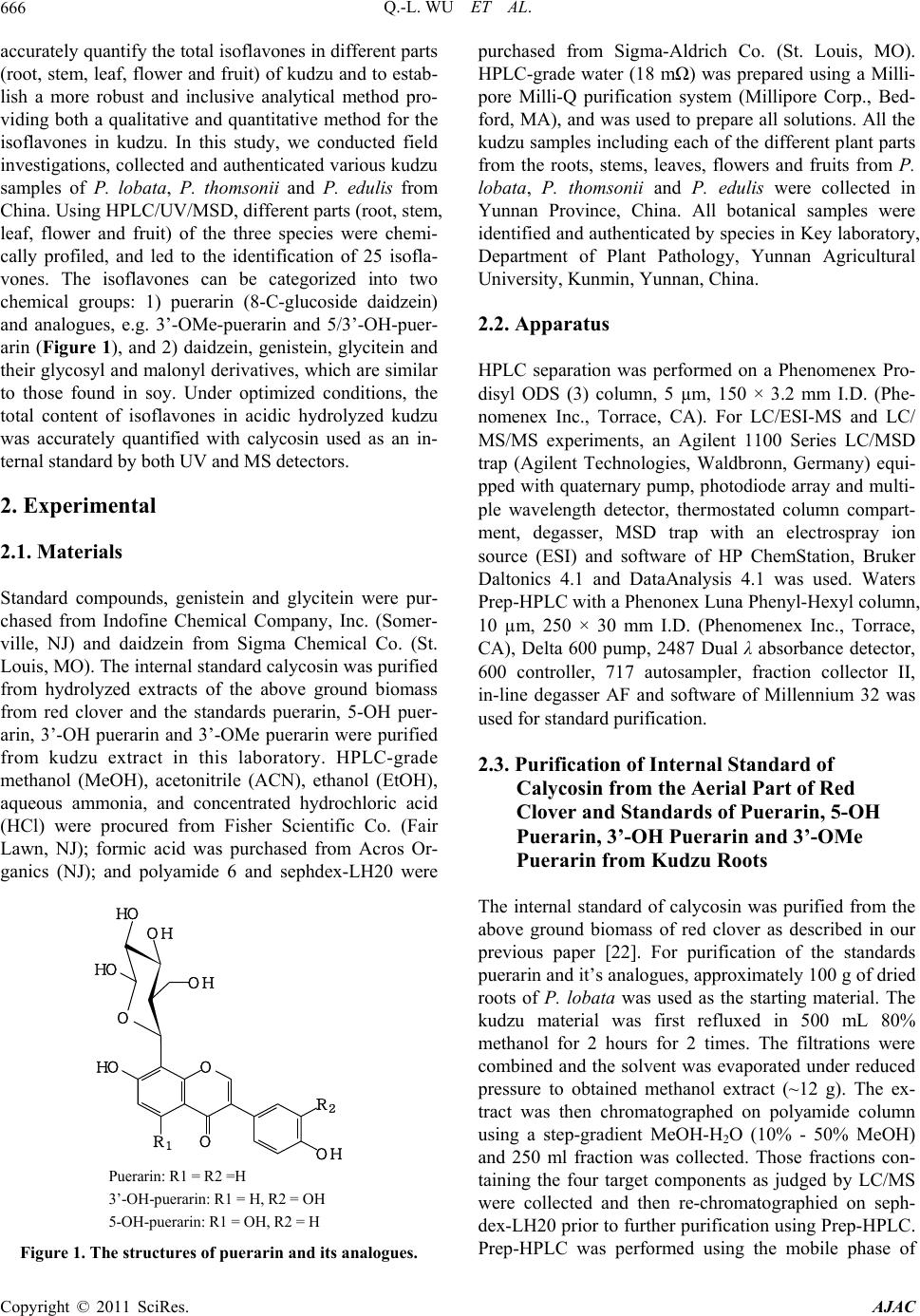 Q.-L. WU ET AL. 666 accurately quantify the total isoflavones in different parts (root, stem, leaf, flower and fruit) of kudzu and to estab- lish a more robust and inclusive analytical method pro- viding both a qualitative and quantitative method for the isoflavones in kudzu. In this study, we conducted field investigations, collected and authenticated various kudzu samples of P. lobata, P. thomsonii and P. edulis from China. Using HPLC/UV/MSD, different parts (root, stem, leaf, flower and fruit) of the three species were chemi- cally profiled, and led to the identification of 25 isofla- vones. The isoflavones can be categorized into two chemical groups: 1) puerarin (8-C-glucoside daidzein) and analogues, e.g. 3’-OMe-puerarin and 5/3’-OH-puer- arin (Figure 1), and 2) daidzein, genistein, glycitein and their glycosyl and malonyl derivatives, which are similar to those found in soy. Under optimized conditions, the total content of isoflavones in acidic hydrolyzed kudzu was accurately quantified with calycosin used as an in- ternal standard by both UV and MS detectors. 2. Experimental 2.1. Materials Standard compounds, genistein and glycitein were pur- chased from Indofine Chemical Company, Inc. (Somer- ville, NJ) and daidzein from Sigma Chemical Co. (St. Louis, MO). The internal standard calycosin was purified from hydrolyzed extracts of the above ground biomass from red clover and the standards puerarin, 5-OH puer- arin, 3’-OH puerarin and 3’-OMe puerarin were purified from kudzu extract in this laboratory. HPLC-grade methanol (MeOH), acetonitrile (ACN), ethanol (EtOH), aqueous ammonia, and concentrated hydrochloric acid (HCl) were procured from Fisher Scientific Co. (Fair Lawn, NJ); formic acid was purchased from Acros Or- ganics (NJ); and polyamide 6 and sephdex-LH20 were Puerarin: R1 = R2 =H 3’-OH-puerarin: R1 = H, R2 = OH 5-OH- uerarin: R1 = OH, R2 = H Figure 1. The structures of puerarin and its analogues. purchased from Sigma-Aldrich Co. (St. Louis, MO). HPLC-grade water (18 mΩ) was prepared using a Milli- pore Milli-Q purification system (Millipore Corp., Bed- ford, MA), and was used to prepare all solutions. All the kudzu samples including each of the different plant parts from the roots, stems, leaves, flowers and fruits from P. lobata, P. thomsonii and P. edulis were collected in Yunnan Province, China. All botanical samples were identified and authenticated by species in Key laboratory, Department of Plant Pathology, Yunnan Agricultural University, Kunmin, Yunnan, China. 2.2. Apparatus HPLC separation was performed on a Phenomenex Pro- disyl ODS (3) column, 5 µm, 150 × 3.2 mm I.D. (Phe- nomenex Inc., Torrace, CA). For LC/ESI-MS and LC/ MS/MS experiments, an Agilent 1100 Series LC/MSD trap (Agilent Technologies, Waldbronn, Germany) equi- pped with quaternary pump, photodiode array and multi- ple wavelength detector, thermostated column compart- ment, degasser, MSD trap with an electrospray ion source (ESI) and software of HP ChemStation, Bruker Daltonics 4.1 and DataAnalysis 4.1 was used. Waters Prep-HPLC with a Phenonex Luna Phenyl-Hexyl column, 10 µm, 250 × 30 mm I.D. (Phenomenex Inc., Torrace, CA), Delta 600 pump, 2487 Dual λ absorbance detector, 600 controller, 717 autosampler, fraction collector II, in-line degasser AF and software of Millennium 32 was used for standard purification. 2.3. Purification of Internal Standard of Calycosin from the Aerial Part of Red Clover and Standards of Puerarin, 5-OH Puerarin, 3’-OH Puerarin and 3’-OMe Puerarin from Kudzu Roots The internal standard of calycosin was purified from the above ground biomass of red clover as described in our previous paper [22]. For purification of the standards puerarin and it’s analogues, approximately 100 g of dried roots of P. lobata was used as the starting material. The kudzu material was first refluxed in 500 mL 80% methanol for 2 hours for 2 times. The filtrations were combined and the solvent was evaporated under reduced pressure to obtained methanol extract (~12 g). The ex- tract was then chromatographed on polyamide column using a step-gradient MeOH-H2O (10% - 50% MeOH) and 250 ml fraction was collected. Those fractions con- taining the four target components as judged by LC/MS were collected and then re-chromatographied on seph- dex-LH20 prior to further purification using Prep-HPLC. Prep-HPLC was performed using the mobile phase of Copyright © 2011 SciRes. AJAC  Q.-L. WU ET AL.667 MeCN-H2O (7% MeCN) at a flow rate of 7 mL/min to get puerarin (40 mg), 5-OH puerarin(12 mg), 3’-OH pu- erarin (14 mg) and 3’-OMe puerarin (8 mg). The struc- tures of these four compounds were then determined and verified by UV, MS and NMR spectrometric methods. 2.4. Preparation of Stock Solutions and Calibration Standards Individual stock solutions of 7 standards were prepared by dissolving the appropriate amounts of ~5.0 mg in 15.0 mL of diluent (water and MeOH, 3:7). The final volume of each solution was then diluted to 25 mL with diluent. Calibration standards were prepared by diluting the stock solutions with diluent and spiked with same amount of internal standard of calycosin. The calibration curve ranges for UV and MS methods show excellent linearity (Table 1). In the calibration plots, 8 and 6 different con- centration levels were used for UV and MS detection, respectively. 2.5. Plant Sample Preparation For qualitative study, ~200 mg of finely ground material was extracted with 10 mL 80% methanol using sonica- tion for 1 hour at room temperature. The extracts were filtered through 0.45 µm filter and 20 μL extract was injected for each analysis. The extraction procedure for quantitative analysis was adopted from our prior studies [22,23]. Approximately 1000 mg of powdered kudzu material was placed into a 250 mL flask along with 50 mL of ethanol, 20 mL of DI water, and 8 mL of concen- trated HCl. The mixture was refluxed for 2 hours pro- tected by N2. The solution was filtered and diluted to volume of 100 mL. Each hydrolyzed sample of 5 μL filtered over 0.45 µm filter was analyzed by triplicate injections. 2.6. Liquid Chromatographic and Mass Spectrometric Conditions for Identification of Isoflavones HPLC separation was performed with the mobile phase consisting of solvent A and B in gradient, where A was 0.1% formic acid (v/v) in water and B was 0.1% formic acid (v/v) in acetonitrile. The linear gradient profile was from 10% to 40% B in 40min. The wavelength of UV detection was 254 nm. Column compartment was set at 25˚C. The flow rate was 1.0 mL/min. The electrospray ion mass spectrometer (ESI-MS) was operated under positive ion and auto MS/MS mode (Threshold, 30,000) and optimized collision energy level of 80%, scanned from m/z 100 to 700. ESI was conducted using a needle voltage of 3.5 kV. High-purity nitrogen (99.999%) was used as dry gas and nebulizer at a flow rate of 12 L/Min, and capillary temperature at 350˚C. Helium was used as collision at 60 psi. The ESI interface and mass spec- trometer parameters were optimized to obtain maximum sensitivity. The auto MS/MS total ion chromatogram was processed by extracting the molecular ions of each isoflavone for identification. 2.7. Liquid Chromatographic and Mass Spectrometric Conditions for Quantification of Isoflavones MS detection was conducted under collision energy level of 80% and scanned from m/z 100 to 600. Other MS pa- rameter and LC conditions were the same as described above. Under SIM mode (selected ion monitoring), pro- tonated [M++H] ions were isolated for each isoflavone. The mass spectrometer was set into two time segments: 1) from 0 to 16.5 min for 3’-OH puerarin; puerarin; 3’-OMe puerarin and 5-OH puerarin with isolation of m/z 417, 433 and 447; 2) from 16.5 to 30 min for daidzein, gly- citein, genistein and calycosin of m/z 255, 271 and 285. The isolation width was set as 1.0 m/z. The calibration curves were plotted using a 1/x-weighted quadratic mo- del for the regression of peak area acquired from UV and MS detector versus analyte concentration. 3. Results and Discussion 3.1. Characterization of Isoflavones Simultaneous UV and processed auto MS/MS chroma- tograms of 80% methanol extract of P. lobata root are illustrated in Figure 2. The identities, retention time, protonated [M+H]+ and characteristic fragment ions for individual peaks are listed in Table 2. In kudzu, the car- bon glucoside of daidzein, puerarin (7, peak 6 in Figure 2) is well known to dominate the isoflavone constituents. Therefore, some minor analogues of puerarin e.g. 5-OH puerarin, 3’-OH puerarin, 3’-OMe puerarin and their glycosides are also detected on the basis of MS and MS/MS spectral interpretation and some of them by comparison to the authenticated standards. The MS spec- tra of puerarin and its analogues showing the protonated molecular ions [M+H]+ are illustrated in Figure 3. Based on the above analysis, a total of 10 puerarin analogues were determined in kudzu (Compounds 1-9 and 12 in Table 2), with some of the structures of puerarin and its analogues shown in Figure 1. Daidzein, genistein, gly- citein and their derivatives of glycoside and glycoside malonate, which are well-known to be present in soy, were also detected in kudzu (Compounds 10 , 11 and Copyright © 2011 SciRes. AJAC 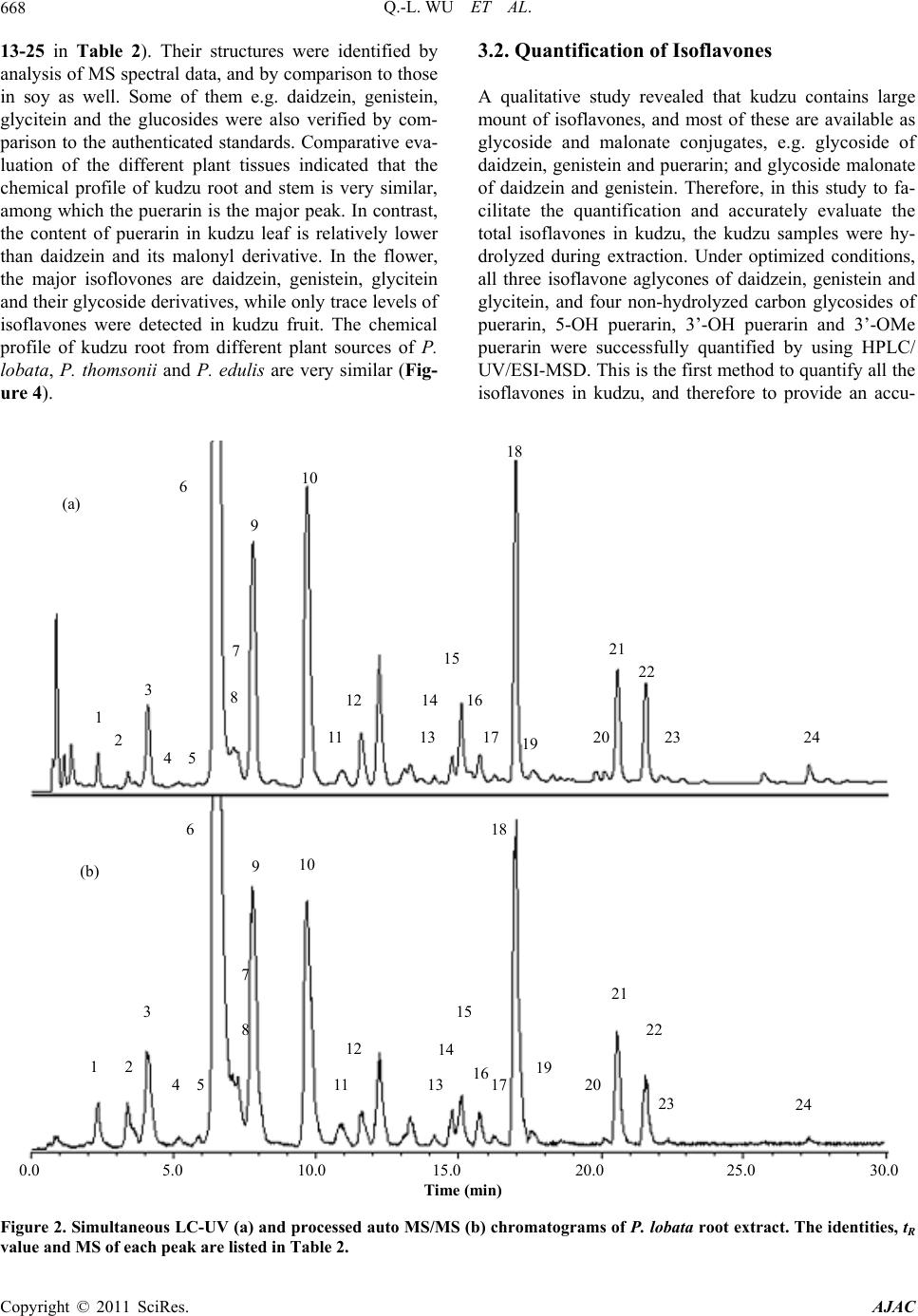 Q.-L. WU ET AL. Copyright © 2011 SciRes. AJAC 668 3.2. Quantification of Isoflavones 13-25 in Table 2). Their structures were identified by analysis of MS spectral data, and by comparison to those in soy as well. Some of them e.g. daidzein, genistein, glycitein and the glucosides were also verified by com- parison to the authenticated standards. Comparative eva- luation of the different plant tissues indicated that the chemical profile of kudzu root and stem is very similar, among which the puerarin is the major peak. In contrast, the content of puerarin in kudzu leaf is relatively lower than daidzein and its malonyl derivative. In the flower, the major isoflovones are daidzein, genistein, glycitein and their glycoside derivatives, while only trace levels of isoflavones were detected in kudzu fruit. The chemical profile of kudzu root from different plant sources of P. lobata, P. thomsonii and P. edulis are very similar (Fig- ure 4). A qualitative study revealed that kudzu contains large mount of isoflavones, and most of these are available as glycoside and malonate conjugates, e.g. glycoside of daidzein, genistein and puerarin; and glycoside malonate of daidzein and genistein. Therefore, in this study to fa- cilitate the quantification and accurately evaluate the total isoflavones in kudzu, the kudzu samples were hy- drolyzed during extraction. Under optimized conditions, all three isoflavone aglycones of daidzein, genistein and glycitein, and four non-hydrolyzed carbon glycosides of puerarin, 5-OH puerarin, 3’-OH puerarin and 3’-OMe puerarin were successfully quantified by using HPLC/ UV/ESI-MSD. This is the first method to quantify all the isoflavones in kudzu, and therefore to provide an accu- 0.0 5.0 10.0 15.0 20.0 25.0 30.0 Time ( mi n) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 1 2 3 4 5 6 9 7 8 10 11 12 14 17 13 15 16 18 19 20 21 22 23 24 (a) (b) Figure 2. Simultaneous LC-UV (a) and pr ocessed auto MS/MS (b) chromatograms of P. lobata root extract. The identities, tR value and MS of each peak are listed in Table 2. 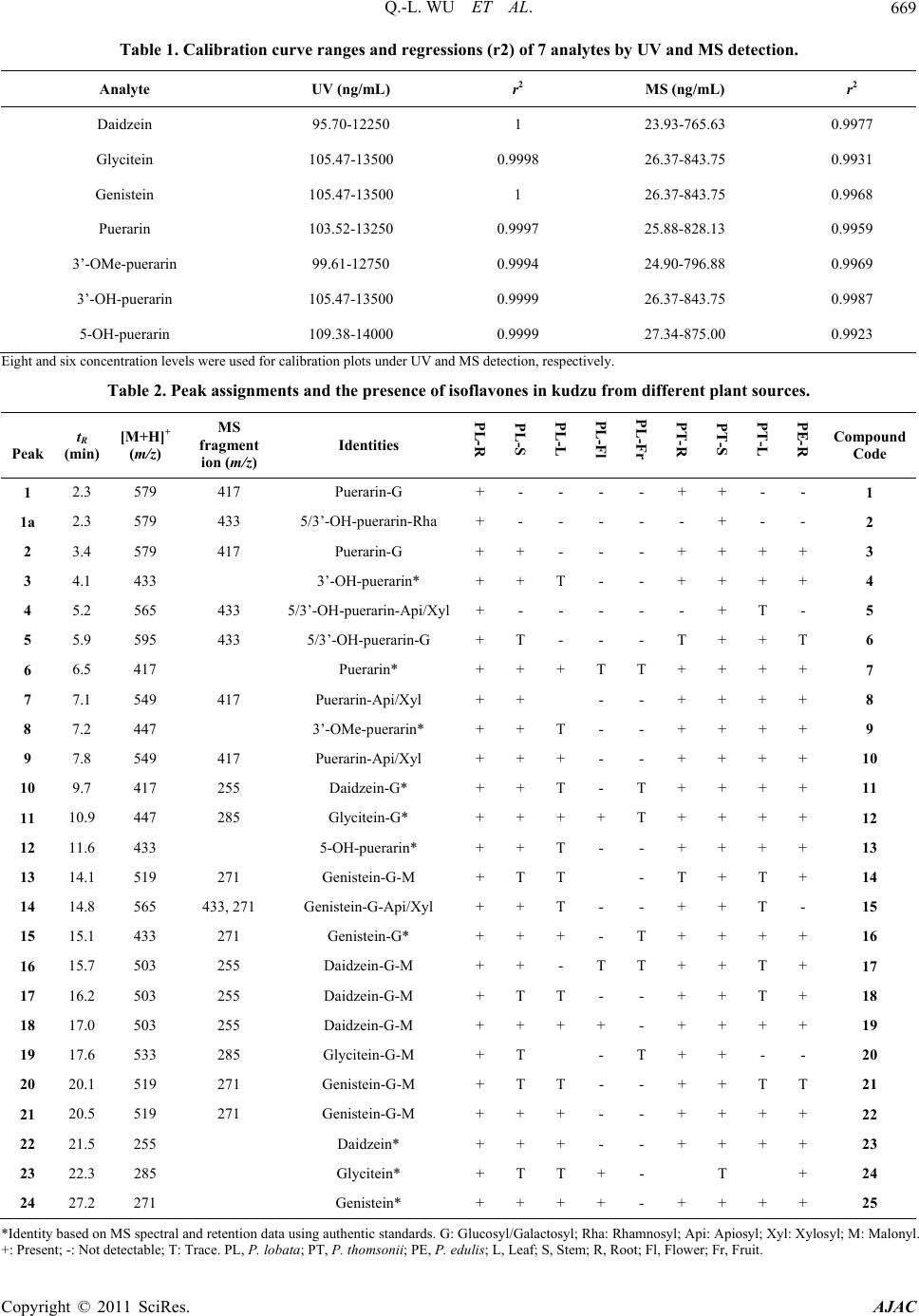 Q.-L. WU ET AL.669 Table 1. Calibration curve ranges and regressions (r2) of 7 analytes by UV and MS detection. Analyte UV (ng/mL) r2 MS (ng/mL) r2 Daidzein 95.70-12250 1 23.93-765.63 0.9977 Glycitein 105.47-13500 0.9998 26.37-843.75 0.9931 Genistein 105.47-13500 1 26.37-843.75 0.9968 Puerarin 103.52-13250 0.9997 25.88-828.13 0.9959 3’-OMe-puerarin 99.61-12750 0.9994 24.90-796.88 0.9969 3’-OH-puerarin 105.47-13500 0.9999 26.37-843.75 0.9987 5-OH-puerarin 109.38-14000 0.9999 27.34-875.00 0.9923 Eight and six concentration levels were used for calibration plots under UV and MS detection, respectively. Table 2. Peak assignments and the presence of isoflavones in kudzu from different plant sources. Peak tR (min) [M+H]+ (m/z) MS fragment ion (m/z) Identities PL-R PL-S PL-L PL-Fl PL-Fr PT-R PT-S PT-L PE-R Compound Code 1 2.3 579 417 Puerarin-G + - - - - + + - - 1 1a 2.3 579 433 5/3’-OH-puerarin-Rha + - - - - - + - - 2 2 3.4 579 417 Puerarin-G + + - - - + + + + 3 3 4.1 433 3’-OH-puerarin* + + T - - + + + + 4 4 5.2 565 433 5/3’-OH-puerarin-Api/Xyl+ - - - - - + T - 5 5 5.9 595 433 5/3’-OH-puerarin-G + T - - - T + + T 6 6 6.5 417 Puerarin* + + + T T + + + + 7 7 7.1 549 417 Puerarin-Api/Xyl + + - - + + + + 8 8 7.2 447 3’-OMe-puerarin* + + T - - + + + + 9 9 7.8 549 417 Puerarin-Api/Xyl + + + - - + + + + 10 10 9.7 417 255 Daidzein-G* + + T - T + + + + 11 11 10.9 447 285 Glycitein-G* + + + + T + + + + 12 12 11.6 433 5-OH-puerarin* + + T - - + + + + 13 13 14.1 519 271 Genistein-G-M + T T - T + T + 14 14 14.8 565 433, 271 Genistein-G-Api/Xyl + + T - - + + T - 15 15 15.1 433 271 Genistein-G* + + + - T + + + + 16 16 15.7 503 255 Daidzein-G-M + + - T T + + T + 17 17 16.2 503 255 Daidzein-G-M + T T - - + + T + 18 18 17.0 503 255 Daidzein-G-M + + + + - + + + + 19 19 17.6 533 285 Glycitein-G-M + T - T + + - - 20 20 20.1 519 271 Genistein-G-M + T T - - + + T T 21 21 20.5 519 271 Genistein-G-M + + + - - + + + + 22 22 21.5 255 Daidzein* + + + - - + + + + 23 23 22.3 285 Glycitein* + T T + - T + 24 24 27.2 271 Genistein* + + + + - + + + + 25 *Identity based on MS spectral and retention data using authentic standards. G: Glucosyl/Galactosyl; Rha: Rhamnosyl; Api: Apiosyl; Xyl: Xylosyl; M: Malonyl. +: Present; -: Not detectable; T: Trace. PL, P. lobata; PT, P. thomsonii; PE, P. edulis; L, Leaf; S, Stem; R, Root; Fl, Flower; Fr, Fruit. Copyright © 2011 SciRes. AJAC 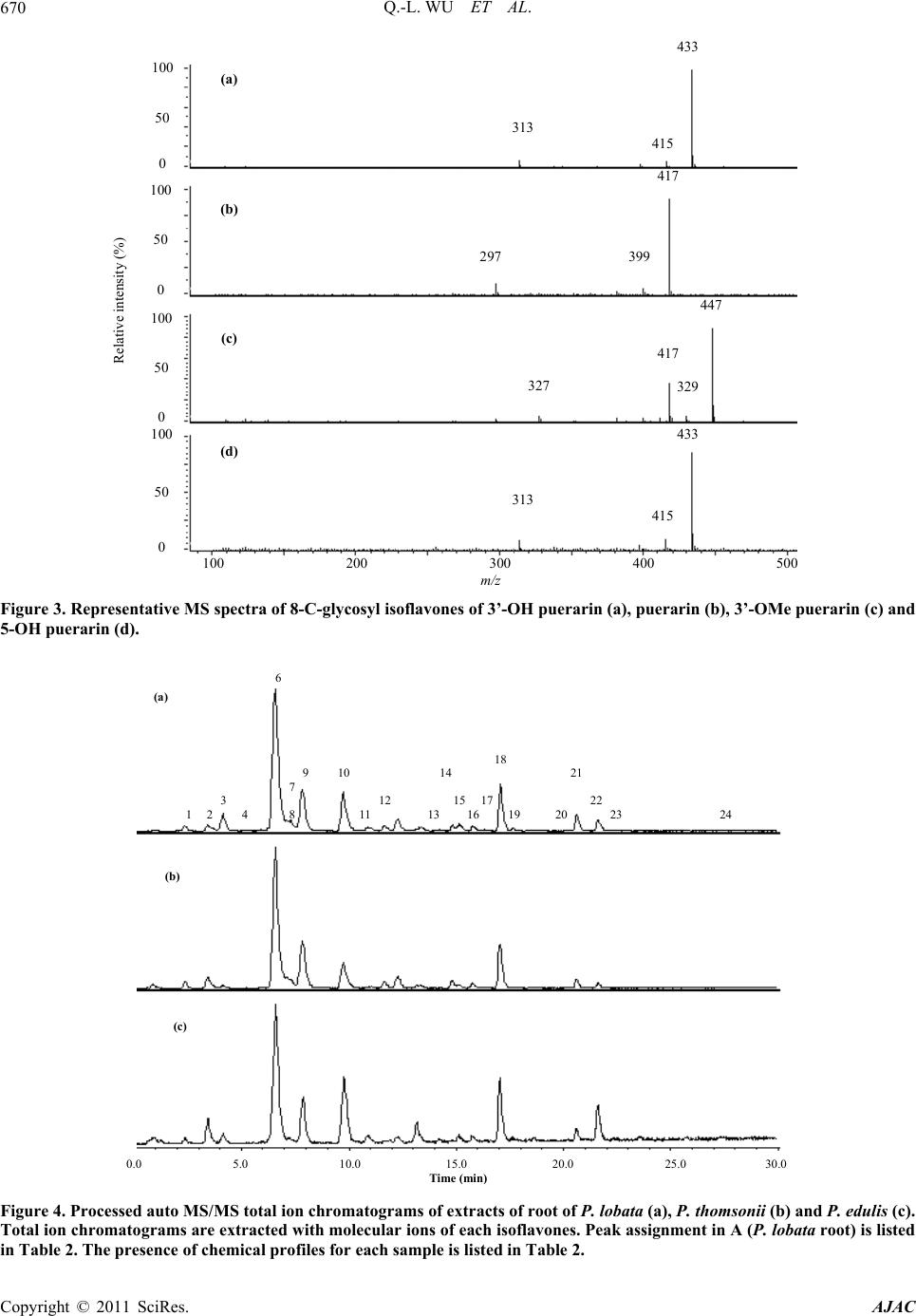 Q.-L. WU ET AL. 670 433 417 447 433 415 399 329 417 415 313 297 327 313 Relative intensity (%) 100 50 0 (a) (b) (c) (d) 100 200 300 400 500 z 100 50 0 100 50 0 100 50 0 Figure 3. Representative MS spectra of 8-C-glycosyl isoflavones of 3’-OH puerarin (a), puerarin (b), 3’-OMe puerarin (c) and 5-OH puerarin (d). 0.0 5.0 10.0 15.0 20.0 25.0 30.0 Time mi n 1 2 3 4 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 (a) (b) (c) Figure 4. Processed auto MS/MS total ion chromatograms of extracts of root of P. lobata (a), P. thomsonii (b) and P. edulis (c). Total ion chromatograms are extracted with molecular ions of each isoflavones. Peak assignment in A (P. lobata root) is listed n Table 2. The presence of chemical profiles for each sample is listed in Table 2. i Copyright © 2011 SciRes. AJAC 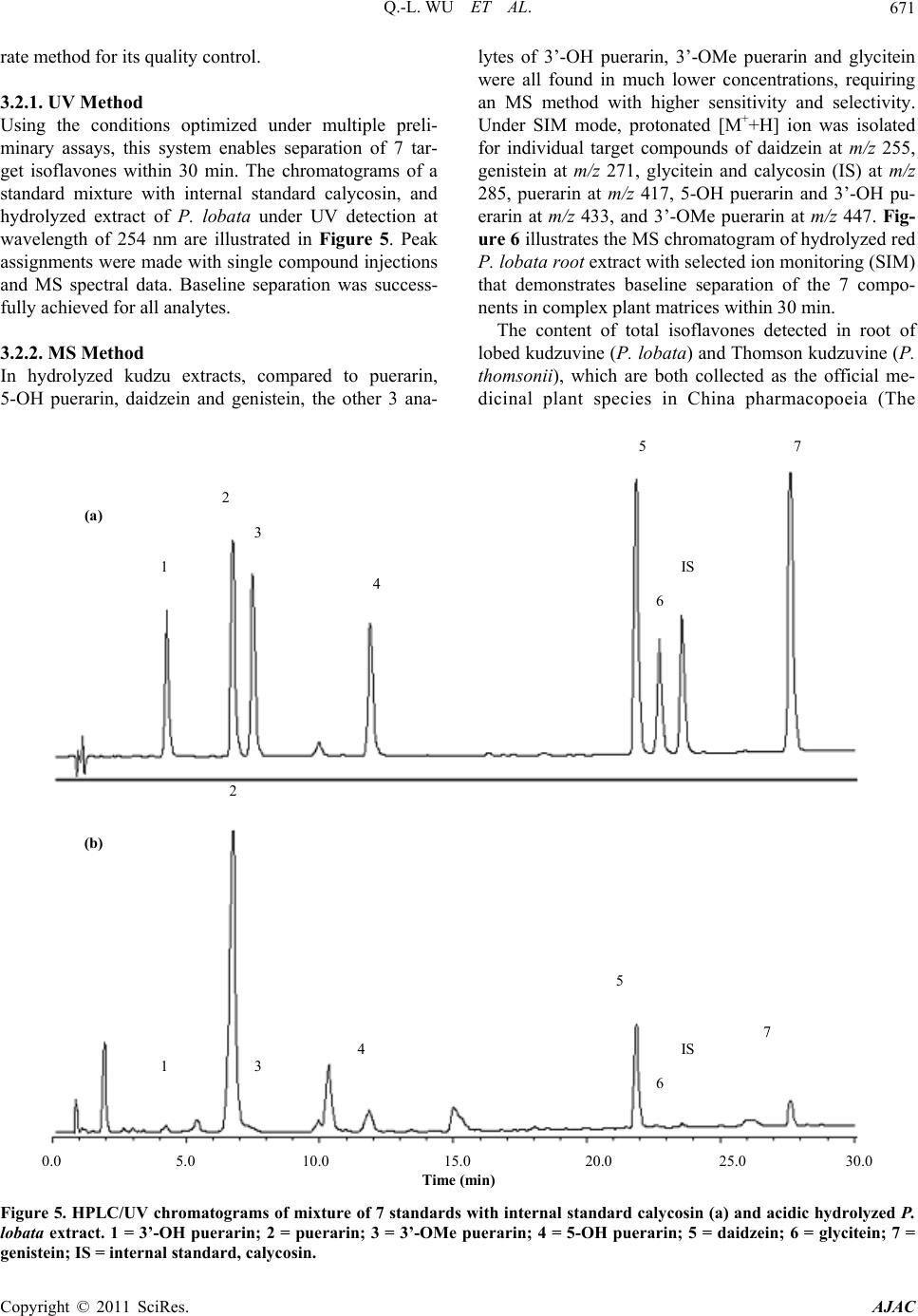 Q.-L. WU ET AL. Copyright © 2011 SciRes. AJAC 671 rate method for its quality control. 3.2.1. U V M ethod Using the conditions optimized under multiple preli- minary assays, this system enables separation of 7 tar- get isoflavones within 30 min. The chromatograms of a standard mixture with internal standard calycosin, and hydrolyzed extract of P. lobata under UV detection at wavelength of 254 nm are illustrated in Figure 5. Peak assignments were made with single compound injections and MS spectral data. Baseline separation was success- fully achieved for all analytes. 3.2.2. MS Me thod In hydrolyzed kudzu extracts, compared to puerarin, 5-OH puerarin, daidzein and genistein, the other 3 ana- lytes of 3’-OH puerarin, 3’-OMe puerarin and glycitein were all found in much lower concentrations, requiring an MS method with higher sensitivity and selectivity. Under SIM mode, protonated [M++H] ion was isolated for individual target compounds of daidzein at m/z 255, genistein at m/z 271, glycitein and calycosin (IS) at m/z 285, puerarin at m/z 417, 5-OH puerarin and 3’-OH pu- erarin at m/z 433, and 3’-OMe puerarin at m/z 447. Fig- ure 6 illustrates the MS chromatogram of hydrolyzed red P. lobata root extract with selected ion monitoring (SIM) that demonstrates baseline separation of the 7 compo- nents in complex plant matrices within 30 min. The content of total isoflavones detected in root of lobed kudzuvine (P. lobata) and Thomson kudzuvine (P. thomsonii), which are both collected as the official me- dicinal plant species in China pharmacopoeia (The (a) (b) 1 2 3 4 5 6 IS 7 1 2 3 4 5 6 IS 7 0.0 5.0 10.0 15.0 20.0 25.0 30.0 Time (min) Figure 5. HPLC/UV chromatograms of mixture of 7 standards with internal standard calycosin (a) and acidic hydrolyzed P. lobata extract. 1 = 3’-OH puerarin; 2 = puerarin; 3 = 3’-OMe puerarin; 4 = 5-OH puerarin; 5 = daidzein; 6 = glycitein; 7 = genistein; IS = internal standard, calycosin. 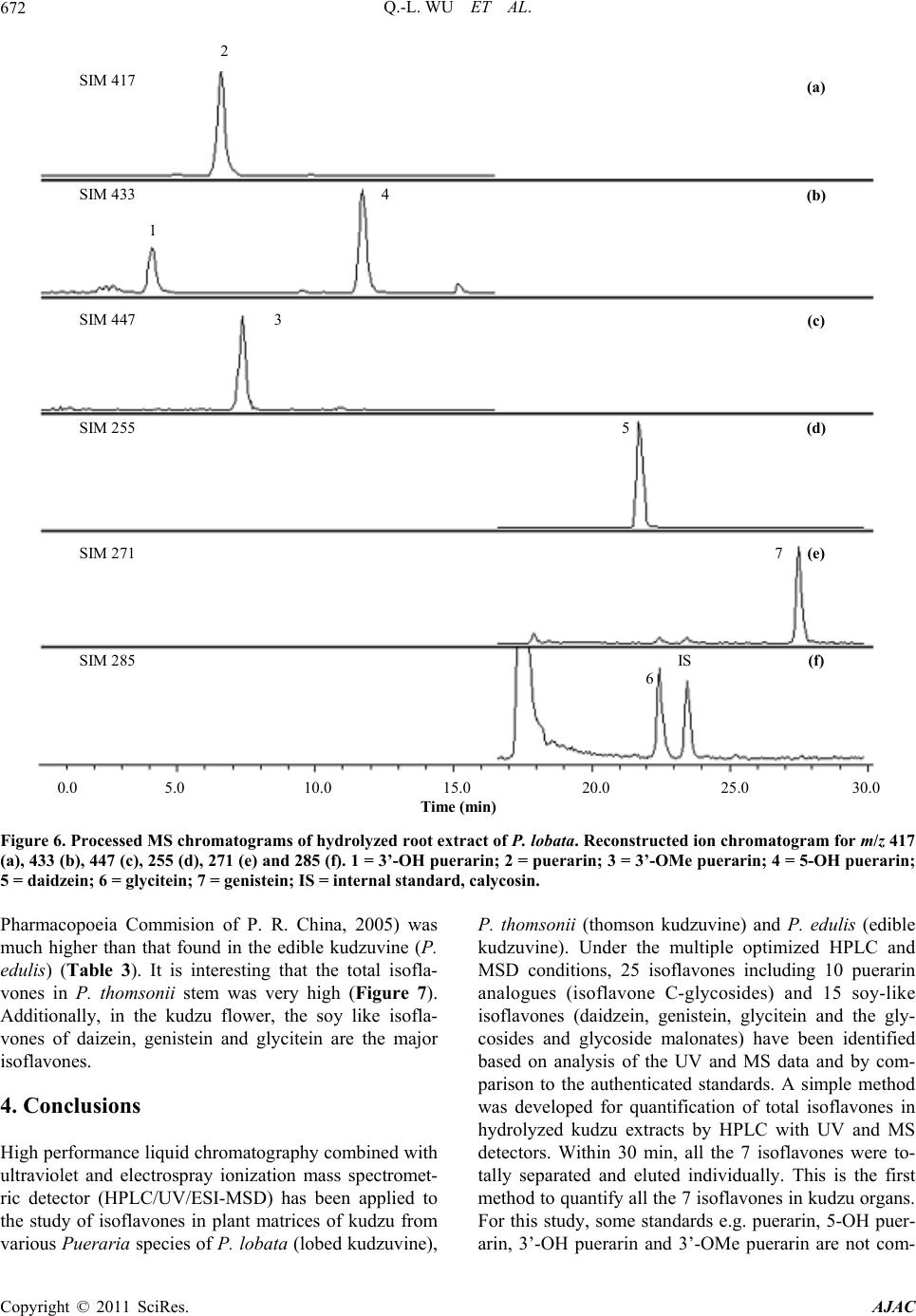 Q.-L. WU ET AL. 672 SIM 417 SIM 433 SIM 447 SIM 271 SIM 255 SIM 285 0.0 5.0 10.0 15.0 20.0 25.0 30.0 Time (min) (a) (b) (c) (e) (d) (f) 1 2 4 5 7 6 IS 3 Figure 6. Processed MS chromatograms of hydrolyzed root extract of P. lobata. Reconstructe d ion chromatogr am for m/z 417 (a), 433 (b), 447 (c), 255 (d), 271 (e) and 285 (f). 1 = 3’-OH puerarin; 2 = puerarin; 3 = 3’-OMe puerarin; 4 = 5-OH puerarin; 5 = daidzein; 6 = glycitein; 7 = genistein; IS = internal standard, calycosin. Pharmacopoeia Commision of P. R. China, 2005) was much higher than that found in the edible kudzuvine (P. edulis) (Table 3). It is interesting that the total isofla- vones in P. thomsonii stem was very high (Figure 7). Additionally, in the kudzu flower, the soy like isofla- vones of daizein, genistein and glycitein are the major isoflavones. 4. Conclusions High performance liquid chromatography combined with ultraviolet and electrospray ionization mass spectromet- ric detector (HPLC/UV/ESI-MSD) has been applied to the study of isoflavones in plant matrices of kudzu from various Pueraria species of P. lobata (lobed kudzuvine), P. thomsonii (thomson kudzuvine) and P. edulis (edible kudzuvine). Under the multiple optimized HPLC and MSD conditions, 25 isoflavones including 10 puerarin analogues (isoflavone C-glycosides) and 15 soy-like isoflavones (daidzein, genistein, glycitein and the gly- cosides and glycoside malonates) have been identified based on analysis of the UV and MS data and by com- parison to the authenticated standards. A simple method was developed for quantification of total isoflavones in hydrolyzed kudzu extracts by HPLC with UV and MS detectors. Within 30 min, all the 7 isoflavones were to- tally separated and eluted individually. This is the first method to quantify all the 7 isoflavones in kudzu organs. For this study, some standards e.g. puerarin, 5-OH puer- arin, 3’-OH puerarin and 3’-OMe puerarin are not com- Copyright © 2011 SciRes. AJAC 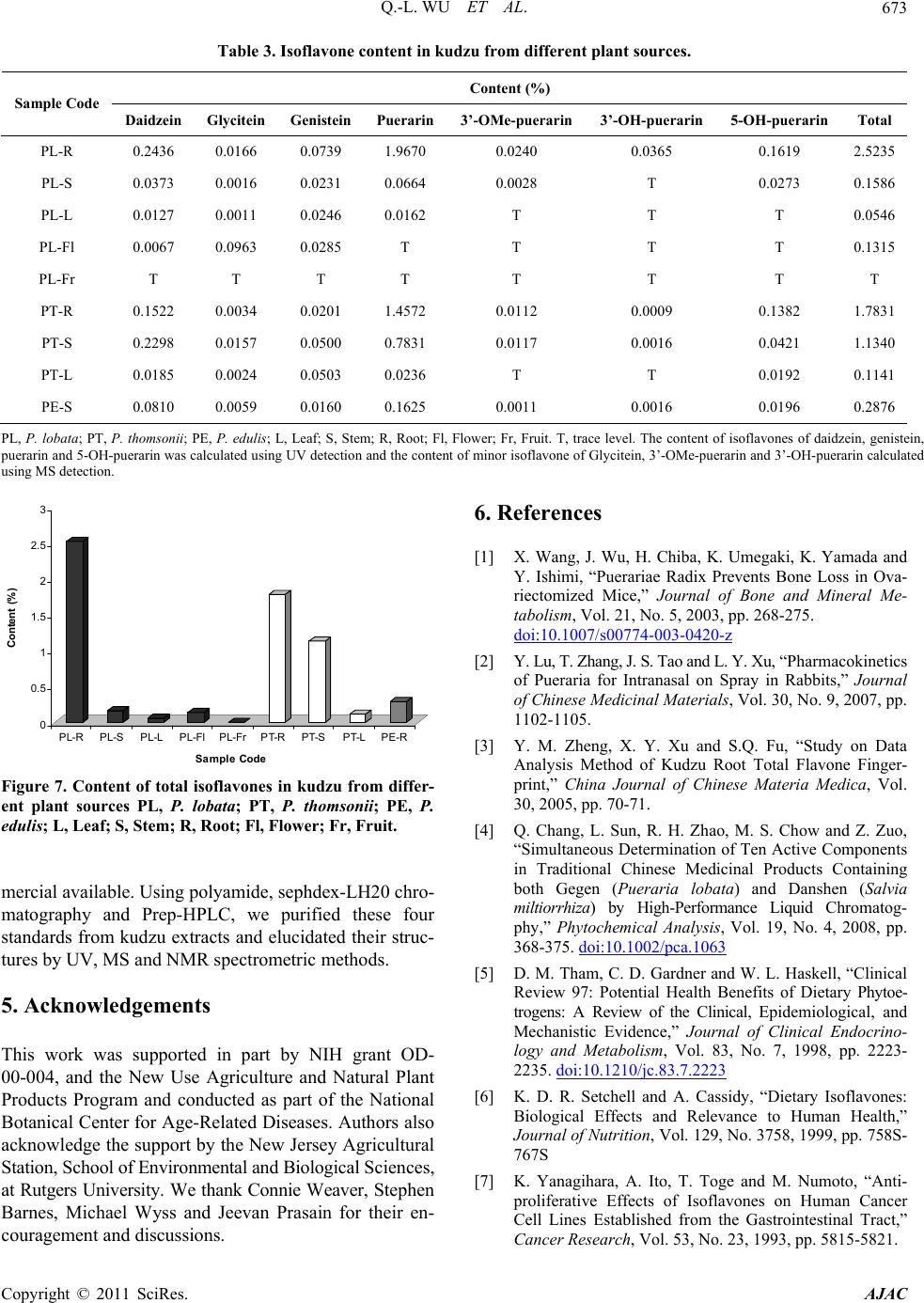 Q.-L. WU ET AL.673 Table 3. Isoflavone content in kudzu from different plant sources. Conten t (%) Sample Code Daidzein Glycitein Genistein Puerarin3’-OMe-puerarin3’-OH-puerarin 5-OH-puerarinTotal PL-R 0.2436 0.0166 0.0739 1.9670 0.0240 0.0365 0.1619 2.5235 PL-S 0.0373 0.0016 0.0231 0.0664 0.0028 T 0.0273 0.1586 PL-L 0.0127 0.0011 0.0246 0.0162 T T T 0.0546 PL-Fl 0.0067 0.0963 0.0285 T T T T 0.1315 PL-Fr T T T T T T T T PT-R 0.1522 0.0034 0.0201 1.4572 0.0112 0.0009 0.1382 1.7831 PT-S 0.2298 0.0157 0.0500 0.7831 0.0117 0.0016 0.0421 1.1340 PT-L 0.0185 0.0024 0.0503 0.0236 T T 0.0192 0.1141 PE-S 0.0810 0.0059 0.0160 0.1625 0.0011 0.0016 0.0196 0.2876 PL, P. lobata; PT, P. thomsonii; PE, P. edulis; L, Leaf; S, Stem; R, Root; Fl, Flower; Fr, Fruit. T, trace level. The content of isoflavones of daidzein, genistein, puerarin and 5-OH-puerarin was calculated using UV detection and the content of minor isoflavone of Glycitein, 3’-OMe-puerarin and 3’-OH-puerarin calculated using MS detection. 0 0.5 1 1.5 2 2.5 3 Conten t (%) PL-R PL-SPL-L PL-FlPL-FrPT-R PT-SPT-LPE-R Sample Code Figure 7. Content of total isoflavones in kudzu from differ- ent plant sources PL, P. lobata; PT, P. thomsonii; PE, P. edulis; L, Leaf; S, Stem; R, Root; Fl, Flower; Fr, Fruit. mercial available. Using polyamide, sephdex-LH20 chro- matography and Prep-HPLC, we purified these four standards from kudzu extracts and elucidated their struc- tures by UV, MS and NMR spectrometric methods. 5. Acknowledgements This work was supported in part by NIH grant OD- 00-004, and the New Use Agriculture and Natural Plant Products Program and conducted as part of the National Botanical Center for Age-Related Diseases. Authors also acknowledge the support by the New Jersey Agricultural Station, School of Environmental and Biological Sciences, at Rutgers University. We thank Connie Weaver, Stephen Barnes, Michael Wyss and Jeevan Prasain for their en- couragement and discussions. 6. References [1] X. Wang, J. Wu, H. Chiba, K. Umegaki, K. Yamada and Y. Ishimi, “Puerariae Radix Prevents Bone Loss in Ova- riectomized Mice,” Journal of Bone and Mineral Me- tabolism, Vol. 21, No. 5, 2003, pp. 268-275. doi:10.1007/s00774-003-0420-z [2] Y. Lu, T. Zhang, J. S. Tao and L. Y. Xu, “Pharmacokinetics of Pueraria for Intranasal on Spray in Rabbits,” Journal of Chinese Medicinal Materials, Vol. 30, No. 9, 2007, pp. 1102-1105. [3] Y. M. Zheng, X. Y. Xu and S.Q. Fu, “Study on Data Analysis Method of Kudzu Root Total Flavone Finger- print,” China Journal of Chinese Materia Medica, Vol. 30, 2005, pp. 70-71. [4] Q. Chang, L. Sun, R. H. Zhao, M. S. Chow and Z. Zuo, “Simultaneous Determination of Ten Active Components in Traditional Chinese Medicinal Products Containing both Gegen (Pueraria lobata) and Danshen (Salvia miltiorrhiza) by High-Performance Liquid Chromatog- phy,” Phytochemical Analysis, Vol. 19, No. 4, 2008, pp. 368-375. doi:10.1002/pca.1063 [5] D. M. Tham, C. D. Gardner and W. L. Haskell, “Clinical Review 97: Potential Health Benefits of Dietary Phytoe- trogens: A Review of the Clinical, Epidemiological, and Mechanistic Evidence,” Journal of Clinical Endocrino- logy and Metabolism, Vol. 83, No. 7, 1998, pp. 2223- 2235. doi:10.1210/jc.83.7.2223 [6] K. D. R. Setchell and A. Cassidy, “Dietary Isoflavones: Biological Effects and Relevance to Human Health,” Journal of Nutrition, Vol. 129, No. 3758, 1999, pp. 758S- 767S [7] K. Yanagihara, A. Ito, T. Toge and M. Numoto, “Anti- proliferative Effects of Isoflavones on Human Cancer Cell Lines Established from the Gastrointestinal Tract,” Cancer Research, Vol. 53, No. 23, 1993, pp. 5815-5821. Copyright © 2011 SciRes. AJAC  Q.-L. WU ET AL. 674 [8] K. K. Hintz and L. Ren, “Phytoestrogenic Isoflavones Daidzein and Genistein Reduce Glucose-Toxicity-Induced Cardiac Contractile Dysfunction in Ventricular Mycytes,” Endocrine Research, Vol. 30, 2004, pp. 215-223. doi:10.1081/ERC-120037730 [9] X. P. Song, P. P. Chen and X. S. Chai, “Effects of Puer- arin on Blood Pressure and Plasma Renin Activity in Spontaneously Hypertensive Rats,” Acta Pharmacologica Sinica, Vol. 9, No. 1, 1988, pp. 55-58. [10] L. P. Yan, S. W. Chan, A. C. Chan, S. L. Chen, X. J. Ma and H. X. Xu, “Puerarin Decreases Serum Total Choles- terol and Enhances Thoracic Aorta Endothelial Nitric Oxide Synthase Expression in Diet-Induced Hypercho- lesterolemic Rats,” Life Sciences, Vol. 79, 2006, pp. 324-330. doi:10.1016/j.lfs.2006.01.016 [11] W. M. Keung and B. L. Vallee, “Kudzu Root: An An- cient Chinese Source of Modern Antidipsotropic Agents,” Phytochemistry, Vol. 47, No. 4, 1998, pp. 499-506. doi:10.1016/S0031-9422(97)00723-1 [12] D. H. Overstreet, J. E. Kralic, A. L. Morrow, Z. Z. Ma, Y. W. Zhang and D. Y. Lee, “NPI-031G (Puerarin) Reduces Anxiogenic Effects of Alcohol Withdrawal or Benzod- azepine Inverse or 5-HT2C Agonists,” Pharmacology Biochemistry and Behavior, Vol. 75, No. 3, 2003, pp. 619-625. doi:10.1016/S0091-3057(03)00114-X [13] R. C. Lin and T. K. Li, “Effects of Isoflavones on Alcohol Pharmacokinetics and Alcohol-Drinking Behavior in Rats,” American Journal of Clinical Nutrition, Vol. 68, No. 6, 1998, pp. 1512S- 1515S. [14] P. Delmonte and J. I. Rader, “Analysis of Isoflavones in Foods and Dietary Supplements,” Journal of AOAC International, Vol. 89, No. 4, 2006, pp. 1138-1146. [15] C. B. Fang, X. C. Wan, H. R. Tan and C. J. Jiang, “Iden- tification of Isoflavonoids in Several Kudzu Samples by High-Performance Liquid Chromatography Coupled with Electrospray Ionization Tandem Mass Spectrometry,” Journal of Chromatographic Science, Vol. 44, No. 2, 2006, pp. 57-63. [16] P. Delmonte, J. Perry and J. I. Rader, “Determination of Isoflavones in Dietary Supplements Containing Soy, Red Clover and Kudzu: Extraction Followed by Basic or Acid Hydrolysis,” Journal of Chromatography A, Vol. 1107, No. 1-2, 2006, pp. 59-69. doi:10.1016/j.chroma.2005.11.060 [17] Y. Zhang, Q. Xu, X. Z. Zhang, J. P. Chen, X. M. Liang and A. Kettrup, “High-Performance Liquid Chromatog- raphy-Tandem Mass Spectrometry for Identification of Isoflavones and Description of the Biotransformation of Kudzu Root,” Analytical and Bioanalytical Chemistry, Vol. 383, No. 5, 2005, pp. 787-796. doi:10.1007/s00216-005-0068-8 [18] E. Benlhabib, J. I. Baker, D. E. Keyler and A. K. Singh, “Quantitative Analysis of Phytoestrogens in Kudzu-Root, Soy and Spiked Serum Samples by High-Pressure Liquid Chromatography,” Biomedical Chromatography, Vol. 18, No. 6, 2004, pp. 367-380. doi:10.1002/bmc.326 [19] J. K. Prasain, K. Jones, M. Kirk, L. Wilson, M. Smith- Johnson, C. Weaver and S. Barnes, “Profiling and Quan- tification of Isoflavonoids in Kudzu Dietary Supplements by High-Performance Liquid Chromatography and Elec- trospray Ionization Tandem Mass Spectrometry,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 15, 2003, pp. 4213-4218. doi:10.1021/jf030174a [20] Y. Niiho, Y. Nakajima, T. Yamazaki, M. Okamoto, R. Tsuchihashi, M. Kodera, J. Kinjo and T. Nohara, “Simul- taneous Analysis of Isoflavones and Saponins in Pueraria Flowers Using HPLC Coupled to an Evaporative Light Scattering Detector and Isolation of a New Isoflavone Diglucoside,” Journal of Natural Medicines, Vol. 64, No. 3, 2010, pp. 313-320. doi:10.1007/s11418-010-0411-z [21] J. G. Mun, M. D. Grannan, P. J. Lachcik, R. B. Rogers, G. G. Yousef, M. H. Grace, E. M. Janle, Q. L. Wu, J. E. Simon, C. W. Weaver and M. A. Lila, “Tracking Depos- tion of a 14C-Radiolabeled Kudzu Hairy Root-Derived Isoflavone-Rich Fraction into Bone,” Experimental Biol- ogy and Medicine (Maywood), Vol. 235, No. 10, 2010, pp. 1224-1235. doi:10.1258/ebm.2010.010134 [22] Q. L. Wu, M. F. Wang and J. E. Simon, “Determination of Isoflavones in Red Clover and Related Species by High-performance Liquid Chromatography Combined with Ultraviolet and Mass Spectrometric Detection,” Journal of Chromatography A, Vol. 1016, No. 2, 2003, pp. 195-209. doi:10.1016/j.chroma.2003.08.001 [23] Q. L. Wu, M. F. Wang, J. S. William and J. E. Simon, “LC/UV/ESI-MS Analysis of Isoflavones in Edamame and Tofu Soybeans,” Journal of Agricultural and Food Chemistry, Vol. 52, No. 10, 2004, pp. 2763-2769. doi:10.1021/jf035053p Copyright © 2011 SciRes. AJAC
|