Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2011, 4, 631-641 JBiSE doi:10.4136/jbise.2011.49079 Published Online September 2011 (http://www.SciRP.org/journal/jbise/). Published Online September 2011 in SciRes. http://www.scirp.org/journal/JBiSE Mathematical model for steady state current at PPO-modified micro-cylinder biosensors Kodhandapani Venu gopal1, Alagu Eswari2, Lakshmanan Rajendran2* 1Department of Mathematics, J.J College of Engineering and Technology, Tiruchirappali, India; 2Department of Mathematics, The Madura College, Madurai, India. Email: *raj_sms@rediffmail.com Received 1 July 2011; revised 18 July 2011; accepted 3 August 2011. ABSTRACT A Mathemataical model for a modified micro- cylinder electrode in which polyphenol oxidase ( PPO) occurs for all values of the concentration of catechol and o-quinone is analysed. This model is based on system of reaction-diffusion equations containing a non-linear term related to Michaelis Menten kinetics of the enzymatic reaction. Here a new analytical technique Homotopy Perturbation Method is used to solve the system of non-linear differential equations. that describe the diffusion coupled with a Michaelis- Menten kinetics law. Here we report an analytical expressions pretaining to the concentration of catechol and o-quinone and corresponding current in terms of dimensionless reaction-diffusion parame- ters in closed form. An excellent agreement with available limiting case is noticed. Keywords: Non-Linear Reaction/Diffusion Equation; Biosensors; Polymer-Modified Micro-Cylinder Electrode; Polyphenol Oxidase; Homotopy Perturbation Method 1. INTRODUCTION Microelectrodes are increasingly being used in biosen- sors [1-3]. This is due to factors such as fast response times, high signal: noise ratios and the ability to operate in low conductivity media, sub-micro volume and in vivo [4]. The most commonly used microelectrode in bio-sensor is microcylinder such as carbon fibres. This is because they are cheap, readily available, their form is suited to implantation [5] and because much is known about their surface characteristics [6]. Immobilization of enzymes is used in biosensors to detect the concentration of a specific analyte as a result of the biological recognition between the analyte and the immobilized enzyme. Enzymes have been immobilized at carbon fibres by many methods. Among all the meth- ods, layer-by-layer (LbL) self assembly process is a simple technique which may be applied to a wide range of enzymes and that it is one of the few immobilization procedures which allows control over the amount and spatial distribution of the enzyme [7]. This property is important both for constructing and modeling studies of biosensors. The layer-by-layer process was first intro- duced by Decher and Hong [7]. This method has been applied to planar electrodes of Au [8,9], carbon elec- trodes [10] and polystyrene latex [11-15]. To analyse the performance of biosensors of any kind, it would be useful to have a mathematical model of the electrode response. Theoretical models of enzyme elec- trodes give information about the mechanism and kinet- ics operating in the biosensor. Unlike experimental in- vestigations of biosensors, where changing one parame- ter inevitably alters others, the influence of individual variables can be assessed in an idealized way. Thus, the information gained from modeling can be useful in sen- sor design, optimization and prediction of the electrodes response. Recently Rijiravanich et al. [16] obtained the steady state concentration profile of o-quinone and dimen- sionless sensor response j for the limiting cases of low substrate concentrations. To the best of our knowledge, no rigorous analytical solutions for the steady state con- centrations for micro-cylinder biosensors for all values of the parameters have been published. In this commu- nication, we have derived the new and simple analytical solutions of the concentration and the current for all values of parameters using the Homotopy Perturbation Method 2. MATHEMATICAL FORMULATION OF THE PROBLEM AND ANALYSIS The system presented here is a cylindrical electrode which is uniformly coated by an enzyme immobilized in non-conducting material which is porous to substrate.  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 632 The electrode is used in a stirred solution containing an excess of supporting electrolyte. The enzyme and elec- trode reaction are [16]: 22 O2catechol2quinone2H Oo (1) + quinone 2H2ecatecholo (2) Hence the catechol/quinone conversion forms an am- plification cycle within the enzyme film. While it is pos- sible in principle to solve for either phenol or catechol as substrate, solving for catechol is simpler, since it in- volves only one enzymic conversion. The actual mecha- nism of that conversion is complex, and involves three different states, oxy, met, deoxy [17] i.e. (where Ca is catechol, Q is quinone). 1 22 22 deoxy oxy CuICuI+O2HOHO CuIIOO CuIIHO k (3) 2 22 2 oxy met HOCuIIOO CuIIHOCuIICaCuII2HO 2H k (4) 3 met deoxy Cu IICaCuIICu ICu I+ kQ (5) It is assumed that the enzyme concentration is uniform and that the enzyme reaction follows Michaelis-Menten kinetics, in which case the reaction in the film is [18] 1 2 112 [] cat kk k SE ESPE (6) where 2 1cat O kkc and 2 12 3 23 () O M kk kc Kkk (7) are the rate constant and Michaelis-Menten constant. The model of a cylindrical electrode modified with both an enzyme and conducting sites/particles (circles) is shown in Figure 1. The mass balance for catechol C c can be written in cylindrical coordinates as follows: d d0 dd CCcatEC CM Dckcc r rrrc K (8) where C c is the concentration profile of catechol, E c is the concentration profile of enzyme, C D and Q D are its diffusion coefficients, and M K is the Michaelis constant and Q c is the concentration profile of quinone. Then the equation of continuum for quinone is generally expressed in the steady-state by [16] d d0 dd QQ cat E C CM Dc kcc r rrrc K (9) At the electrode surface (0 r) and at the film surface (1 r) the boundary conditions are given by [16] * 0 * 1 : , 0 : , 0 CC Q CC Q rrc cc rrccc (10) where * C c is the bulk concentration of catechol scaled by the partition coefficient of the enzyme film. Adding the Eqs.8 and 9 and integrating with boundary condition (10), yields ** () () 1 QQ C CCC Dcr cr cDc (11) The steady-state current can be given as [16]: 0 0 2πdd QQ rr ILr Dcr nF (12) We introduce the following set of dimensionless vari- ables: * C C c Cc , * Q C c Qc , 0 r Rr , * C M c K , 2 0 cat E E CM kcr DK , 2 0cat E S QM kcr DK , Q E CS D D (13) where C and Q are the dimensionless concentration of the catechol and o-quinone. R is the dimensionless distance parameter. , E S and are the dimen- sionless reaction-diffusion parameters and saturation parameter [16]. 2 2 d1d 0 d1 d EC CC RR C R (14) 2 2 d1d 0 d1 d SC QQ RR C R (15) The boundary conditions are represented as follows: 1 , 0 when 1CQ R (16) 10 1 , 0 when CQ Rrr (17) The dimensionless current at the micro-cylinder elec- trode can be given as follows: * 1 2πdd QC R InFLDcQ R (18) 3. ANALYTICAL SOLUTIONS OF THE CONCENTRA TIONS AND THE CURRENT USING THE HOMOTOPY PERTURBATION METHOD Nonlinear phenomena play a crucial role in applied  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 633 Figure 1. Illustration of the model of a cylindrical electrode modified with both an enzyme and conducting sites/particles (circles). mathematics and chemistry. Construction of particular exact solutions for these equations remains an important problem. Finding exact solutions that have a physical, chemical or biological interpretation is of fundamental importance. This model is based on steady-state system of diffusion equations containing a non-linear reaction term related to Michaelis-Menten kinetics of the enzy- matic reactions. It is not possible to solve these equa- tions using standard analytical technique. In the past, many authors mainly had paid attention to study solution of nonlinear equations by using various methods, such as Backlund transformation [19], Darboux transformation [20], Inverse scattering method [21], Bilinear method [22], The tanh method [23], Variational iteration method [24] and Homotopy Perturbation Method [25-28] etc. The Homotopy Perturbation Method [25-28] has been extensively worked out over a number of years by nu- merous authors. The Homotopy Perturbation Method was first proposed by He [24-26] and was successfully applied to autonomous ordinary differential equations to nonlinear polycrystalline solids and other fields. Recently Meena and Rajendran [29], Anitha et al. [30] and Manimozhi et al. [31] implemented Homotopy per- turbation method to give approximate and analytical solutions of nonlinear reaction-diffusion equations con- taining a nonlinear term related to Michaelis-Menten kinetic of the enzymatic reaction. Eswari et al. in series [32,33] solved the coupled non linear diffusion equations analytically for the microdisk and micro-cylinder en- zyme electrode when a product from an immobilized enzyme reacts with the electrode. Using Homotopy Per- turbation Method (see Appendix B), we can obtain the following solutions to the Eqs.14 to 15. 2 1010 1 () 12(1 ) EE E RrrRrr CR (19) 2 10 10 1 () 2(1 ) SS S RrrRrr QR (20) The Eqs.19-20 satisfies the boundary conditions (16) to (17). These equations represent the new and simple analytical expression of the concentration of catechol and o-quinone for all possible values of the parame- ters E , S , and 10 rr. The Eqs.19 and 20 also satisfy the relation () () ()1 ES CR QR . From Eqs.19 and 20, we can obtain the dimensionless current, which is as follows: 10 *12 22(1 ) SS QC rr InFLDc (21) Eq. (21) represents the new and closed form of an analytical expression for the current for all possible val- ues of parameters. 3.1. Limiting Cases for Unsaturated (First Order) Catalytic Kinetics In this case, the catechol concentration C c is less than Michaelis constant M K . Now the Eqs.8 and 9 reduce to the following forms: d d0 dd CCcatEC M Dckcc r rr rK (22) d d0 dd QQ cat E C M Dc kcc r rr rK (23) By solving the Eq.22 using the boundary condition (Eq.10), the concentration of catechol C c can be ob- tained in the form of modified Bessel functions of zeroth order 0() I r and 0() K r . *0000100100 0001 0100 () [()()]() [()()] () ()() ()() CC IrKrKr KrIrIr crc KrIrKrIr (24)  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 634 where 2 cat ECM kc DK (25) Inserting Eqs.24 into Eqs.11, we can obtain the con- centration Q c 0000100100 * 0001 0100 () () [()()]() [()()] 1()()()() QQ CC Dc rIrKrKr KrIrIr KrIrKrIr Dc (26) The sensor response j in terms of modified Bessel function of zeroth order can be obtained as follows: * 0 10010010 0001 0001 0100 2π()()()() ()() ()() ()() CC I jnFLD c r K rI r I rIrK rK r KrIrKrIr (27) 4. COMPARISON WITH LIMITING CASE WORK OF RIJIRAVANICH ET AL. [16] Recently, they [16] have derived the analytical expres- sion of the steady- state concentration Q c (Eq.28 and sensor response j (Eqs.28 and 29) in integral form for the limiting case CM cK . 11 00 00 0 11 11 * 10 () ln( ) ()d ()d()d ()d ln( ) rr rr QQ rr rr CC Dc rrr g fI rrK rrfIrrK rr rr Dc (28) 11 00 010 1011 * 10 1 2π ()()()d()d ln( ) rr rr CC I jgrfIrKrfIrrKrr rr nFLD c (29) where 0 00000010100 1 [()()], [()()][()()]. g fIrKrfKrKr IrIr Rijiravanich et al. [16] obtained the empirical expres- sion of the current 1 2πtanh[(/ 2)(1)] qp jx x (30) where p and q are empirical constants and 110 .rr The value of p and q are given for various values of 0 () x r in the Tables 1-3. This empirical expression is compared our simple closed analytical expression Eq.2 7, in Tables 2-3. The average relative difference between our Eq.27 and the empirical expression Eq.30 is 0.71% when 11.5 and 0.59% when 15 . 6. DISCUSSION Figures 2 and 3 shows the dimensionless concentration profile of catechol ()CR using Eq.19 for all Table 1. Values of p and qwhich fit Eq.30 to Eq.29 with < 5% error [16]. x p q 9.0-7.0 1.00 1.01 6.0-4.0 1.03 1.05 3.0 1.04 1.10 2.0 1.02 1.14a/1.25b a Valid for 12.0 ; b Valid for 12. 0 various values of the parameters 10 , , and SE rr . Thus it is concluded that there is a simultaneous increase in the values of the concentration of catechol as well as in saturated parameter for small values of E . Also the value of catechol concentration C is approximately equal to 1 when 1 andR 10 Rrrfor all values of E and . Figures 4 and 5 show the concentration profile of o quinone ()QR in R space for various values of and S calculated using Eq.20. The plot was con- structed for 101.5 and 5rr . From these figures, it is confirmed that the value of the concentration of o- quinone increases when 0.1 S for small values of . From the Figures 2-5, we can observed that the di- mensionless concentration of catechol should vary be- tween 0 and 1. Because catechol is converted to o quinone, the o- quinone concentration should be the in- verse of catechol. The substrate catechol C is mini- mum and product o-quinone Q is maximum when 10 0.5 2Rrr for all values of and S . The minimum value of concentration profile of catechol is 2 11 min 88 2 8(1 ) EE E C (31) 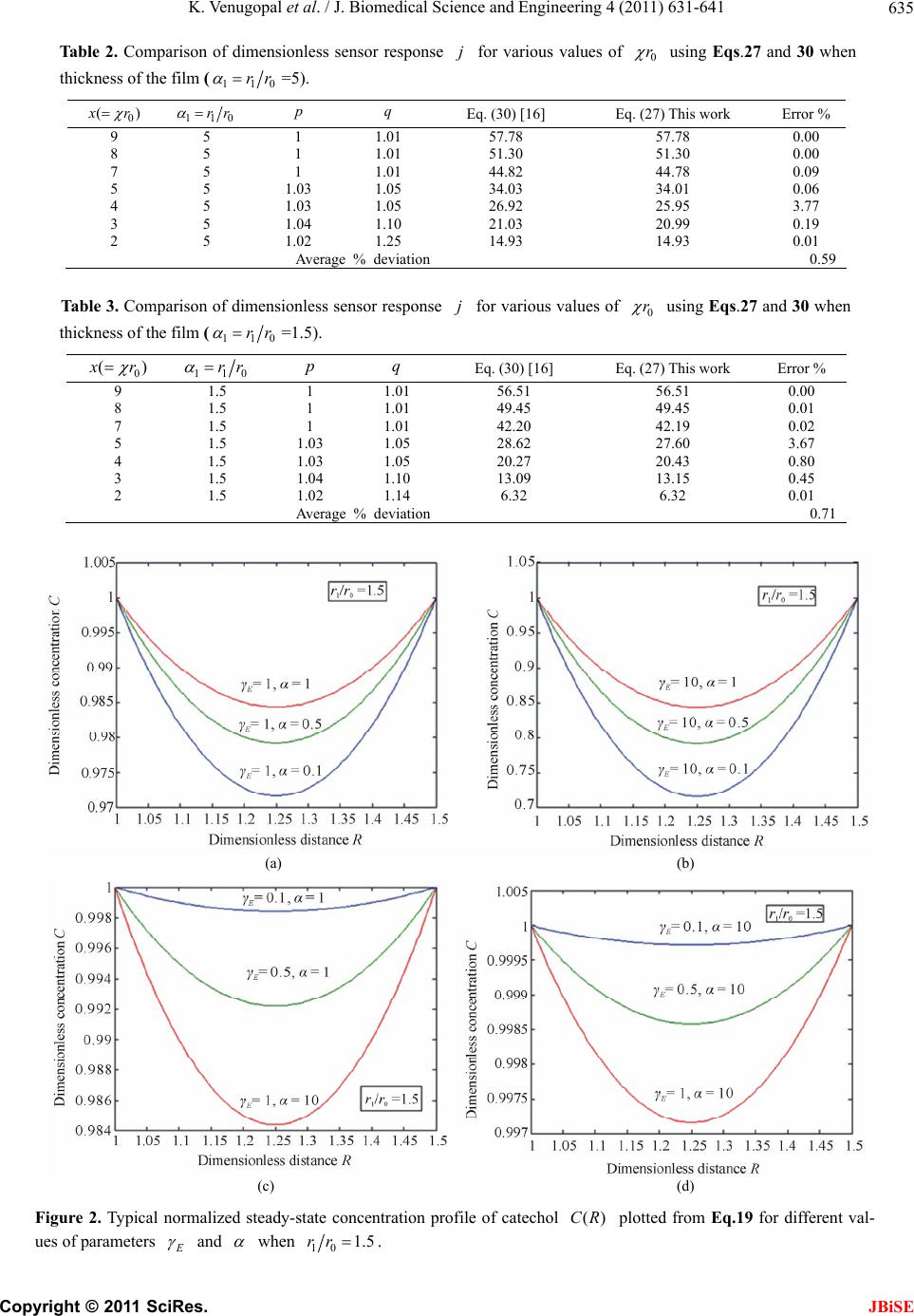 K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 635 Table 2. Comparison of dimensionless sensor response j for various values of 0 r using Eqs.27 and 30 when thickness of the film (110 rr =5). 0 () x r 110 rr p q Eq. (30) [16] Eq. (27) This work Error % 9 5 1 1.01 57.78 57.78 0.00 8 5 1 1.01 51.30 51.30 0.00 7 5 1 1.01 44.82 44.78 0.09 5 5 1.03 1.05 34.03 34.01 0.06 4 5 1.03 1.05 26.92 25.95 3.77 3 5 1.04 1.10 21.03 20.99 0.19 2 5 1.02 1.25 14.93 14.93 0.01 Average % deviation 0.59 Table 3. Comparison of dimensionless sensor response j for various values of 0 r using Eqs.27 and 30 when thickness of the film (110 rr =1.5). 0 () x r 110 rr p q Eq. (30) [16] Eq. (27) This work Error % 9 1.5 1 1.01 56.51 56.51 0.00 8 1.5 1 1.01 49.45 49.45 0.01 7 1.5 1 1.01 42.20 42.19 0.02 5 1.5 1.03 1.05 28.62 27.60 3.67 4 1.5 1.03 1.05 20.27 20.43 0.80 3 1.5 1.04 1.10 13.09 13.15 0.45 2 1.5 1.02 1.14 6.32 6.32 0.01 Average % deviation 0.71 (a) (b) (c) (d) Figure 2. Typical normalized steady-state concentration profile of catechol ()CR plotted from Eq.19 for different val- ues of parameters E and when 10 1.5rr. 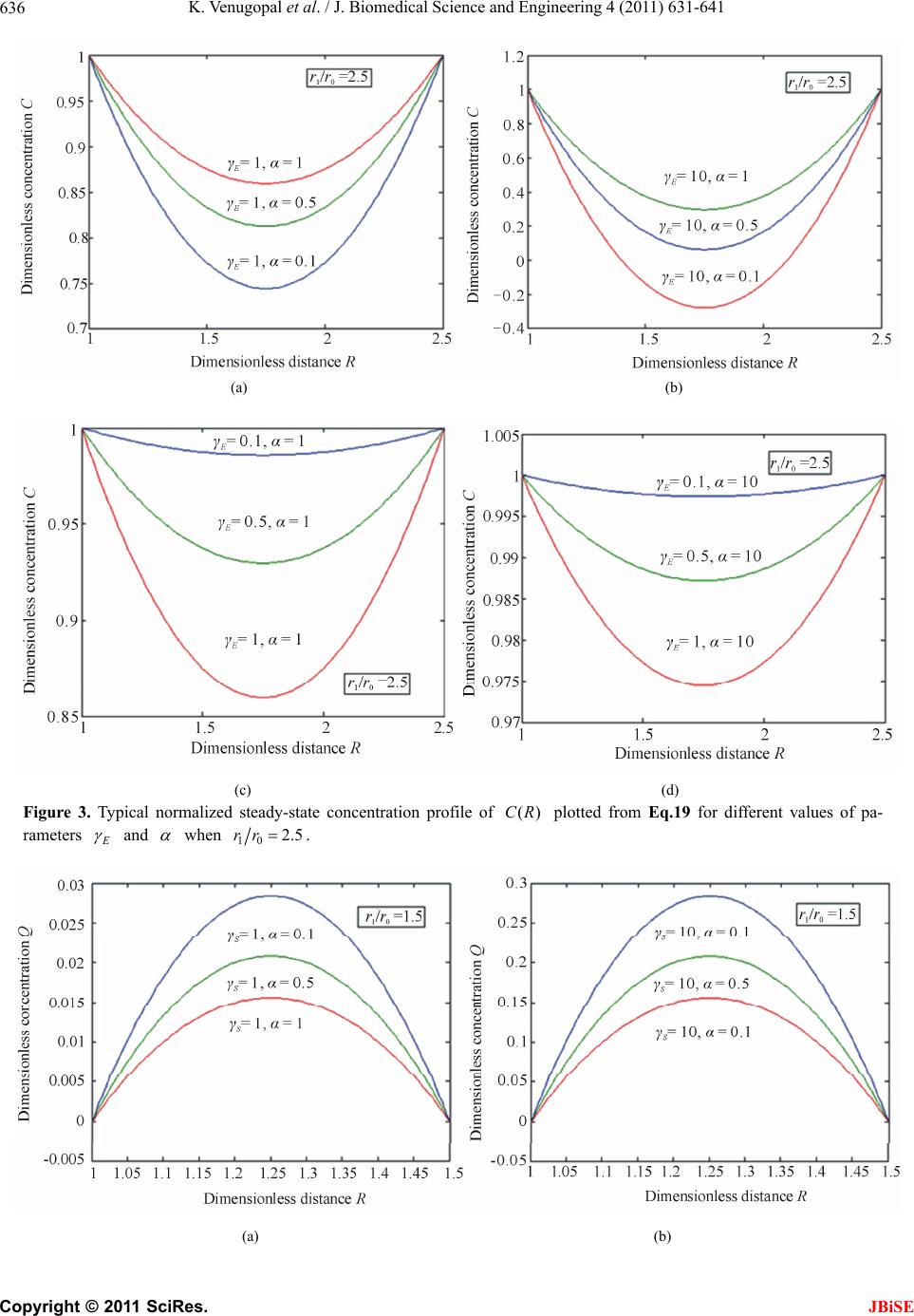 K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 636 (a) (b) (c) (d) Figure 3. Typical normalized steady-state concentration profile of ()CR plotted from Eq.19 for different values of pa- rameters E and when 102.5rr. (a) (b) 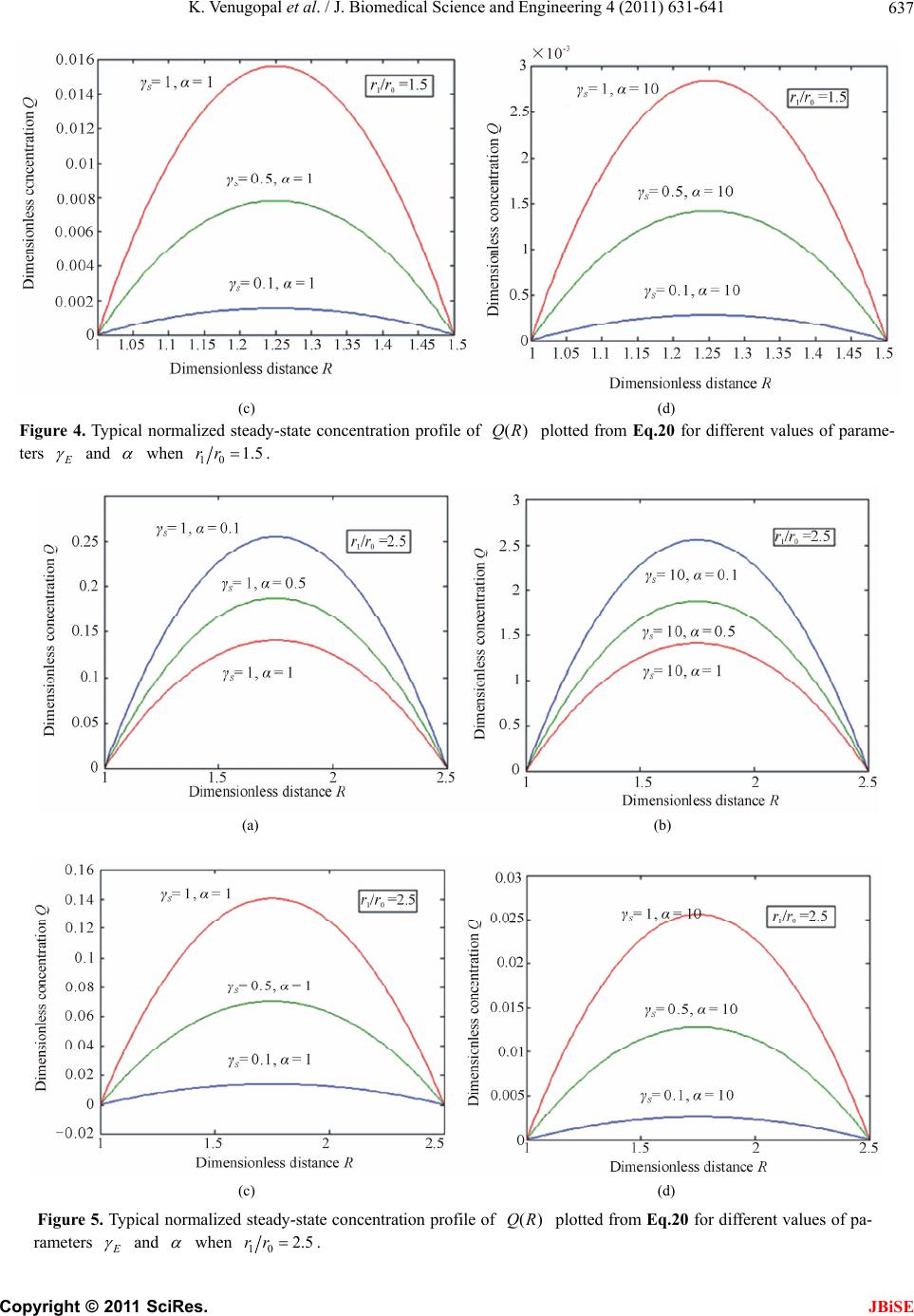 K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 637 (c) (d) Figure 4. Typical normalized steady-state concentration profile of ()QR plotted from Eq.20 for different values of parame- ters E and when 10 1.5rr . (a) (b) (c) (d) Figure 5. Typical normalized steady-state concentration profile of ()QR plotted from Eq.20 for different values of pa- rameters E and when 102.5rr.  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 638 (a) (b) Figure 6. Plot of dimensionless current versus 10 .rr Current is calculated in the Eq.21. and the maximum value of concentration profile of quinone is 2 11 max (1 2) 8(1 ) S Q (32) where 10 1 rr . The dimensionless current versus 10 rr using Eq.21 is plotted in Figure 6. The value of current increases when thickness of the film 10 rr and dimensionless reaction-diffusion parameter S is increases or decreases. 7. CONCLUSIONS A non-linear time independent ordinary differential equation has been formulated and solved analytically. Analytical expression for the concentration of catechol and o-quinone and steady state current are derived by contains significant non-linear contributions using the Homotopy Perturbation Method. The primary result of this work is simple approximate calculation of concen- tration of catechol, o-quinone and current for all values of E , S , and 10 rr and 0 r . Formerly in polyphenol oxidase micro-cylinder biosensor models are [16] have only considered the first order kinetics of the enzyme and therefore could only be applied to the sen- sor’s linear range. However, in this paper, calibration curves of many of the catechol/phenol biosensors con- tain most important non-linear contributions are reported. Also, the length of the linear range is an important ana- lytical parameter. In developing a sensor, experimental scientists would like this range to cover all concentra- tions expected in actual samples, as this makes calibra- tion of the sensor in the field much easier. In Tables 2-3, our analytical results are compared with limiting case of first order catalytic kinetics [16] results, which yield a good agreement with the previous limiting case results. 8. ACKNOWLEDGEMENTS This work was supported by the Council of Scientific and Industrial Research (CSIR No.: 01(2442)/10/EMR-II), Government of India. The authors also thank Mr. M. S. Meenakshisundaram, Secretary, The Madura College Board, Dr. R. Murali, The Principal,nd S.Thia- gara- jan, Head of the Department, Madura College, Madurai, India for their constant encouragement. The authors K. Venugopal and A. Eswari are very thankful to the Manonmaniam Sundaranar University, Tirunelveli for allowing to do the research work. REFERENCES [1] Revzin, A.F., Sirkar, K., Simonian, A. and Pishko, M.V. (2002) Glucose, lactate and pyruvate biosensor arrays based on redox polymer/oxidoreductase nanocomposite thin films deposited on photolithographically patterned gold electrodes. Sensor and Actuators B, 81, 359. doi:10.1016/S0925-4005(01)00982-0 [2] Shi, G., Liu, M., Zhu, M., Zhou, T., Chen, J., Jin, L. and Jin, J.-Y. (2002) The study of nafion/xanthine oxidase/au colloid chemically modified biosensor and its application in the determination of hypoxanthine in myocardial cells in vivo. Analyst, 127, 396. doi:10.1039/b108462n [3] Gue, A.-M., Tap, H., Gros, P. and Maury, F. (2002) A miniaturized silicon based enzymatic biosensor: Towards a generic structure and technology for multi-analytes as- says. Sensor and Actuators B, 82, 227. doi:10.1016/S0925-4005(01)01009-7 [4] Edmonds, T.E. (1985) Electroanalytical application of carbon fiber electrodes, Analytica Chimica Acta, 175, 1. doi:10.1016/S0003-2670(00)82713-0 [5] Gonon, F., Suaud-Changny, M.F. and Buda, M. (1992) Proceedings of satellite symposium on neu-roscience and technology, Lyon, 215. [6] Donnet, J.B. and Basal, R.C. (1984) International Fiber Science and Technology, Carbon Fibers, Dekker, New York , 3.  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 639 [7] Decher, G. and Hong, J.D. (1991) Buildup of ultrathin multilayer films by a self-assembly process: II. Consecu- tive adsorption of anionic and cationic bipolar amphi- philes and polyelectrolytes on charged surfaces. Berichte der Bunsengesellschaft Für Physikalische Chemie, 95, 1430. [8] Hodak, J., Etchenique, R., Calvo, E.J., Singhal, K. and Bartlett, P.N. (1997) Layer by layer self assembly of glucose oxidase with a poly(allylmanine)-ferrocene re- dox mediator. Langmuir, 13, 2708. doi:10.1021/la962014h [9] Forzani, E.S., Solis, V.M. and Calvo, E.S. (2000) Elec- trochemical behavior of polyphenol oxidase immobilized in self-assembled structures layer by layer with cationic polyallylamine. Analytical Chemistry, 72, 5300. doi:10.1021/ac0003798 [10] Coche-Guerante, L., Labbe, P. and Mengeand, V. (2001) Analytical Chemistry, 73, 3206. doi:10.1021/ac001534l [11] Lvov, Y. and Caruso, F. (2001) Biocolloids with ordered urease multilayer shells as enzymatic reactors. Analytical Chemistry, 73, 4212. doi:10.1021/ac010118d [12] Fang, M., Grant, P.S., McShane, M.J., Sukhorukov, G. B., Golub, V.O. and Lvov, Y. (2002) Magnetic bio/nan- oreactor with multilayer shells of glucose oxidase and inorganic nanoparticles. Langmuir, 18, 6338. doi:10.1021/la025731m [13] Caruso, F. and Schuler, C. (2000) Enzyme multilayers on colloid particles: Assembly, stability, and enzymatic ac- tivity. Langmuir, 16, 9595. doi:10.1021/la000942h [14] Caruso, F., Fiedler, H. and Haage, K. (2000) Assembly of β-glucosidase multilayers on spherical colloidal particles and their use as active catalyst. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 169, 287. doi:10.1016/S0927-7757(00)00443-X [15] Sun, H. and Hu, N. (2004) 1. Voltammetric studies of hemoglobin-coated polystyrene latex bead films on py- rolytic graphite electrodes. Biophysical Chemistry, 110, 297. sdoi:10.1016/j.bpc.2004.03.005 [16] Rijiravanich, P., Aoki, K. and Chen, J., Surareungchai, W. and Somasundrum, M. (2006) Micro-cylinder biosensors for phenol and catechol based on layer-by-layer immobi- lization of tyrosinase on latex particles: Theory and ex- periment. Journal of Electroanalytical Chemistry, 589, 249-258. doi:10.1016/j.jelechem.2006.02.019 [17] Wilcox, D.E., Porras, A.G., Hwang, Y.T., Lerch, K., Winkler, M.E. and Solomon, E.I. (1985) Substrate Ana- logue Binding to the Coupled Binuclear Copper Active Site in Tyrosinase. Journal of American Chemical Soci- ety, 107, 4015. doi:10.1021/ja00299a043 [18] Carbanes, J., Garcia-Canovas, F., Lozano, J.A. and Gar- cia-Carmona. F (1987) A kinetic study of the melaniza- tion pathway between L-tyrosine and dopachrome. Bio- chimica et Biophysica Acta-General Subjects, 923, 187. [19] Coely, A., et al. (2001) Backlund and darboux transfor- mation. American Mathematical Society, Providence, RI. [20] Wadati, M., Sanuki, H. and Konno, K. (1975) Relation- ships among inverse method, bäcklund transformation and an infinite number of conservation laws. Progress of Theoretical Physics, 53, 419. doi:10.1143/PTP.53.419 [21] Gardener, C.S., Green, J.M., Kruskal, M.D. and Miura, R.M. (1967) Method for solving the Korteweg–de Vries equation. Physical Review Letter, 19, 1095. doi:10.1103/PhysRevLett.19.1095 [22] Hirota, R. (1971) Exact solutions to the equation. de- scribing cylindrical solitons. Physical Review Letter, 27, 1192. doi:10.1103/PhysRevLett.27.1192 [23] Malfliet, W. (1992) Solitary wave solutions of nonlinear wave equations. American Journal of Physics, 60, 650. doi:10.1119/1.17120 [24] He, J.H. (1998) Approximate analytical solution for seepage flow with fractional derivatives in porous media. Computer Methods in Applied Mechanics and Engineer- ing, 167, 57. doi:10.1016/S0045-7825(98)00108-X [25] He, J.H. (2005) Approximate solution of nonlinear dif- ferential equations with convolution product nonlineari- ties. Computer Methods in Applied Mechanics and En- gineering, 26, 695-700. [26] He, J.H. (2006) Homotopy perturbation method for solv- ing boundary value problems. Physical Letter A, 350, 87-88. doi:10.1016/j.physleta.2005.10.005 [27] Ariel, P.D. (2010) Homotopy perturbation method and the natural convection flow of a third grade fluid through a circular tube. Nonlinear Science Letters A, 1, 43-52. [28] Ganji, D.D. and Rafei, M. (2006) Solitary wave solutions for a generalized hirota-satsuma coupled KdV equation by homotopy perturbation Method. Physical Letter A, 356, 131-137. doi:10.1016/j.physleta.2006.03.039 [29] Meena, A. and Rajendran, L. (2010) Mathematical mod- eling of amperometric and potentiometric biosensors and system of non-linear equations-Homotopy perturbation approach. Journal of Electroanalytical Chemistry, 644, 50-59. doi:10.1016/j.jelechem.2010.03.027 [30] Anitha, S., Subbiah, A., Rajendran, L. and Ashok K.J. (2010) Solutions of the coupled reaction and diffusion equations within polymer-modified ultramicroelectrodes. Physical Chemistry C, 114, 7030-7037. [31] Manimozhi, P., Subbiah, A. and Rajendran, L. (2010) Solution of steady-state substrate concentration in the ac- tion of biosensor response at mixed enzyme kinetics. Sensor and Actuators B, 147, 290-297. doi:10.1016/j.snb.2010.03.008 [32] Eswari, A. and Rajendran, L. (2010) Analytical solution of steady state current at a microdisk biosensor. Journal of Electroanalytical Chemistry, 641, 35-44. doi:10.1016/j.jelechem.2010.01.015 [33] Eswari, A. and Rajendran, L. (2010) Analytical solution of steady-state current an enzyme-modified microcylin- der electrodes. Journal of Electroanalytical Chemistry, 648, 36-46. doi:10.1016/j.jelechem.2010.07.002 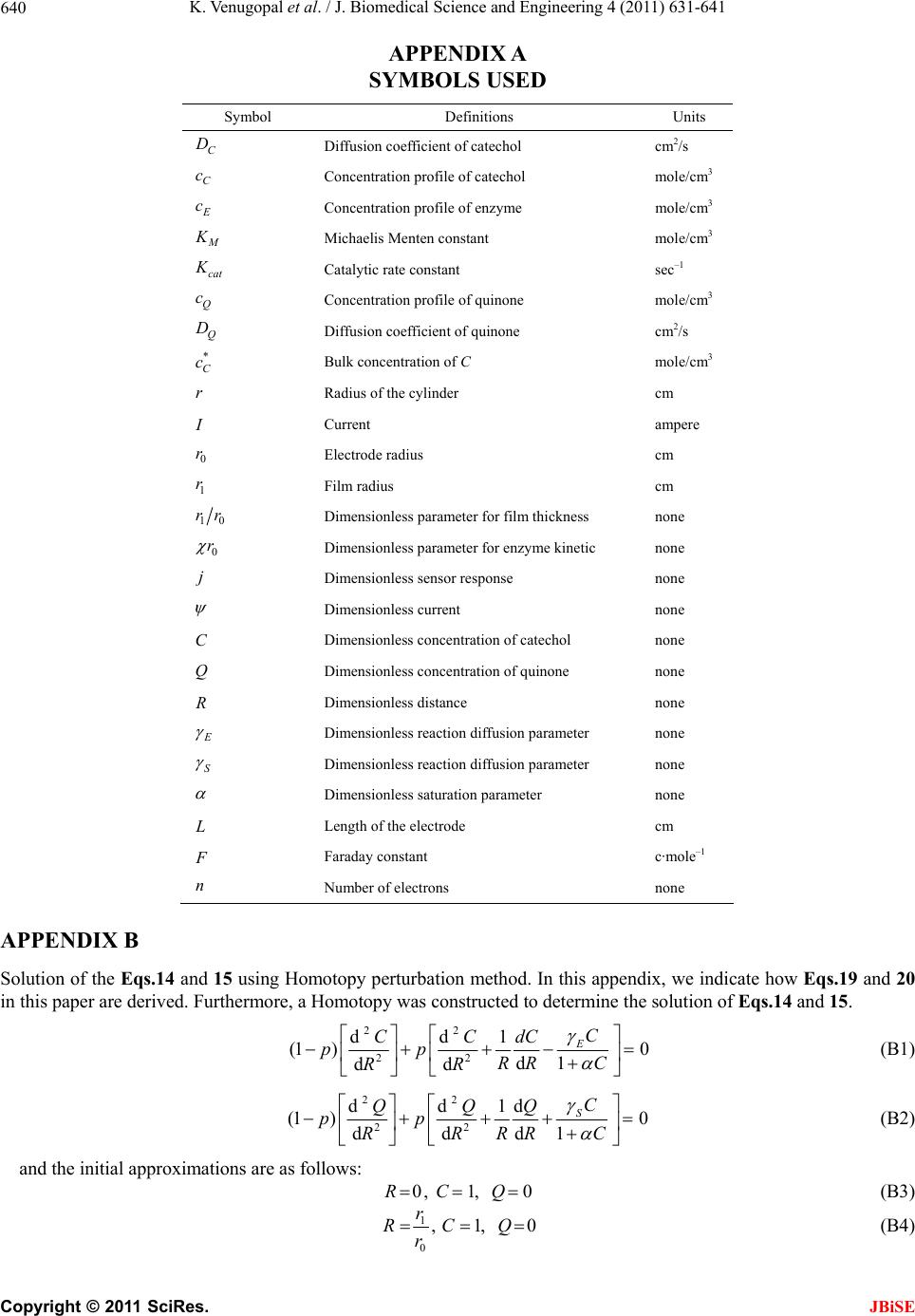 K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 640 APPENDIX A SYMBOLS USED Symbol Definitions Units C D Diffusion coefficient of catechol cm2/s C c Concentration profile of catechol mole/cm3 E c Concentration profile of enzyme mole/cm3 M K Michaelis Menten constant mole/cm3 cat K Catalytic rate constant sec–1 Q c Concentration profile of quinone mole/cm3 Q D Diffusion coefficient of quinone cm2/s * C c Bulk concentration of C mole/cm3 r Radius of the cylinder cm I Current ampere 0 r Electrode radius cm 1 r Film radius cm 10 rr Dimensionless parameter for film thickness none 0 r Dimensionless parameter for enzyme kinetic none j Dimensionless sensor response none Dimensionless current none C Dimensionless concentration of catechol none Q Dimensionless concentration of quinone none R Dimensionless distance none E Dimensionless reaction diffusion parameter none S Dimensionless reaction diffusion parameter none Dimensionless saturation parameter none L Length of the electrode cm F Faraday constant c·mole–1 n Number of electrons none APPENDIX B Solution of the Eqs.14 and 15 using Homotopy perturbation method. In this appendix, we indicate how Eqs.19 an d 20 in this paper are derived. Furthermore, a Homotopy was constructed to determine the solution of Eqs.14 and 15. 22 22 dd1 (1 )0 d1 dd EC CCdC pp RRC RR (B1) 22 22 dd1d (1 )0 d1 dd SC QQQ pp RR C RR (B2) and the initial approximations are as follows: 0, 1, 0 RCQ (B3) 1 0 ,1, 0 r RCQ r (B4)  K. Venugopal et al. / J. Biomedical Science and Engineering 4 (2011) 631-641 Copyright © 2011 SciRes. JBiSE 641 The approximate solutions of (B1) and (B2) are 23 01 2 3 CCpC pCpC (B5) and 23 01 2 3 QQpQpQpQ (B6) Substituting Eqs.B5 and B6 into Eqs .B1 and B2 and comparing the coefficients of like powers of p 2 00 2 d : 0 d C pR (B7) 2 100 1 2 0 d d1 : 0 d1 d E CC C pRR C R (B8) and 2 00 2 d : 0 d Q pR (B9) 2 100 1 2 0 d d1 : 0 d1 d S QC Q pRR C R (B10) Solving the Eqs.B7 to B1 0, and using the boundary conditions (B3) and (B4), we can find the following results 0() 1CR (B11) 2 10 10 1 1 () 2(1 ) EE E Rrr rrR CR (B12) and 0() 0QR (B13) 2 1010 1 1 () 2(1 ) SSS rr RrrR QR (B14) According to the HPM, we can conclude that 012 1 ()lim() p CRCRCC C (B15) 01 2 1 ()lim() p QRQRQ QQ (B16) Using Eqs.B11 and (B12) in Eq.B15 and Eqs.B13 and B14 in Eq.B16, we obtain the final results as described in Eqs.19 and 20. |

