Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2011, 4, 583-590 JBiSE doi:10.4236/jbise.2011.49074 Published Online September 2011 (http://www.SciRP.org/journal/jbise/). Published Online September 2011 in SciRes. http://www.scirp.org/journal/JBiSE Fabrication and characterization of the Ti-Ca-P composites by vacuum sintering Dibakar Mondal1, Swapan Kumar Sarkar1, Dong-Won Lee2, Young-Seon Lee2, Byong-Taek Lee1* 1Department of Biomedical Engineering and Materials, College of Medicine, Soonchunhyang University, Chungnam, Korea; 2Department of Materials Processing, Korea Institute of Materials Science, Changwon, Kyungsangnam-Do, Korea. Email: lbt@sch.ac.kr Received 14 February 2011; revised 25 April 2011; accepted 23 May 2011. ABSTRACT Using Ti and biphasic calcium phosphate (BCP) pow- ders, Ti-Ca-P composites which contained 0 - 30 vol.% BCP powders initially, were fabricated by vac- uum sintering at two different sintering temperatures, 1300˚C and 1400˚C. Detailed microstructural char- acteristics of the resulting composites were investi- gated. Mechanical properties like compressive stren- gth, Vickers hardness were evaluated and they sh ow- ed decreasing trend with the increasing initial BCP content. The x-ray diffraction (XRD) profiles revealed that extensive chemical reaction occurred and the initial BCP was degraded and formed CaO, TiO2, TiP, CaTiO3. However, the cell viability by MTT assay and cell proliferation behavior through one cell mor- phology analysis showed excellent increasing trend in biocompatibility which makes this materials suitable for hard tissue aid material. And the composite con- taining 30 vol.% BCP content with Ti sintered at 1400˚C showed excellent biocompatibility with the Vickers Hardness value 108.8 HV and the compres- sive strength value 303.7 MPa. Keywords: Ti; Calcium Phosphate; Biocompatibility; Bio-Ceramic 1. INTRODUCTION Biocompatible metals such as Ti and its alloys, Co-Cr alloys are still the most preferred implant materials for applications that require load bearing conditions, whether it is in dense form or porous. Titanium and its alloys are corrosion resistant, biocompatible, and self- passivating materials that have a much lower elastic modulus than Co-Cr alloys and stainless steel. The me- chanical properties of titanium and its alloys are good enough for load-bearing implants, but their biocompati- bility is much lower than that of calcium phosphate ce- ramics [1,2]. In addition, the interface between titanium and host bone is a simple interlocking bonding owing to the bio-inert nature of titanium metal, which can lead to the loosening of the implant and the eventual failure of implantation [3]. The lack of biological bonding between the metal and host bone can cause wear and associated debris which can lead to particle induced inflammation. The implantation of metal in place of the damaged bone can cause the stress shielding effect, due to the dissimi- larity of elastic modulus between bone and the implant materials, which weakens new bone formation and causes severe damages to the whole bone structure in the long run. The skeleton grows more bone tissue in re- gions where the load on the skeleton is large and the net result is a more closely packed and stronger skeleton that has the strength to sustain the increased load. In areas with diminished load, the skeleton retains only enough bone tissue necessary to sustain the diminished load. Thus, the skeleton in unloaded areas is weaker [4-6]. Surgeons believe that stress shielding is harmful because the weaker skeleton may fracture. The stress shielding effect depends on the difference between the stiffness of the shaft component and the stiffness of the bone. This only can be avoided by matching the elastic modulus of the implant and bone closely. The problems with tita- nium metals are obvious and only could be addressed with a systemic approach to incorporate features to im- prove the biocompatibility and modify the mechanical properties. BCP, which is a mixture of Hydroxyapatite, Ca10(PO4)6(OH)2 and Tri Calcium Phosphate, Ca3(PO4)2, has a similar crystallographic structure to bones mineral phase. Several studies have demonstrated that BCP is biocompatible with hard tissues and exhibits osseocon- ductive properties [5-9]. It is also well known that BCP forms a direct bond with surrounding tissue after bone implantation. However, its poor mechanical properties, especially the fracture toughness less by an order of  D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 584 magnitude, are the most serious obstacles for applica- tions as load-bearing implants [10]. Many efforts have been made to improve the me- chanical properties of Hydroxyapatite (HAp) [11,12] and the biological properties of titanium and its alloys [13, 14]. Achieving a good combination of the bioactivity of HAp and the favorable mechanical properties of metals are considered a promising approach to fabricate more perfect biomedical devices for load-bearing applications in hard tissue engineering. This could be achieved by using appropriate metallic reinforcing materials with hydroxyapatite [15-17]. Functionally graded materials consisting of metallic and ceramic components [18] have been shown to improve the properties of several systems such as medical implant devices. Several studies have examined the potential of coating of Hydroxyapatite on Ti with or without combination of Ti to improve the biocompatibility of the metallic system in terms of biological fixation with the host site. Several methods of coating Ti have been developed such as plasma spraying, dip-spin, electrochemical etc. [19-22]. However, coating Ti is complex because the bonding strength of the interface of Ti and BCP is very low. Therefore, it is very difficult to prepare a uniform coat- ing on an implant with a complex structure using this technique. The most widely used coating method is plasma spray coating, which severely damages the Hy- droxyapatite [19-21]. BCP and Ti duplex metal-matrix composites can be viewed as a potential alternative but there are also some unique problems using this approach. Due to severe oxidation of titanium in air, sintering of the Ti-BCP composites has to be done in vacuum or un- der the protection of an inert atmosphere. However, un- der such sintering conditions, dehydration and decompo- sition temperatures of HAp will decrease remarkably [23], which decreases its mechanical properties as well as the excellent biocompatibility of the HAp is lost. However, a comprehensive investigation is still to be reported with the aim to incorporate HAp in titanium matrix with a nano-structured HAp phase inclusion and the improvement of biocompatibility along with the modification of mechanical properties with in favorable range of magnitude. In this study, Ti-BCP composites were fabricated us- ing the vacuum sintering method to combine the bioac- tivity of BCP and the mechanical properties of titanium. Two sintering temperatures, 1300˚C and 1400˚C, were used to investigate the sintering behaviors. In addition, four compositions at each sintering temperatures were examined; pure Ti or 0% BCP as the controls, 10 vol.% BCP, 20 vol.% BCP and 30 vol.% BCP. These experi- ments were conducted to investigate changes in the properties of the composite as a function of BCP content. Especially using MTT assay and observation of single cell growth the cell viability and biocompatibility of Ti-Ca-P based composites were investigated. 2. MATERIALS AND METHODS 2.1. Preparation of the Composite The BCP powder was prepared at room temperature by mixing calcium nitrate tetrahydrate (Ca(NO3)2.4H2O) (Samchun Chemicals, 98.5% pure) and ammonium phosphate dibasic ((NH4)3PO4·2H2O) (Samchun Chemi- cals, 98.5% pure) in an ultrasonic bath [24]. NH4OH was added to the solution to bring the pH > 9 and the sample was ultrasonciated for 4hrs. The solution was then al- lowed to precipitate for 24 hr. Then, the precipitated BCP was washed to remove NH4OH and filtrated. After filtration the cake was dried at 80˚C for 72 hr in an oven and then crushed. The BCP powder was then calcined at 750˚C for 2 hr. Commercially pure titanium powder (–325 mesh, 99.5%, Alfa Aesar, USA) and BCP were mixed with alcohol and ball milled for 24 hr. Four dif- ferent compositions were fabricated; pure Ti powder, 10 vol.% BCP, 20 vol.% BCP and 30 vol.% BCP powder with Ti. The mixture was then dried at 80˚C for 3 hr. For the characterization of microstructure and material prop- erties, pellets of 1.3 cm diameter were made in a hydrau- lic pressure unit (Carver Inc., USA) with 5 tons force. Then, the composite pellets were sintered in vacuum at a temperature of 1300˚C and 1400˚C for 1 hr. 2.2. Characterization The density of the composites was measured using the Archimedes method. The Vickers’ hardness was meas- ured using a hardness testing machine (Microvicker, Akashi, Japan) and the compressive strength was meas- ured using universal testing machine (Unitech TM, R&B, Korea). The microstructure of the composites was char- acterized using a Scanning Electron Microscope (JSM- 6701F, JEOL Ltd, Japan). In addition, the composites composition and crystal structure of composites were determined by X-Ray diffraction (Miniflex II, Rigaku, Japan). 2.3. In Vitr o Study The in vitro properties of the composite were character- ized by cytotoxicity test for L929 cell line with a MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) solution and the cell morphology of individual cells using MG63 cell line. BCP-Ti composites pellets were first chemically etched with a 20% H2SO4 solution for 5 minutes in an ultrasonic bath. Then, they were cleaned with acetone, alcohol and deionized water sequentially in an ultrasonic bath for 5 minutes each. The pellets were placed in fal-  D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 585 con tubes and sterilized in an autoclave. The pellets were then submerged in 12 ml solution of Roswell Park Memorial Institute medium (RPMI). The mixture is then incubated 5% CO2 atmospheres for 72 hrs in a shaking incubator. After that medium was fil- trated and the extract was taken in 50 ml falcon tube. Previously cultured L929 cells were seeded into 96-well microtiter plates (Nunclon™, Nunc, Wiesbaden, Ger- many) at a density of 7 × 104 cells/well. After washing with PBS the extract and medium solution were pour into the well and moved those in CO2 incubator for 72 hrs. After that the medium was removed and in each well 20 μl of 5 mg·ml–1 MTT was added including one set of wells with MTT but no cells (control). Then Incubated for 3.5 hours at 37˚C in culture hood media was re- moved carefully. With adding 150 μl MTT solvent the plate was covered with Al foil and agitates cells on or- bital shaker for 15 min, then the absorbance was read at 590 nm using an HP 8453 spectrophotometer. For one cell morphology the MG63 cells were seeded onto the composite pellets in a 24-multiwell plate at a final density 10,000 cells·cm–2. After 1 hr and 24 hrs, the media was removed and after dehydratationin ethanol solutions of 75%, 90%, 95% and 100%specimens were fixed with 4% glutaraldehyde in PBS (pH 7.2) and keep at room temperature for drying. After drying the samples were sputter-coated with Pt. The surface of the speci- mens was finally examined with backscattered (BSE) mode and secondary electrons (SE) modeby scanning electron microscopy (SEM) under a voltage of 15 kV. 3. RESULTS 3.1. Microstructure and Morphologies of Ti-Ca-P Composites SEM images of sintered composites body had shown in Fig.1, as the amount of initial BCP in composites in- creases so does the porosity in the composites. This trend was common for both of the sintering temperature of 1300˚C and 1400˚C as shown in Figures 1(a-d), re- spectively. From the SEM images, we could easily see that the titanium surface was barely visible except for the polished surface. 3.2. Phase Composition of the Composites The SEM microstructure and energy dispersive X-ray spectroscopy (EDS) profile of the Ti-10% BCP compos- ite sintered at 1400˚C shown in Figure 2. As shown in Figure 2(b), in selected point Pon the titanium metal surface, significant amounts of titanium were observed as expected. In addition, in point Q contained more Ca and P (Figure 2(c)); however, a significant amount of Ti was Figure 1. SEM morphologies of (a) 10% BCP-Ti; (b) 30% BCP-Ti vacuum sintered at 1300˚C and (c) 10% BCP-Ti; (d) 30% BCP-Ti vacuum sintered at 1400˚C.  D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 586 1 2 3 4 5 6 1 2 3 4 5 6 Figure 2. (a) SEM image of a small area of 10% BCP-Ti based composites surface sin- tered at 1400˚C and Energy dispersive spectroscopy (EDS) profiles of (b) point P on tita- nium surface and (c) point Q on Ca-P surface. also there, which revealed that Ti in the composite body got reacted with the initial BCP phase, which was the reason for the decomposition of the calcium phosphate phase. Ti reacted with BCP and formed TiO2, TiP and CaTiO3. By analyzing the x-ray diffraction data (XRD) in Figure 3, the phase can be mostly detected. From Figure 3(a) for pure Ti, no TiO2 or CaO or CaTiO3was observed. But for 90% Ti with 10% initial BCP and both for vacuum sintered at (b) 1300˚C and (d) 1400˚C, some amount of TiO2, CaO and CaTiO3had formed. In addi- tion, for 70% Ti with 30% initial BCP, the amount of all these components increased. At all compositions and both sintering temperatures, no BCP phase was detected (for BCP major peak occurs at 2θ value of 31). 3.3. Mechanical Characteristics of the Composites As shown in Figure 4(a), the density of the Ti based composites decreasedat increasing initial BCP content for vacuum sintered composites both at 1300˚C and 1400˚C. Pure Ti had almost the same value as the theo- retical value near 4.50 g·cm–3. The hardness and com- pressive strength also showed the same tendency, there was a decrease in the mechanical properties at higher initial BCP contents as shown in Figures 4(b) and (c). From these figures it can be seen that at the same initial Ti-BCP composition, composites sintered at 1400˚C had better mechanical properties than composites sintered at 1300˚C due to enhanced densification at higher tem- perature, although the composition reacted at high tem- perature to form different reaction product and the initial BCP phase was almost entirely disappeared. For pure titanium, this effect was not significant. The hardness value was drastically fallen after introducing the ceramic phase but kept steady afterwards with minor declination with the increased BCP content. Compressive strength value showed steadily declination as the initial BCP content was increasing. 3.4. In Vitro Experiments To evaluate the cytotoxicity of the composites, cell vi- ability and cell proliferation on the composites were de- termined through in vitro experiments with MTT assay and by examining the morphology of surface grown in- dividual cells. These experiments were conducted with the composites sintered at a temperature of 1400˚C. It was expected that the 70% Ti based composite would show the best results relative to the other compositions because of high initial Ca-P content. As expected, after 60 minutes of incubation, the cells on the 70% Ti com-  D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 587 Figure 3. XRD pattems of (a) pure Ti sintered at 1400℃, (b) 10% BCP-Ti and (c) 30% BCP-Ti sintered at 1300˚C, (d) 10% BCP-Ti and (e) 30% BCP-Ti sintered at 1400˚C. Unmarked peaks are TiP. (a) (b) (c) Figure 4. Density, (b) Vikers, Hardness and (c) compressive strength of Ti-BCP composites at two different sintering temperatures. posites had divided daughter cells were observed in the SEM images; however, no daughter cells were observed on the 80% Ti based composites and others. 4. DISCUSSION Prior to mixing, the biphasic calcium phosphate and tita- nium powders consisted of different particle size which made a non uniform mixing with a bimodal particle dis- tribution with two sharp peaks (Figure 1). BCP powder consisted of nano size particles (<100 nm particle size) with spherical morphology [25] and the Ti powder con- sisted of micro size particle (less than 43 μm) with ir- regular spongy shape [26]. The nano sized BCP covered the much larger Ti particles and make a soft coating like layer. This prevented the grain diffusion of the Ti and sintering was hindered. The BCP particles themselves were sintered in the mean time and the inter-particular space of the Ti particles remained barely unaltered to make the residual pores. However there were some necking zones among the Ti particles that facilitate sin- tering. The XRD (Figure 3) and EDS (Figure 2) profiles in- dicated that there was a reaction between titanium and BCP. And BCP degraded into CaTiO3, CaO, TiO2and TiP. The reaction [27] between the titanium and BCP is, Ti + Ca10(PO4)6(OH)2 → CaTiO3 + CaO + TiO2 + TiP + H2O. Soas desired, no Hydroxyapatite or tri-calcium phos- phate was found in the final sintered sample as shown in the XRD profiles of Figure 3. The SEM images of the composite bodies as shown in Fig.1demonstrated that the surface of the titanium particles was degraded. The tita- nium particles were wrapped by the other materials pro- duced after the sintering. As the BCP concentration in- creased so did the porosity and surface degradation. This is due to the thicker covering of the Ti particles by in- 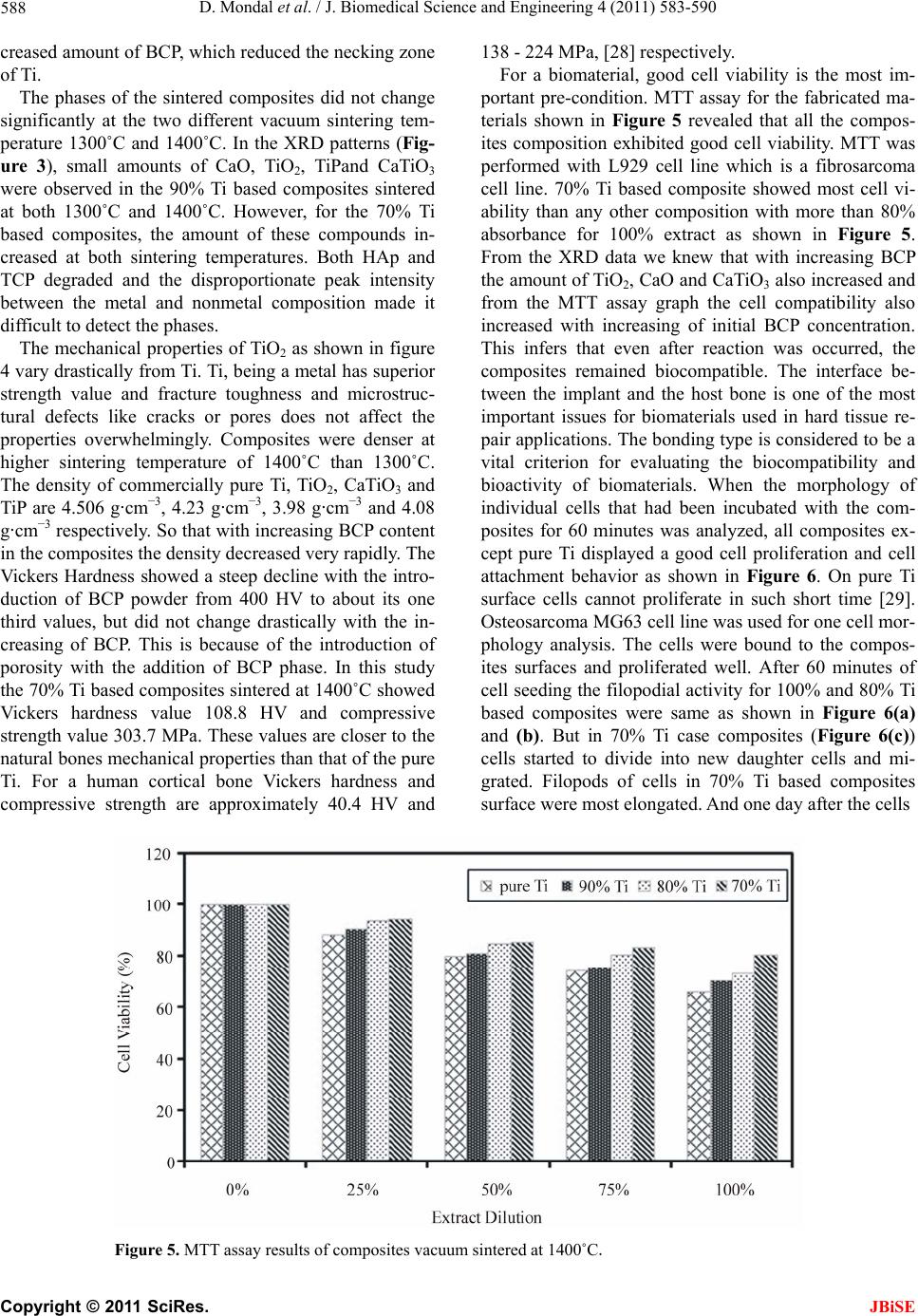 D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 588 creased amount of BCP, which reduced the necking zone of Ti. The phases of the sintered composites did not change significantly at the two different vacuum sintering tem- perature 1300˚C and 1400˚C. In the XRD patterns (Fig- ure 3), small amounts of CaO, TiO2, TiPand CaTiO3 were observed in the 90% Ti based composites sintered at both 1300˚C and 1400˚C. However, for the 70% Ti based composites, the amount of these compounds in- creased at both sintering temperatures. Both HAp and TCP degraded and the disproportionate peak intensity between the metal and nonmetal composition made it difficult to detect the phases. The mechanical properties of TiO2 as shown in figure 4 vary drastically from Ti. Ti, being a metal has superior strength value and fracture toughness and microstruc- tural defects like cracks or pores does not affect the properties overwhelmingly. Composites were denser at higher sintering temperature of 1400˚C than 1300˚C. The density of commercially pure Ti, TiO2, CaTiO3 and TiP are 4.506 g·cm–3, 4.23 g·cm–3, 3.98 g·cm–3 and 4.08 g·cm–3 respectively. So that with increasing BCP content in the composites the density decreased very rapidly. The Vickers Hardness showed a steep decline with the intro- duction of BCP powder from 400 HV to about its one third values, but did not change drastically with the in- creasing of BCP. This is because of the introduction of porosity with the addition of BCP phase. In this study the 70% Ti based composites sintered at 1400˚C showed Vickers hardness value 108.8 HV and compressive strength value 303.7 MPa. These values are closer to the natural bones mechanical properties than that of the pure Ti. For a human cortical bone Vickers hardness and compressive strength are approximately 40.4 HV and 138 - 224 MPa, [28] respectively. For a biomaterial, good cell viability is the most im- portant pre-condition. MTT assay for the fabricated ma- terials shown in Figure 5 revealed that all the compos- ites composition exhibited good cell viability. MTT was performed with L929 cell line which is a fibrosarcoma cell line. 70% Ti based composite showed most cell vi- ability than any other composition with more than 80% absorbance for 100% extract as shown in Figure 5. From the XRD data we knew that with increasing BCP the amount of TiO2, CaO and CaTiO3 also increased and from the MTT assay graph the cell compatibility also increased with increasing of initial BCP concentration. This infers that even after reaction was occurred, the composites remained biocompatible. The interface be- tween the implant and the host bone is one of the most important issues for biomaterials used in hard tissue re- pair applications. The bonding type is considered to be a vital criterion for evaluating the biocompatibility and bioactivity of biomaterials. When the morphology of individual cells that had been incubated with the com- posites for 60 minutes was analyzed, all composites ex- cept pure Ti displayed a good cell proliferation and cell attachment behavior as shown in Figure 6. On pure Ti surface cells cannot proliferate in such short time [29]. Osteosarcoma MG63 cell line was used for one cell mor- phology analysis. The cells were bound to the compos- ites surfaces and proliferated well. After 60 minutes of cell seeding the filopodial activity for 100% and 80% Ti based composites were same as shown in Figure 6(a) and (b). But in 70% Ti case composites (Figure 6(c)) cells started to divide into new daughter cells and mi- grated. Filopods of cells in 70% Ti based composites surface were most elongated. And one day after the cells Figure 5. MTT assay results of composites vacuum sintered at 1400˚C. 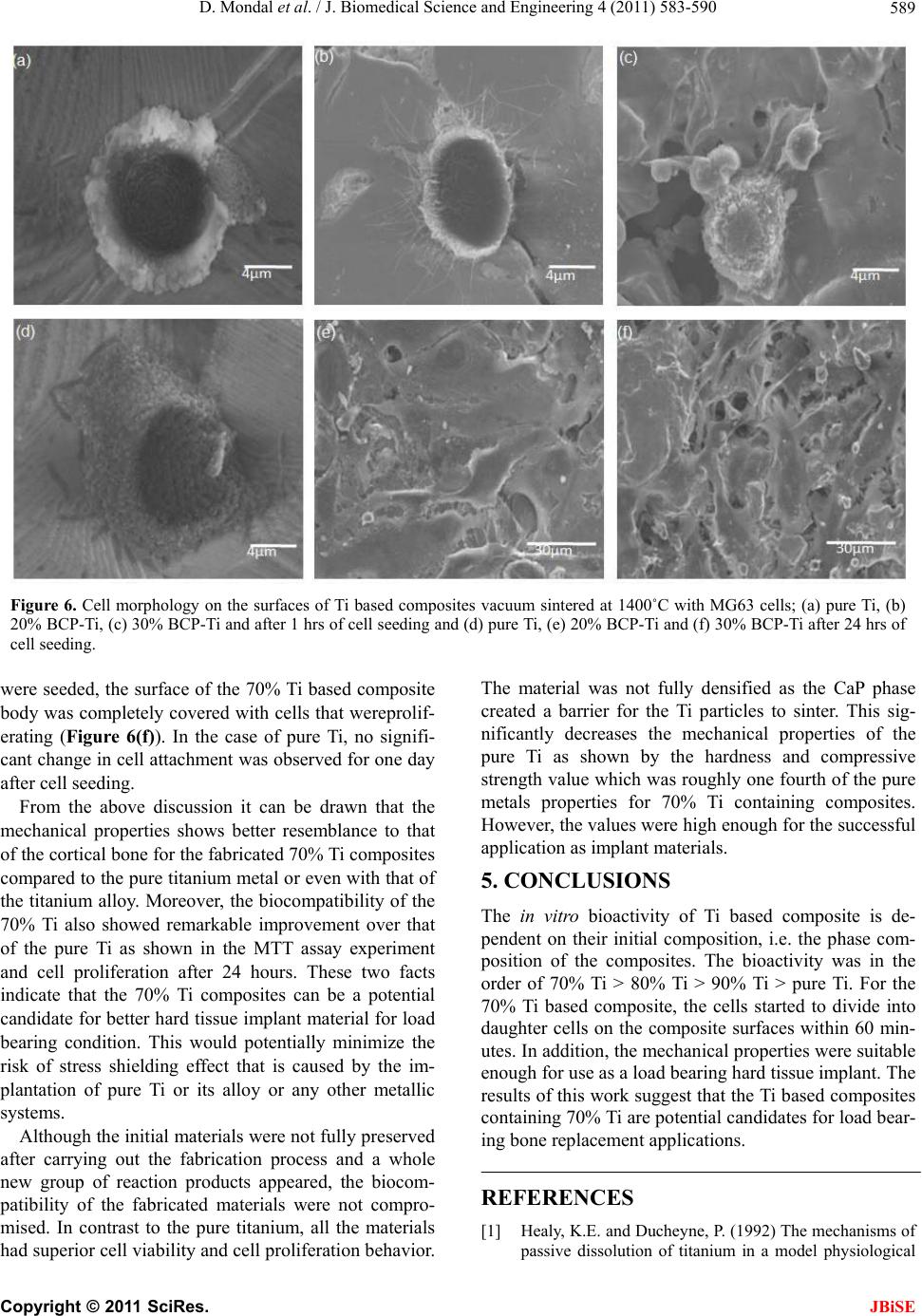 D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 589 Figure 6. Cell morphology on the surfaces of Ti based composites vacuum sintered at 1400˚C with MG63 cells; (a) pure Ti, (b) 20% BCP-Ti, (c) 30% BCP-Ti and after 1 hrs of cell seeding and (d) pure Ti, (e) 20% BCP-Ti and (f) 30% BCP-Ti after 24 hrs of cell seeding. were seeded, the surface of the 70% Ti based composite body was completely covered with cells that wereprolif- erating (Figure 6(f)). In the case of pure Ti, no signifi- cant change in cell attachment was observed for one day after cell seeding. From the above discussion it can be drawn that the mechanical properties shows better resemblance to that of the cortical bone for the fabricated 70% Ti composites compared to the pure titanium metal or even with that of the titanium alloy. Moreover, the biocompatibility of the 70% Ti also showed remarkable improvement over that of the pure Ti as shown in the MTT assay experiment and cell proliferation after 24 hours. These two facts indicate that the 70% Ti composites can be a potential candidate for better hard tissue implant material for load bearing condition. This would potentially minimize the risk of stress shielding effect that is caused by the im- plantation of pure Ti or its alloy or any other metallic systems. Although the initial materials were not fully preserved after carrying out the fabrication process and a whole new group of reaction products appeared, the biocom- patibility of the fabricated materials were not compro- mised. In contrast to the pure titanium, all the materials had superior cell viability and cell proliferation behavior. The material was not fully densified as the CaP phase created a barrier for the Ti particles to sinter. This sig- nificantly decreases the mechanical properties of the pure Ti as shown by the hardness and compressive strength value which was roughly one fourth of the pure metals properties for 70% Ti containing composites. However, the values were high enough for the successful application as implant materials. 5. CONCLUSIONS The in vitro bioactivity of Ti based composite is de- pendent on their initial composition, i.e. the phase com- position of the composites. The bioactivity was in the order of 70% Ti > 80% Ti > 90% Ti > pure Ti. For the 70% Ti based composite, the cells started to divide into daughter cells on the composite surfaces within 60 min- utes. In addition, the mechanical properties were suitable enough for use as a load bearing hard tissue implant. The results of this work suggest that the Ti based composites containing 70% Ti are potential candidates for load bear- ing bone replacement applications. REFERENCES [1] Healy, K.E. and Ducheyne, P. (1992) The mechanisms of passive dissolution of titanium in a model physiological  D. Mondal et al. / J. Biomedical Science and Engineering 4 (2011) 583-590 Copyright © 2011 SciRes. JBiSE 590 environment. Journal of Biomedical Materials Research, 26, 319-338. doi:10.1002/jbm.820260305 [2] Nanci, A., Wuest, J.D., Peru, L., Brunet, P., Sharma, V., Zalzal S. and McKee, M.D. (1998) Chemical modifica- tion of titanium surfaces for covalent attachment of bio- logical molecules. Journal of Biomedical Materials Re- search, 40, 324-335. doi:10.1002/(SICI)1097-4636(199805)40:2<324::AID-J BM18>3.0.CO;2-L [3] Albrektsson, T. and Hansson, H.A. (1986) An ultrastruc- tural characterization of the interface between bone and sputtered titanium or stainless steel surfaces. Biomate- rials, 7, 201-205. doi:10.1016/0142-9612(86)90103-1 [4] Damien C.J. and Persons, J.R. (1992) Bone graft and bone graft substitutes: A review of current technology and applications. Journal of Applied Biomaterials, 2, 187- 208. doi:10.1002/jab.770020307 [5] Hench, L.L. (1999) Bioactive Glasses and Glass-Ceram- ics. Materials Science Forum, 293, 37-64. doi:10.4028/www.scientific.net/MSF.293.37 [6] McGrory, B.J., Morrey, B.F., Cahalan, T.D. and Cabanela M.E. (1995) Effect of femoral offset on range of motion and abductor muscle strength after total hip arthroplasty. Journal of Bone and Joint Surgery, 77, 865-869. [7] Elliott, J.C., Mackie P.E. and Young, R.A. (1973) Mono- clinic Hydroxyapatite. Science, 108, 1055-1057. doi:10.1126/science.180.4090.1055 [8] Hong, L., Xu H.C. and De Groot, K. (1992) Tensile strength of the interface between hydroxyapatite and bone. Journal of Biomedical Materials Research, 26, 7-18. doi:10.1002/jbm.820260103 [9] Edwards, J.T., Brunski, J.B. and Higuchi, H.W. (1997) Mechanical and morphologic investigation of the tensile strength of a bone hydroxyapatite interface. Journal of Biomedical Materials Research, 36, 454-468. doi:10.1002/(SICI)1097-4636(19970915)36:4<454::AID -JBM3>3.0.CO;2-D [10] Gautier, S., Champion, E., Bernache-Assollant D. and Chartier T., (1999) Rheological characteristics of aluminia platelet-hydroxyapatite composite suspension. Journal of the European Ceramic Society, 19, 469-477. doi:10.1016/S0955-2219(98)00224-6 [11] Kong, Y., Kim, S., Kim, H. and Lee, I. (1999) Rein- forcement of hydroxyapatite bioceramics by addition of ZrO2 coated Al2O3. Journal of the European Ceramic Society, 82, 2963-2968. doi:1 0. 1111/j.1151 -2916.1999.tb02189.x [12] Li, J., Hermansson L. and Soremark, R. (1993) High strength biofunctional zirconia: Mechanical properties and static fatigue behaviour of zirconia-apatite composite. Journal of Materials Science: Materials in Medicine, 4, 50-54. doi:10.1007/BF00122977 [13] T. Kokubo, T. Matsushita and H. Takadama (2007) tita- nia-based bioactive materials. Journal of the European Ceramic Society, 27, 1553-1558. doi:10.1016/j.jeurceramsoc.2006.04.015 [14] Vehof, J.W.M., Spauwen P.H.M. and Jansen, J.A. (2000) Bone formation in calcium-phosphate-coated titanium mesh. Biomaterials, 21, 2003-2009. doi:10.1016/S0142-9612(00)00094-6 [15] Miyazaki, T., Kim, H.M., Miyaji, F., Kokubo, T. and Nakamura, T. (1997) Bioceramics 10. Elsevier Science LTD, New York. [16] Kim, H.M., Miyaji, F., Kokubo, T. and Nakamura, T. (1997) Apatite-forming ability of alkali-treated ti metal in body environment. Journal of the ceramic Society of Ja- pan, 105, 111-116. doi:10.2109/jcersj.105.111 [17] Cortes, D.A., Escobedo, J.C., Nogiwa A. and Munoz, A. (2003) Biomimetic bonelike apatite coating on cobalt based alloys. Materials Science Forum, 442, 61-66. doi:10.4028/www.scientific.net/MSF.442.61 [18] Aboudi J., Pindera, M.-J. and Arnold, S.M. (2001) Linear thermoelastic higher-order theory for periodic multiphase materials, Journal of Applied Mechanics, 68, 697-707. doi:10.1115/1.1381005 [19] De Groot, K., Geesink, R., Klein C. and Serekian, P. (1987) Plasma sprayed coatings of Hydroxyapatite. Journal of Biomedical Materials Research, 21, 1375- 1381. doi:10.1002/jbm.820211203 [20] Yang, Y.C. (2007) Influence of residual stress on bonding strength of the plasma-sprayed hydroxyapatite coating after the vacuum heat treatment, Surface and Coatings Technology, 201, 7187-7193. doi:10.1016/j.surfcoat.2007.01.027 [21] Chen C.C. and Ding, S.J. (2006) Effect of heat treatment on characteristics of plasma sprayed hydroxyapatite coat- ings. Materials Transactions, 47, 935-940. doi:10.2320/matertrans.47.935 [22] Yang, X.D., Lu, X., Zhang, Q.Y., Zhang, X.D., et al. (2007) BCP coatings on pure titanium plates by CD method. Materials Science and Engineering C, 27, 781- 786. doi:10.1016/j.msec.2006.08.011 [23] Ducheyne P. and Hasting, G.W. (1984) Metal and Ce- ramic Biomaterials. CRC Press, Boca Raton, 144-166. [24] Byong-Taek, L., Min-Ho, Y., RajatKanti, P., Kap-Ho, L. and Ho-Yeon, S. (2007) In situ synthesis of spherical BCP nanopowders by microwave assisted process. Mate- rials Chemistry and Physics, 104, 249-253. doi:10.1016/j.matchemphys.2007.02.009 [25] Cao, L., Zhang, C. and Huang, J. (2005) Synthesis of hydroxyapatite nanoparticles in ultrasonic precipitation. Ceramics International, 31, 1041-1044. doi:10.1016/j.ceramint.2004.11.002 [26] Choi, M. G. et al. (2005) Effects of titanium particle size on osteoblast functions in vitro and in vivo. PNAS, 102, 4578-4583. doi:10.1073/pnas.0500693102 [27] Ning C.Q. and Zhou, Y. (2004) On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials, 25, 3379-3387. doi:10.1016/j.biomaterials.2003.10.017 [28] Reilly D.T. and Burstein, A.H. (1975) The elastic and ultimate properties of compact bone tissue. Journal of Biomechanics, 8, 393-396. doi:10.1016/0021-9290(75)90075-5 [29] Schmidt, C., Ignatius A.A. and Claes, L.E. (2001) Prolif- eration and differentiation parameters of humanosteo- blasts on titanium and steel surfaces. Journal of Biomedi- cal Materials Research, 54, 209-215. doi:10.1002/1097-4636(200102)54:2<209::AID-JBM7>3 .0.CO;2-7 |

