 International Journal of Organic Chemistry, 2011, 1, 97-104 doi:10.4236/ijoc.2011.13015 Published Online September 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC Reactivity of 3-Cyanoacetylindole Derivatives: Synthesis of 3-Hydrazonopyrazolyl and 3-Thiadiazolyl Indole Derivatives Hamdi M. Hassaneen*, Huwaida M. E. Hassaneen, Zakaria Ahmed Gomaa Department of Chemistry, Faculty of Science, Cairo University, Giza, Egypt E-mail: *huwaidahassaneen@hotmail.com Received May 27, 2011; revised June 27, 2011; accepted July 5, 2011 Abstract The coupling reaction of 3-cyanoacetyl-2-methylindole 1a with the aromatic diazonium salts gave the corre- sponding arylhydrazones 2a-e. Compounds 2 were used for synthesis of 4-aminopyrazole-5-carbonitrile 4a-e and 5-amino-4-arylazo-3-pyrazoles 5a-e derivatives. Also, treatment of 3-cyanoacetyl-2-phenylindole 1b with phenyl isothiocyanate gave the corresponding thioacetanilide 7. The later compound 7 was utilized as the key intermediate for the synthesis of some new thiadiazole derivatives 9a-r. The structures of all new compounds were elucidated on the basis of elemental analysis and spectral data. Keywords: 3-Methyl Indole, 3-Phenyl Indole, Phenyl Isothiocyanate, Cyanoacetic Acid 1. Introduction The indole moiety is found in various pharmacologically and biologically active compounds [1,2]. Many indole alkaloids are recognized as one of the rapidly growing groups of marine invertebrate metabolites for their broad spectrum of biological properties [3-6]. For example, five novel indole alkaloids [7,8], tunicate aplidium me- ridianum A-E, have been isolated from tunical splidium meridianum. They show cytotoxicity toward murine tu- mor cell lines and have potent inhibition against several protein kinases [9,10]. Along with these, the substitution at the 3-position of the indole ring can take place by connecting an additional heterocyclic ring, such as imi- dazole (topsentins [11,12], nortopsentins [13]), dihy- droimidazole (disc odermindole [14]), oxazole (mar- tefragin [15], amazole [16]), oxadiazine (alboinon [17]), maleimide (didemidines [18]), and piperazine (dragma- cidone [19]). Therefore, 3-substituted indoles still repre- sent a significant synthetic challenge. 2. Results and Discussion As a part of our program aimed at developing a synthesis for pyrazole [20] and thiadiazole derivatives [21-23], we report here an efficient synthesis for aminopyrazoles, which are used as precursors for biologically active fused pyrazoles. Thus, reacting 2-methylindole with cyanoace- tic acid in acetic anhydride, utilizing a literature proce- dure [24], led to formation of 3-cyanoacetyl-2-methyl- indole 1a. The latter compound 1 reacted with aromatic diazonium salts to yield the corresponding arylhydra- zones 2a-e in excellent yields (Scheme 1). The E-struc- ture for hydrazones 2A was preferred over possible hy- drogen-bonded Z-structure 2B based on analog of the recently-reported structure of 3-substituted-2-aryl-hy- drazono-3-oxoalkanenitriles, whose E-structure has con- firmed by X-ray crystal structure determination [25] and supported by theoretical calculation [26]. Compound 2 reacted readily with chloroacetonitrile in presence of triethylamine to give products of molecular Scheme 1. MCR of α-cyanoketenes S, S-acetals, amine and guanidine carbonate. 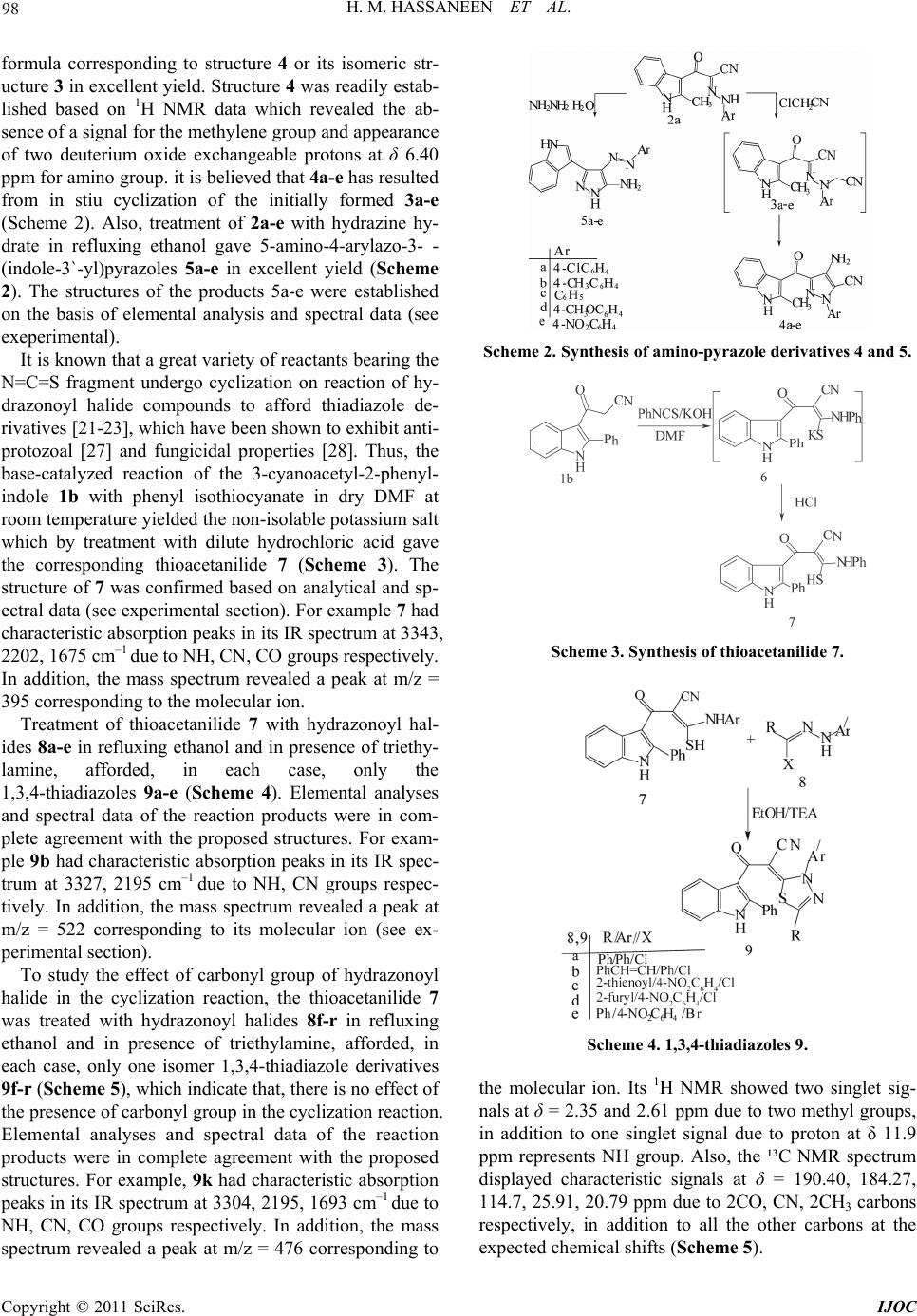 H. M. HASSANEEN ET AL. 98 formula corresponding to structure 4 or its isomeric str- ucture 3 in excellent yield. Structure 4 was readily estab- lished based on 1H NMR data which revealed the ab- sence of a signal for the methylene group and appearance of two deuterium oxide exchangeable protons at δ 6.40 ppm for amino group. it is believed that 4a-e has resulted from in stiu cyclization of the initially formed 3a-e (Scheme 2). Also, treatment of 2a-e with hydrazine hy- drate in refluxing ethanol gave 5-amino-4-arylazo-3- - (indole-3`-yl)pyrazoles 5a-e in excellent yield (Scheme 2). The structures of the products 5a-e were established on the basis of elemental analysis and spectral data (see exeperimental). It is known that a great variety of reactants bearing the N=C=S fragment undergo cyclization on reaction of hy- drazonoyl halide compounds to afford thiadiazole de- rivatives [21-23], which have been shown to exhibit anti- protozoal [27] and fungicidal properties [28]. Thus, the base-catalyzed reaction of the 3-cyanoacetyl-2-phenyl- indole 1b with phenyl isothiocyanate in dry DMF at room temperature yielded the non-isolable potassium salt which by treatment with dilute hydrochloric acid gave the corresponding thioacetanilide 7 (Scheme 3). The structure of 7 was confirmed based on analytical and sp- ectral data (see experimental section). For example 7 had characteristic absorption peaks in its IR spectrum at 3343, 2202, 1675 cm–1 due to NH, CN, CO groups respectively. In addition, the mass spectrum revealed a peak at m/z = 395 corresponding to the molecular ion. Treatment of thioacetanilide 7 with hydrazonoyl hal- ides 8a-e in refluxing ethanol and in presence of triethy- lamine, afforded, in each case, only the 1,3,4-thiadiazoles 9a-e (Scheme 4). Elemental analyses and spectral data of the reaction products were in com- plete agreement with the proposed structures. For exam- ple 9b had characteristic absorption peaks in its IR spec- trum at 3327, 2195 cm–1 due to NH, CN groups respec- tively. In addition, the mass spectrum revealed a peak at m/z = 522 corresponding to its molecular ion (see ex- perimental section). To study the effect of carbonyl group of hydrazonoyl halide in the cyclization reaction, the thioacetanilide 7 was treated with hydrazonoyl halides 8f-r in refluxing ethanol and in presence of triethylamine, afforded, in each case, only one isomer 1,3,4-thiadiazole derivatives 9f-r (Scheme 5), which indicate that, there is no effect of the presence of carbonyl group in the cyclization reaction. Elemental analyses and spectral data of the reaction products were in complete agreement with the proposed structures. For example, 9k had characteristic absorption peaks in its IR spectrum at 3304, 2195, 1693 cm–1 due to NH, CN, CO groups respectively. In addition, the mass spectrum revealed a peak at m/z = 476 corresponding to Scheme 2. Synthesis of amino-pyrazole derivatives 4 and 5. Scheme 3. Synthesis of thioacetanilide 7. Scheme 4. 1,3,4-thiadiazoles 9. the molecular ion. Its 1H NMR showed two singlet sig- nals at δ = 2.35 and 2.61 ppm due to two methyl groups, in addition to one singlet signal due to proton at δ 11.9 ppm represents NH group. Also, the ¹³C NMR spectrum displayed characteristic signals at δ = 190.40, 184.27, 114.7, 25.91, 20.79 ppm due to 2CO, CN, 2CH3 carbons respectively, in addition to all the other carbons at the expected chemical shifts (Scheme 5). Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. 99 Scheme 5. Synthesis of 1,3,4-thiadiazoles 9. 3. Experimental Section 3.1. General All melting points were determined on an electrothermal GallenKamp melting point apparatus and are uncorrected. The IR spectra were recorded as KBr Pellets on a Jasco FTIR-460 plus Fourier transform infrared spectropho- tometer.1H and 13C NMR spectra were recorded at (300 MHz) and (75 MHz) respectively on Varian EM-300 MHz spectrometer. Chemical shifts (δ) are given from TMS (ppm) as internal standard for ¹H NMR and ¹³C NMR. Mass spectra were recorded on AEI MS 30 mass spectrometer operating at 70 eV. The elemental analyses were performed at the Microanalytical Center of Cairo University. 3.2. General Method for Preparation of (E)-N’-aryl-2-(2-methyl-1H-indol-3-yl) -2-oxo-acetohydrazonoyl cyanide 2a-e A cold solution of aryldiazonium salt (10 mmol) was prepared by adding a solution of sodium nitrite (10 mmol in water) to a cold solution of the aromatic amine hy- drochloride (10 mmol) with stirring. The resulting solu- tion of the diazonium salt was added to a cold solution of compound 1a (10 mmol) in pyridine (100 mL). The reac- tion mixture was stirred at room temperature for 30 min. the solid product so formed was collected, washed with water, and crystallized from suitable solvent to afford 2a-e. (E)-N'-(4-Chlorophenyl)-2-(2-methyl-1H-indol-3-yl)- 2-oxoacetohydrazonoyl cyanide 2a: Orange crystals; m.p: 244˚C (acetonitrile); yield (85%); IR (KBr): υ = 3299 (NH), 2204 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 7.01-7.72 (m, 8H, ArH), 11.91 (s, 1H, NH), 12.02 (s, 1H, NH). MS: m/z (%) = 336 [M+], 307, 210, 158, 130. Anal. for C18H13ClN4O: calcd. C, 64.19; H, 3.89; Cl, 10.53; N, 16.64. found C, 63.88; H, 3.56; Cl, 10.20; N, 16.31. (E)-2-(2-Methyl-1H-indol-3-yl)-2-oxo-N'-p-tolylaceto- hydrazonoyl cyanide 2b: Yellow crystals; m.p: 207˚C (methanol); yield ( 87%); IR(KBr): υ = 3348 (NH), 2216 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.21 (s, 3H, CH3), 2.50 (s, 3H, in- dole-CH3), 7.04 - 7.75 (m, 8H, Ar H), 11.85 (s, 1H, NH), 11.98 (s, 1H, NH).¹³C NMR (DMSO-d6): δ = 23.92, 29.81, 120.05, 120.12, 123.0, 194.94, 125.01, 130.01, 130.42, 131.01, 136.97, 139.15, 143.0, 144.32, 149.22, 153.32, 192.56. MS: m/z (%) = 316 [M+], 287, 210, 158, 130, 91. Anal. for C19H16N4O: Calcd. C, 72.13; H, 5.1; N, 17.71. found C, 71.81; H, 4.7; N, 17.39. (E)-2-(2-Methyl-1H-indol-3-yl)-2-oxo-N'-phenyl-Acet- ohydrazonoyl cyanide 2c: Yellow crystals ; m.p: 235˚C (ethanol); yield (83%); IR (KBr): υ = 3366 (NH), 2200 (CN) cm–1; 1 H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 7.03 - 7.71 (m, 9H, ArH), 11.94 (s, 1H, NH),11.98 (s, 1H, NH).MS: m/z (%) = 302 [M+], 273, 210, 158, 130, 77. Anal. for C18H14N4O: calcd. C, 71.51; H, 4.67; N, 18.53. found C, 71.12; H, 4.41; N, 18.28. (E)-N'-(4-Methoxyphenyl)-2-(2-methyl-1H-indol-3-yl)- 2-oxoacetohydrazonoyl cyanide 2d: Yellow crystals; m.p: 194˚C (ethanol); yield (77%); IR (KBr): υ = 3265 (NH), 2201 (CN) cm–1; ¹H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 3.69 (s, 3H, OCH3), 6.82 - 7.69 (m, 8H, Ar H), 11.89 (s, 1H, NH), 11.98 (s, 1H, NH). MS: m/z (%) = 332 [M+], 158, 130. Anal. for C19H16N4O2: calcd. C, 68.66; H, 4.85; N, 16.86. found C, 68.29; H, 4.46; N, 16.38. (E)-2-(2-Methyl-1H-indol-3-yl)-N'-(4-nitrophenyl)-2- oxoacetohydrazonoyl cyanide 2e: Orange crystals; m.p: 274˚C (acetonitrile); yield (88%); IR (KBr): υ = 3266 (NH), 2218 (CN) cm-1; 1H NMR (DMSO-d6): δ = 2.52 (s, 3H, indole-CH3), 7.02 - 8.16 (m, 8H, ArH), 12.06 (s, 1H, NH), 12.39 (s, 1H, NH).MS: m/z (%) = 347 [M+], 318, 158, 130. Anal. for C18H13N5O3: calcd. C, 62.24; H, 3.77; N, 20.16. found C, 61.90; H, 3.46; N, 19.89. 3.3. General Method for Preparation of 4-amino-1-aryl-3-(2-methyl-1H-indole-3-car bonyl)-1H-pyrazole-5-carbonitrile Derivatives 4a-e To a solution of 2 (5 mmol) in triethylamine (10 mmol), chloroacetonitrile (16 mmol) was added. The reaction mixture was refluxed for 2 h, and then poured onto cold dilute HCl. The solid product formed was filtered off and crystallized from suitable solvent to afford 4a-e. 4-Amino-1-(4-chlorophenyl)-3-(2-methyl-1H-indole-3- Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. 100 carbonyl)-1H-pyrazole-5-carbonitrile 4a: Yellow crystals; m.p: 252˚C (acetic acid); yield (88%); IR (KBr ): υ = 3471 & 3353 (NH2), 3313 (NH), 2212 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, in- dole-CH3), 6.40 (s, 2H, NH2), 7.04 - 7.81 (m, 8H, ArH), 12.02 (s, 1H, NH). MS: m/z (%) = 375 [M+], 360, 158, 130. Anal. for C20H14ClN5O: calcd. C, 63.92; H, 3.75; Cl, 9.43; N, 18.64. found C, 63.51; H, 3.50; Cl, 9.03; N,18.22. 4-Amino-3-(2-meth yl-1H-indole-3-carbonyl)-1-p-tolyl- 1H-pyrazole-5-carbonitrile 4b: Brown crystals; m.p: 188˚C (methanol); yield (91%); IR (KBr): υ = 3473 & 3356 (NH2), 3312 (NH), 2212 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.21 (s, 3H, CH3), 2.50 (s, 3H, indole-CH3), 6.41 (s, 2H, NH2), 7.05 - 7.79 (m, 8H, ArH), 11.98 (s, 1H, NH). MS: m/z (%) = 355 [M+], 340, 225, 158, 130, 91. Anal. for C21H17N5O: calcd. C, 70.97; H, 4.82; N, 19.71. found C, 70.55; H, 4.61; N, 19.33. 4-Amino-3-(2-methyl-1H-indole- 3-carbonyl)-1-phenyl- 1H-pyrazole-5-carbonitrile 4c: Brown crystals; m.p: 204˚C (ethanol); yield (87%); IR (KBr): υ = 3470 & 3358 (NH2), 3311 (NH), 2213 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 6.42 (s, 2H, NH2), 7.03 - 7.81 (m, 9H, ArH) , 12.0 (s, 1H, NH). MS: m/z (%) = 341 [M+], 326, 158, 130, 77. Anal. for C20H15N5O: calcd. C, 70.37; H, 4.43; N, 20.52. found C, 69.98; H, 4.11; N, 20.13. 4-Amino-1-(4-methoxyphenyl)-3-(2-methyl-1H-indole- 3-carbonyl)-1H-pyrazole-5-carbonitrile 4d: Brown crystals; m.p: 214˚C (acetonitrile); yield (89%); IR (KBr): υ = 3471 & 3358 (NH2), 3313 (NH), 2213 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 3.82 (s, 3H, OCH3), 6.40 (s, 2H, NH2), 7.10 - 7.83 (m, 8H, Ar H), 11.95 (s, 1H, NH). MS: m/z (%) = 371 [M+], 356, 158, 130, 77. Anal. for C21H17N5O2: calcd. C, 67.91; H, 4.61; N, 18.86. found C, 67.50; H, 4.33; N, 18.43. 4-Amino-3-(2-methyl-1H- indole-3-carbonyl)-1-(4- nitrophenyl)-1H-pyrazole-5-carbonitrile 4e: Brown crystals; m.p: 289˚C (acetonitrile); yield (88%); IR (KBr): υ = 3472 & 3355 (NH2), 3311 (NH), 2217 (CN) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 6.54 (s, 2H, NH2), 7.08 - 8.46 (m, 8H, ArH), 12.06 (s, 1H, NH). MS: m/z (%) = 386 [M+], 371, 158, 130, 77. Anal. for C20H14N6O3: calcd. C, 62.17; H, 3.65; N, 21.75. found C, 61.80; H, 3.41; N, 21.33. 3.4. General Method for Preparation of (Z)-4-(2-Aryl-hydrazono)-5-(2-methyl-1H-in dol-3-yl)-4H-pyrazol-3-amine derivatives 5a-e To an appropriate compounds 2 (10 mmol) hydrazine hydrate (10 mmol) was added in ethanol (20 mL). The reaction mixture was refluxed for 6 h, the solvent was evaporated and the crude product was collected then crystallized from benzene to afford the corresponding compounds 5a-e. (Z)-4-(2-(4-Chlorophenyl)hydrazono)-5-(2-methyl- 1H-indol-3-yl)-4H-p yrazol-3-amine 5a: Orange crystals; m.p: 232˚C; yield (87%); IR (KBr): υ = 3473 & 3454 (NH2), 3356 (NH) cm–1; 1H NMR (DMSO-d6): δ = 2.49 (s, 3H, indole-CH3), 6.19 (s, 2H, NH2), 7.02 - 7.64 (m, 8H, ArH), 11.48 (s, 1H, NH), 12.30 (s, 1H, NH). MS: m/z (%) = 350 [M+], 335, 224. Anal.for C18H15ClN6: calcd. C, 61.63; H, 4.31; Cl, 10.11; N, 23.96. found C, 61.29; H, 4.10; Cl, 9.75; N, 23.48. (Z)-5-(2-Methyl-1H-indol-3-yl)-4-(2-p-tolylhydra-zono )-4H-pyrazol-3-amine 5b: Yellow crystals; m.p: 235˚C; yield (89%); IR (KBr): υ = 3468 & 3450 (NH2), 3355 (NH) cm–1; 1H NMR (DMSO-d6): δ = 2.29 (s, 3H, CH3), 2.49 (s, 3H, in- dole-CH3), 6.15 (s, 2H, NH2), 7.01 - 7.53 (m, 8H, ArH), 11.45 (s, 1H, NH) ,12.28 (s, 1H, NH).MS: m/z (%) = 330 [M+], 315, 224, 91. Anal. for C19H18N6: Calcd. C, 69.07; H, 5.49; N, 25.44. found C, 68.72; H, 5.30; N, 25.01. (Z)-5-(2-Methyl-1H-indol-3-yl)-4-(2-phenylhydrazono) -4H-pyrazo l-3-amine 5c: Yellow crystals; m.p: 202˚C; yield (85%); IR (KBr): υ = 3468 & 3450 (NH2), 3353 (NH) cm–1; 1H NMR (DMSO-d6) : δ = 2.50 (s, 3H, indole-CH3), 6.21 (s, 2H, NH2), 7.0 - 7.62 (m, 9H, ArH), 11.43 (s, 1H, NH), 12.28 (s, 1H, NH). M: m/z (%) = 316 [M+], 301, 224, 77. Anal. for C18H16N6: calcd. C, 68.34; H, 5.10; N, 26.56. found C, 67.98; H, 4.82; N, 26.13. (Z)-4-(2-(4-Methoxyphenyl)hydraono)-5-(2-methyl- 1H-indol-3-yl)-4H-pyrazo l-3-amine 5d: Yellow crystals; m.p: 180˚C; yield (80%);IR (KBr): υ = 3389 & 3187 (NH2) cm–1; 1H NMR (DMSO-d6): δ = 2.49 (s, 3H, indole-CH3), 3.76 (s, 3H, OCH3), 6.18 (s, 2H, NH2), 6.93 - 7.60 (m, 8H, ArH), 11.41 (s, 1H, NH), 12.11 (s, 1H, NH). MS: m/z (%) = 346 [M+], 331, 224, 123. Anal. for C19H18N6O: calcd. C, 65.88; H, 5.24; N, 24.26. found: C, 65.45; H, 5.01; N, 23.86. (Z)-5-(2-Methyl-1H-indol-3-yl)-4-(2-(4-nitrophenyl) hydrazono)-4H-pyrazo l-3-amine 5e: Red crystals; m.p: 285˚C; yield (81%); IR (KBr): υ = 3467 & 3399 (NH2), 3349 (NH) cm–1; 1H NMR (DMSO-d6): δ = 2.50 (s, 3H, indole-CH3), 6.50 (s, 2H, NH2), 7.0 - 8.26 (m, 8H, ArH), 11.30 (s, 1H, NH), 12.18 (s, 1H, NH). MS: m /z (%) = 361 [M+], 346, 224. Anal. for C18H15N7O2: calcd. C, 59.83; H, 4.18; N, 27.13. found C, 59.44; H, 4.01; N, 26.87. Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. 101 3.5. General Method for Preparation of (Z)-3mercapto-2-(2-phenyl-1H-indole-3-car bonyl)-3-(phenylamino) Acrylonitrile 7. To a stirred solution of potassium hydroxide (0.56 g, 10 mmol) in dimethylformamide (50 mL), 3-cyan-oace- tyl-2-phenylindole 1b (2.6 g, 10 mmol) was added. After stirring for 30 min, phenyl isothiocyanate (1.4 g, 10 mmol) was added, and stirring was continued for further 6 h. The mixture was then poured over crushed ice con- taining hydrochloric acid. The solid product was filtered off, washed with water, dried and finally recrystallized from acetonitrile to afford 7. (Z)-3-Mercapto-2-(2-phenyl-1H-indole-3-carbonyl)-3- (phenylamino)acrylonitrile 7: Yellow crystals; m.p. 186˚C; yield (78%). IR (KBr): υ = 3343 (NH), 2202 (CN), 1676 (CO) cm–1. MS: m/z (%) = 395 [M+]. Anal. for C24H17N3OS: calcd. C, 72.89; H, 4.33; N, 10.63; S, 8.11.found C, 72.45; H, 4.10; N, 10.47; S, 7.87. 3.6. General Procedure for the Preparation of 2-(3,5-Diaryl-1,3,4-thiadiazol-2-ylidene)-3-(2 -phenyl-1H-indole-3-yl)-3-oxo-propionitriles 9a-r . To a solution of the thioacetanilide 7 (0.79 g, 2 mmol) in absolute ethanol (20 mL), hydrazonoyl halides 8 (2 mmol) was added. To the resulting mixture triethylamine (0.3 mL) was added, and the reaction mixture was re- fluxed for 3 h and then cooled. The solid product was filtered off, washed with ethanol and crystallized from suitable solvent to afford the corresponding thiadiazole derivatives 9a-r. (Z)-2-(3,5-diphenyl-1,3,4-thiadiazol-2(3H)-ylidene)-3- oxo-3-(2-phenyl-1H-indol-3-yl)propane-nitrile 9a: Yellow crystals; m.p. 305˚C (dioxane); yield (79%). IR (KBr): υ = 3277 (NH), 2199 (CN) cm–1. MS: m/z (%) = 496 [M+]. Anal. for C31H20N4OS: calcd. C, 74.98; H, 4.06; N, 11.28; S, 6.46.found C, 74.49; H, 3.89; N, 10.92; S, 6.17. (Z)-3-Oxo-3-(2-phenyl-1H-indol-3-yl)-2-(3-phenyl-5-s tyryl-1,3,4-thiadiazol-2(3H)-ylidene)prop-anenitrile 9b: Yellow crystals; m.p. 299˚C (acetonitrile); yield (73%). IR (KBr): υ = 3327 (NH), 2195 (CN) cm–1. 1H NMR (CDCl3): δ = 7.18 - 7.9 (m, 21H, ArH), 8.8 (s, 1H, NH). MS: m/z (%) = 522 [M+]. Anal. for C33H22N4OS: calcd. C, 75.84; H, 4.24; N, 10.72; S, 6.14.found C, 75.40; H, 4.01; N, 10.43; S, 5.86. (Z)-2-(3-(4-Nitrophenyl)-5-(thiophen-2-yl)-1,3,4-thiad ia-zol-2(3H)-ylidene)-3-oxo-3-(2-phenyl-1H-indol-3-yl) propanenitrile 9c: Yellow crystals; m.p. 361˚C (dioxane); yield (80%). IR (KBr): υ = 3340 (NH), 2203 (CN) cm–1. MS: m/z (%) = 547 [M+]. Anal. for C29H17N5O3S2: calcd. C, 63.61; H, 3.13; N, 12.79 S, 11.71.found C, 63.19; H, 3.01; N, 12.45; S, 11.35. (Z)-2-(5-(Furan-2-yl)-3-(4-nitrophenyl)-1,3,4-thiadiaz ol-2(3H)-ylidene)-3-oxo-3-(2-phenyl-1H-indol-3-yl) propanenitrile 9d: Yellow crystals; m.p. 328˚C (acetonitrile); yield (78%). IR (KBr): υ = 3345 (NH), 2203 (CN) cm–1. MS: m/z (%) = 531 [M+].Anal. for C29H17N5O4S: calcd. C, 65.53; H, 3.22; N, 13.18; S, 6.03. found: C, 65.11; H, 3.0; N, 12.84; S, 5.73. (Z)-2-(3-(4-Nitrophenyl)-5-phenyl-1,3,4-thiadiazol-2(3 H)-ylidene)-3-oxo-3-(2-phenyl-1H-indol-3-yl)propane- nitrile 9e: Yellow crystals; m.p.310˚C (dioxane); yield (81%). IR (KBr): υ = 3336 (NH), 2200 (CN) cm–1. MS: m/z (%) = 541 [M+]. Anal. for C31H19N5O3S: calcd. C, 68.75; H, 3.54; N, 12.93; S, 5.92. found: C, 68.24; H, 3.31; N, 12.61; S, 5.70. (Z)-2-(5-Acetyl-3-phenyl-1,3,4-thiadizol-2(3H)-ylidene) -3-oxo-3-(2-phenyl-1H-indol-3-yl)propanenitrile 9f: Yellow crystals; m.p. 311˚C (acetonitrile); yield (76%). IR (KBr): υ = 3300 (NH), 2194 (CN), 1693 (CO) cm–1.1H NMR (CDCl3): δ = 2.67 (s, 3H, indole-CH3), 7.19 - 8.21 (m, 14H, Ar H), 8.56 (s, 1H, NH). MS: m/z (%) = 462 [M+]. Anal. for C27H18N4O2S: calcd. C, 70.11; H, 3.92; N, 12.11; S, 6.93. found C, 69.51; H, 3.73; N, 11.81; S, 6.70. (Z)-Methyl-5-(1-cyano-2-oxo-2-(2-phenyl-1H-indol-3- yl) ethylidene)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2- carboxylate 9g: Yellow crystals; m.p.247˚C (acetonitrile); yield (73%). IR (KBr): υ = 3322 (NH), 2200 (CN), 1752 (CO), 1725 (CO) cm–1. 1H NMR (CDCl3): δ = 4.05 (s, 3H, es- ter-CH3), 7.19 - 7.90 (m, 14H, Ar H), 8.57 (s, 1H, NH). MS: m/z (%) = 478 [M+]. Anal. for C27H18N4O3S: calcd. C, 67.77; H, 3.79; N, 11.71; S, 6.70.found C, 67.40; H, 3.58; N, 11.43; S, 6.49. (Z)-Ethyl-5-(1-cyano-2-oxo-2-(2-phenyl-1H-indol-3-yl) ethylidene)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-car- boxylate 9h: Yellow crystals; m.p.272˚C (acetonitrile); yield (74%). IR (KBr): υ = 3309 (NH), 2202 (CN), 1743 (CO), 1712 (CO) cm–1. 1H NMR (DMSO-d6): δ = 1.36 (t, 3H, es- ter-CH3), 4.46 (q, 2H, ester-CH2), 7.09 - 7.63 (m, 14H, Ar H), 11.94 (s, 1H, NH). MS: m/z (%) = 492 [M+]. Anal. for C28H20N4O3S: calcd. C, 68.28; H, 4.09; N, 11.37; S, 6.51.found C, 67.91; H, 3.92; N, 11.02; S, 6.25. (Z)-2-(5-Benzoyl-3-phenyl-1,3,4-thiadiazol-2(3H)-ylid ene)-3-oxo-3-(2-phenyl-1H-indol-3-yl)-propanenitrile 9i: Yellow crystals; m.p. 282˚C (dioxane); yield (78%). Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. 102 IR (KBr): υ = 3316 (NH), 2202 (CN), 1650 (CO) cm–1. MS: m/z (%) = 524 [M+]. Anal. for C32H20N4O2S: calcd. C, 73.27; H, 3.84; N, 10.68; S, 6.11. found C, 72.92; H, 3.61; N, 10.29; S, 5.89. (Z)-3-Oxo-3-(2-phenyl-1H-indol-3-yl)-2-(3-phenyl-5- (thiophene-2-carbonyl)-1,3,4-thiadiazol-2(3H)-ylidene) propanenitrile 9j: Yellow crystals; m.p. 303˚C (acetonitrile); yield (72%). IR (KBr): υ = 3264 (NH), 2199 (CN), 1632 (CO) cm–1. MS: m/z (%) = 530 [M+]. Anal. for C30H18N4O2S2: calcd. C, 67.91; H, 3.42; N, 10.56, S, 12.09. found C, 67.48; H, 3.21; N, 10.28; S, 11.81. (Z)-2-(5-Acetyl-3-p-tolyl-1,3,4-thiadiazol-2(3H)- ylidene)-3-oxo-3-(2-phenyl-1H-indol-3-yl)propanenitrile 9k: Yellow crystals; m.p. 327˚C (dioxane); yield (78%). IR (KBr): υ = 3304 (NH), 2195 (CN), 1693 (CO) cm–1. 1H NMR (DMSO-d6): δ = 2.35 (s, 3H, CH3), 2.61 (s, 3H, CH3), 7.12-7.63 (m, 13H, ArH), 11.9 (s, 1H, NH).¹³C NMR (DMSO-d6): δ = 20.79, 25.91, 66.26, 79.28, 111.45, 114.70, 119.68, 120.51, 122.32, 126.58, 127.17, 128.31, 128.51, 129.38, 131.80, 135.55, 139.90, 140.25, 155.68, 164.65, 184.27, 190.40. MS: m/z (%) = 476 [M+]. Anal. for C28H20N4O2S: calcd. C, 70.57; H, 4.23; N, 11.76; S, 6.73.found C, 70.09; H, 4.02; N, 11.47; S, 6.49. (Z)-Methyl5-(1-cyano-2-oxo-2-(2-phenyl-1H-indol-3-yl) ethylidene-4-p-tolyl-4,5-dihydro-1,3,4-thiadiazole-2- carboxylate 9l: Yellow crystals; m.p. 270˚C (dioxane); yield (82%). IR (KBr): υ = 3342 (NH), 2199 (CN), 1752 (CO), 1726 (CO) cm–1. MS: m/z (%) = 492 [M+]. Anal. for C28H20N4O3S: calcd. C, 68.28; H, 4.24; N, 11.37; S, 6.51.found C, 67.93; H, 4.01; N, 11.03; S, 6.28. (Z)-Ethyl-5-(1-cyano-2-oxo-2-(2-phenyl-1H-indol-3-yl) ethylidene-4-p-tolyl-4,5-dihydro-1,3,4-thiadiazole-2- carboxylate 9m: Yellow crystals; m.p. 280˚C (acetonitrile); yield (73%). IR (KBr): υ = 3317 (NH), 2206 (CN), 1752 (CO), 1720 (CO) cm–1.1H NMR (DMSO-d6): δ = 1.35 (t, 3H, es- ter-CH3), 2.34 (s, 3H, CH3), 4.45 (q, 2H, ester-CH2), 7.09 - 7.62 (m, 13H, Ar H), 11.93 (s, 1H, NH). MS: m/z (%) = 506 [M+]. Anal.for C29H22N4O3S: calcd. C, 68.76; H, 4.38; N, 11.06; S, 6.33.found C, 68.33; H, 4.15; N, 10.31; S, 6.14. (Z)-5-(1-Cyano-2-oxo-2-(2-phenyl-1H-indo l-3-yl)ethyl idene)-N-phenyl-4-p-tolyl-4,5-dihydro-1,3,4-thiadiazole- 2-carboxamide 9n: Yellow crystals; m.p. 298˚C (dioxane); yield (81%). IR (KBr): υ =3276 (NH), 2207 (CN), 1667 (CO) cm–1.1H NMR (DMSO-d6): δ = 2.36 (s, 3H, CH3), 7.24 - 7.82 (m, 18H, Ar H), 10.92 (s, 1H, NH), 11.87 (s, 1H, NH).13C NMR (DMSO-d6): δ = 20.79, 66.24, 78.92, 111.53, 114.91, 119.72, 120.41, 120.65, 120.93, 122.23, 124.73, 126.99, 127.19, 128.19, 128.49, 128.60, 129.23, 131.82, 135.53, 137.28, 139.63, 140.24, 153.82, 155.96, 164.63, 184.18. MS: m/z (%) = 553 [M+]. Anal. for C33H23N5O2S: calcd. C, 71.59; H, 4.19; N, 12.65; S, 5.79.found C, 71.12; H, 3.98; N, 12.30; S, 5.61. (Z)-2-(5-Acetyl-3-(4-chlorophenyl)-1,3,4-thiadiazol-2(3H )-ylidene)-3-oxo-3-(2-phenyl-1H-indol-3-yl)propanenitrile 9o: Yellow crystals; m.p. 347˚C (dioxane); yield (81%). IR (KBr): υ = 3312 (NH), 2194 (CN), 1692 (CO) cm–1. MS: m/z (%) = 496 [M+]. Anal. for C27H17ClN4O2S: calcd. C, 65.25; H, 3.45; Cl, 7.13; N, 11.27; S, 6.45. found C, 64.76; H, 3.22; Cl, 6.85; N, 10.88; S, 6.21. (Z)-Methyl-4-(4-chlorophenyl)-5-(1-cyano-2-(2-oxo-2- (2-phenyl-1H-indol-3-yl)ethylidene)-4,5-dihydro-1,3,4- thiadiazole-2-carboxylat 9p: Yellow crystals; m.p.260˚C (dioxane); yield (80%). IR (KBr): υ =3350 (NH), 2197(CN), 1757(CO) cm–1. 1 H NMR (DMSO-d6): δ = 3.98 (s, 3H, ester-CH3), 7.11 - 7.68 (m, 13H, Ar H), 11.99 (s, 1H, NH).¹³C NMR (DMSO-d6): δ = 53.84, 66.32, 79.15, 111.14, 111.70, 114.91, 119.82, 120.72, 122.49, 127.25, 128.43, 128.49, 128.67, 128.87, 129.09, 131.85, 135.14, 135.63, 136.82, 140.37, 149.24, 158.33, 164.59, 183.98. MS: m/z (%) = 512 [M+]. Anal.for C27H17ClN4O3S: calcd. C, 63.22; H, 3.34; Cl, 6.91; N, 10.92; S, 6.25.found: C, 62.81; H, 3.20; Cl, 6.65; N, 10.59; S, 6.01. (Z)-Ethyl4-(4-chlorophenyl)-5-(1-cyano-2-(2-oxo-2-(2- phenyl-1H-indol-3-yl)ethylidene)-4,5-dihydro-1,3,4- thiadiazole-2-carboxylate 9q: Yellow crystals; m.p. 266˚C (acetonitrile); yield (79%). IR (KBr): υ = 3302 (NH), 2202 (CN), 1758 (CO), 1720 (CO) cm–1. MS: m/z (%) = 526 [M+]. Anal. for C28H19ClN4O3S: calcd. C, 63.81; H, 3.63; Cl, 6.73; N, 10.63; S, 6.08.found C, 63.39; H, 3.37; Cl, 6.45; N, 10.30; S, 5.81. (Z)-4-(4-Chlorophenyl)-5-(1-cyano-2-(2-oxo-2-(2- phenyl-1H-indol-3-yl)ethylidene)-N-phenyl-4,5-dihydro- 1,3,4-thiadiazole-2-carboxamide 9r: Yellow crystals; m.p. 328˚C (dioxane); yield (83%). IR (KBr): υ = 3289 (NH), 2205 (CN), 1664 (CO) cm–1. MS: m/z (%) = 573 [M+]. Anal. for C32H20ClN5O2S: calcd. C, 66.95; H, 3.51; Cl, 6.18; N, 12.20; S, 5.59. found C, 66.47; H, 3.28; Cl, 5.92; N, 11.78; S, 5.24. 4. References [1] W. J. Houlihan, W. A. Remers and R. K. Brown, Wiley, New York, 1992. [2] R. J. Sundberg, “The Chemistry of Indoles: Part I,” Aca- demic Press, New York, 1996. [3] A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, “New Bisindole Alkaloids of the Topsentin and Hamacanthin Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. 103 Classes from the Mediterranean Marine Sponge Rhaphi- sia Lacazei,” Journal of Natural Products, Vol. 63, No. 4, 2000, pp. 447-451. doi:10.1021/np9903292 [4] B. Bao, Q. Sun, X. Yao, J. Hong, C. O. Lee, C. J. Sim, K. S. Im and J. H. Jung, “Bisindole Alkaloids from a Marine Sponge Spongosorites sp,” Journal of Natural Products, Vol. 68, No. 5, 2005, pp. 711-715. doi:10.1021/np049577a [5] T. Kouko, K. Matsumura and T. Kawasaki, “Total Syn- thesis of Marine Bisindole Alkaloids, (+)-Hamacanthins A, B and (−)-Antipode of Cis-Dihydrohamacanthin B,” Tetrahedron, Vol. 61, 2005, pp. 2309-2318. [6] K. Kaniwa, M. A. Arai, X. Li and M. Ishibashi, “Synthe- sis, Determination of Stereochemistry, and Evaluation of New Bisindole Alkaloids from the Myxomycete Arcyria ferruginea: An Approach for Wnt Signal Inhibitor,” Bio- organic & Medicinal Chemistry Letters, Vol. 17, No. 5, 2007, pp. 4254-4257. doi:10.1016/j.bmcl.2007.05.033 [7] L. H. Franco, E. B. K. Joffe, L. Puricelli, M. Tatian, A. M. Seldes and J. A. Palermo, “Indole Alkaloids from the Tunicate Aplidium Meridianum,” Journal of Natural Pro- ducts, Vol. 61, No. 9, 1998, pp. 1130-1132. doi:10.1021/np970493u [8] A. A. R. Mohamed and E.-S. Mahmound, “Synthesis and Antitumor Activity of Indolylpyrimidines: Marine Natu- ral Product Meridianin D Analogue,” Bioorganic & Me- dicinal Chemistry Letters, Vol. 15, No. 3, 2007, pp. 1206- 1217. doi:10.1016/j.bmc.2006.11.023 [9] B. Jiang, C. G. Yang, W. N. Xiong and J. Wang, “Syn- thesis and Cytotoxicity Evaluation of Novel Indo- lylpyrimidines and Indolylpyrazines as Potential Antitu- mor Agents,” Bioorganic & Medicinal Chemistry Letters, Vol. 9, No. 5, 2001, pp. 1149-1154. doi:10.1016/S0968-0896(00)00337-0 [10] M. Gompel, M. Leost, E. B. K. Joffe, L. Puricelli, L. H. Franco, J. Palermo and L. Meijer, “Meridianins, a New Family of Protein Kinase Inhibitors Isolated from the As- cidian Aplidium meridianum,” Bioorganic & Medicinal Chemistry Letters, Vol. 14, No. 7, 2004, pp. 1703-1707. doi:10.1016/j.bmcl.2004.01.050 [11] I. Kawasaki, M. Yamashita and S. Ohta, “Successive Diarylation at the Carbon Positions of 1H-Imidazole and Its Application to the Total Synthesis of Nortopsentin D,” Journal of the Chemical Society, Chemical Communica- tions, No. 18, 1994, pp. 2085-2086. doi:10.1039/c39940002085 [12] I. Kawasaki, M. Yamashita and S. Ohta, “Total Synthesis of Nortopsentins A-D, Marine Alkaloids,” Chemical and Pharmaceutical Bulletin, Vol. 44, No. 10, 1996, pp. 1831- 1839. [13] I. Kawasaki, H. Katsuma, Y. Nakayama, M. Yamashita and S. Ohta, “Total Synthesis of Topsentin, Antiviral and Antitumor Bis(indolyl)imidazole,” Heterocycles, Vol. 48, 1998, pp. 1887-1901. [14] H. H. Sun and J. Sakemi, “A Brominated (Aminoimida- zolinyl)indole from the Sponge Discodermia Polydiscus,” The Journal of Organic Chemistry, Vol. 56, No. 13, 1991, pp. 4307-4308. doi:10.1021/jo00013a045 [15] H. C. Vervoort, S. E. Richards-Gross, W. Fenical, A. Y. Lee and J. Clardy, “Didemnimides A-D: Novel, Preda- tor-Deterrent Alkaloids from the Caribbean Mangrove Ascidian Didemnum conchyliatum,” The Journal of Or- ganic Chemistry, Vol. 62, No. 5, 1997, pp. 1486-1490. doi:10.1021/jo961789s [16] S. Takahashi, T. Matsunaga, C. Hasegawa, H. Saito, D. Fujita, F. Kiuchi and Y. Tsuda, “Martefragin A, a Novel Indole Alkaloid Isolated from Red Alga, Inhibits Lipid Peroxidation,” Chemical & Pharmaceutical Bulletin, Vol. 46, No. 10, 1996, pp. 1527-1529. [17] I. N’Diaye, G. Guella, G. Chiasera, Y. Mancini and F. Pietra, “Almazole A and Almazole B, Unusual Marine Alkaloids of an Unidentified Red Seaweed of the Family Delesseriaceae from the Coasts of Senegal,” Tetrahedron Letters, Vol. 35, No. 27, 1994, pp. 4827-4830. doi:10.1016/S0040-4039(00)76979-6 [18] T. Bergmann, D. Schories and B. Steffan, “Alboinon, an Oxadiazinone Alkaloid from the Ascidian Dendrodoa grossularia,” Tetrahedron, Vol. 53, 1997, pp. 2055-2060. [19] S. Kohmoto, Y. Kashman, O. J. McConnell, K. L. Rine- hart Jr., A. Wright and F. Koehn, “Dragmacidin, a New Cytotoxic Bis(Indole) Alkaloid from a Deep Water Ma- rine Sponge, Dragmacidon sp,” The Journal of Organic Chemistry, Vol. 53, No. 13, 1988, pp. 3116-3118. doi:10.1021/jo00248a040 [20] H. M. E. Hassaneen, H. M. Hassaneen and M. H. Elnagdi, “Enamines in Heterocyclic Synthesis: A Route to 4-Subs- tituted Pyrazoles and Condensed Pyrazoles,” Z. Natur- forch., Vol. 59b, 2004, p. 1132. [21] N. M. Elwan, H. M. E. Hassaneen and H. M. Hasssaneen, “Synthesis and Reactions of Indane-1,3-Dione-2-Thiocar- boxanilides with Hydrazonoyl Halides and Active Chlo- romethylene Compounds,” Heteroatom Chemistry, Vol. 13, No. 7, 2002, pp. 585-591. doi:10.1002/hc.10132 [22] M. A. A. Radwan, E. A. Ragab, N. M. Sabry and S. M. El-Shenawy, “Synthesis and Biological Evaluation of New 3-Substituted Indole Derivatives as Potential Anti-In- flammatory and Analgesic Agents,” Bioorganic & Me- dicinal Chemistry, Vol. 15, No. 11, 2007, pp. 3832-3841. doi:10.1016/j.bmc.2007.03.024 [23] H. M. E. Hassaneen and R. M. Pagni, “Synthesis of New 3-Substituted Indole Derivatives,” Verlag der Zeitschrift Naturforschung, Vol. 65b, 2010, pp. 1491-1497. [24] J. Slatt, I. Romero and J. Bergman, “Cyanoacetylation of Indoles, Pyrroles and Aromatic Amines with the Combi- nation Cyanoacetic Acid and Acetic Anhydride,” Synthe- sis, 2004, pp. 2760-2765. [25] O. M. E. El-Dusouqui, M. M. Abdelkhalik, N. A. Al- Awadi, H. Dib, B. J. George and M. H. Elnagdi, “Chem- istry of 2-Arylhydrazonals: Utility of Substituted 2-Ary- lhydrazono-3-oxoalkanals as Precursors for 3-oxoalka- nonitriles, 3-Aminoisoxazole and 1,2,3- and 1,2,4-Tria- zoles,” Journal of Chemical Research, Vol. 2006, No. 5, 2006, pp. 295-302. [26] I. M. Kenawi and M. H. Elnagdi, “DFT and FT-IR Copyright © 2011 SciRes. IJOC  H. M. HASSANEEN ET AL. Copyright © 2011 SciRes. IJOC 104 Analyses of Hydrogen Bonding in 3-Substistuted-3-oxo- arylhydrazonopropanenitriles,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 65, No. 3-4, 2006, pp. 805-810. doi:10.1016/j.saa.2006.01.017 [27] S. K. Mallick and A. R. Martin, “Synthesis and Antim- icrobial Evaluation of Some 5-(5-Nitrofurylidene)rho- danines, 5-(5-Nitrofurylidene)thiazolidine-2,4-diones, and Their Vinylogs,” Journal of Medicinal Chemistry, Vol. 14, No. 6, 1971, pp. 528-532. doi:10.1021/jm00288a017 [28] S. R. Singh, Journal of the Indian Chemical Society, Vol. 52, 1975, pp. 734-735.
|