 Open Journal of Safety Science and Technology, 2011, 1, 31-42 doi:10.4236/ojsst.2011.12004 Published Online September 2011 (http://www.SciRP.org/journal/ojsst) Copyright © 2011 SciRes. OJSST Blast Wave Parameters for Spherical Explosives Detonation in Free Air I. Sochet1, D. Gardebas2, S. Calderara2, Y. Marchal2, B. Longuet3 1Ecole Nationale Supérieu re d’Ingénieurs de Bourges , Institut PRISME, Bourges, France 2Institut de Recherche Criminelle de la Gendarmerie Nationale (IRCGN), Division Criminalistique Physique et Chimie, Rosny-sous-Bois, France 3 Direction Générale de l’Armement (DGA) Techniques Terrestres, Bourges, France E-mail: isabelle.sochet@ensi-bourges.fr Received May 24, 2011; revised July 19, 2011; accepted July 25, 2011 Abstract Several formulations have been published to define the characteristic parameters of an incident blast wave. In almost all previous work, the charge examined has been TNT explosive and overpressure has been the main parameter examined. In this paper, we describe an investigation based on three explosives, TNT, PETN and ANFO, which has been conducted by considering three parameters: overpressure, duration and impulse of the positive blast wave phase. Calculations of the three parameters were conducted using TM5-855 through the tool CONWEP and AUTODYN. The positive overpressures were calculated using the new fo- rensic software ASIDE. The evolution of these blast wave parameters is expressed by combining the laws of two approaches: the forensic approach and the security approach. TNT equivalents are expressed in terms of pressure and impulse for the comparisons of ANFO and PETN. Keywords: ANFO, Blast Wave, Duration, Explosive, Impulse, Overpressure, PETN, TNT 1. Introduction To protect property and persons working on sites that handle, store or transport large quantities of flammable materials, it is necessary to estimate the effects of pres- sure resulting from an explosion, whether the explosion occurs at a close or a distant location. In this work, we consider explosions that are caused by pyrotechnic charges, whether intentional or accidental. Applications of pyrotechnic materials cover a variety of areas, includ- ing satellite technology, tactical and ballistic missiles, ammunitions, law enforcement, security, space launch vehicles, aerospace, automotive safety (airbags), railway signal devices and charges for the oil industry, demoli- tion mines, quarries and buildings. The objective of this work is to establish relationships to evaluate the mechanical effects of shock waves in free fields. We propose a dual approach using both forensic and security analyses. The forensic analysis uses obser- vations of damage occurring at varying distances from the blast site to estimate the pressure and deduce the corresponding mass equivalent of TNT. The security approach, on the other hand, assumes that the mass of products stored or the equivalent TNT charge is known, such that the range of the resulting pressure, and there- fore the resultant damage, must be determined. This study was conducted for three explosives: TriNi- troToluene (TNT), PEntaerythritol TetraNitrate (PETN) and Ammonium Nitrate/Fuel Oil (ANFO). TNT is a chemical compound that consists of an aromatic hydro- carbon crystal. In its refined form, TNT is relatively sta- ble and is not sensitive to shock or friction. TNT is one of the most commonly used military and industrial ex- plosives and is often used as a reference explosive. PETN is one of the most powerful explosives known. It is more sensitive to shock, friction and electrostatic dis- charge than TNT. It is mainly used as principal com- pound in some military explosives compositions (plas- trite, semtex…), in detonators. In medicine, PETN is used as a vasodilator. ANFO is a highly explosive mix- ture consisting of ammonium nitrate and diesel fuel. The diesel fuel can be replaced by kerosene, gasoline or bio- fuels, but the cost and low volatility of diesel makes it ideal. Ammonium nitrate is water-soluble and very hy-  I. SOCHET ET AL. 32 groscopic, i.e., it readily absorbs water from the air. This absorption interferes with its ability to explode, and it must therefore be stored in a dry location. The popularity of ANFO is largely based on its low cost and high stabil- ity. Because of its relative ease of manufacture, its low cost compared to other types of similar explosives and the stability of its two components, ANFO has been used in several terrorist attacks (1970, University of Wiscon- sin-Madison; 1995, Oklahoma City). of CO2, H2O and N2, respectively. The term oxygen balance (OB) represents the concentration of oxygen atoms in an oxidant and indicates its oxidation potential. It expresses the number of molecules of oxygen remain- ing after the oxidation of H, C, Mg, and Al into H2O, CO2, MgO2 and Al2O3, respectively. The oxygen balance can be expressed more directly for a conventional explo- sive by the following equation: abcd CHNO In this study, we will first explore the thermochemical data on these explosives and then analyze the effects of blast waves. 2. Thermodynamics of Explosions 1600d2ab 2 OB MWexp losive (1) where MW is the molecular weight. In this equation, the factor 1600 is the product of the molecular weight of oxygen expressed as a percentage (MW (O) × 100%). The OB provides information about the products that are formed in the reaction. A positive value indicates an excess of oxygen in the explosive, whereas a negative value indicates oxygen levels that are insufficient to ob- tain a complete oxidation reaction. If the oxygen balance is strongly negative, there is not enough oxygen to form CO2, and toxic gases such as CO are formed instead. Note that the oxygen balance provides no information about the exchange of energy during the explosion. The explosives studied here highlight the difficulty in choosing an appropriate value for detonation energy. “Heat of detonation” is defined as the heat of the reac- tion of the explosive that results in the detonation prod- ucts. This heat does not include the heat generated by secondary reactions of the explosive or its products with air. There is some confusion in terminology related to detonation energy and heat of detonation, and the two are often used interchangeably. The heat of detonation is determined using calorimetric methods in a closed chamber and does not take into account the energy available from the highly compressed gases in the prod- ucts, which can significantly contribute to the energy transmitted by a blast wave, as outlined in Scilly [1]. Therefore, the term “detonation energy” will hereafter be devoted to the calculated energy of the detonation of an explosive without considering the presence of air. De- termination of the detonation energy is therefore based on prior knowledge of the decomposition of the explo- sive, which itself depends on the oxygen balance. Therefore, we will examine the oxygen balance of each of the explosives under consideration, as well as their decomposition-based models. Calculation of the oxygen balance of TNT and PETN is relatively simple because the chemical formulas of these materials are distinctly defined. However, this is not the case for ANFO, which is a mixture of ammonium nitrate (AN) and fuel oils (FO) in a ratio of 94:6 AN:FO. Table 1 indicates that TNT has the highest oxygen deficit and that ANFO, PETN do not have sufficient oxygen to obtain complete oxidation reactions and form H2O and CO2. Hence, large amount of toxic gases like carbon monoxide will be present. In case of ANFO, the oxygen balance approaches zero. It means that the sensi- tivity, strength and brisance of ANFO tend to the maxi- mum. 2.2. Decomposition Rules 2.1. Oxygen Balance To clarify the formation of decomposition products [2], a set of rules, known as the “Kistiakowsky-Wilson” rules (K-W rules), has been developed. The rules are used for explosives with moderate oxygen deficits and an oxygen balance greater than −40% and can be described as fol- The detonation of an explosive is an oxidative reaction that is based on the assumption that the available carbon, hydrogen and nitrogen are used solely for the formation Table 1. The oxygen balance of TNT, PETN and ANFO. Name Chemical Formulation MW (g·mol−1) OB (%) 2,4,6-Trinitrotoluene (TNT) C7H5N3O6 227 −74 Pentaerythritol Tetranitrate or Pentrite (PETN) C5H8N4O12 316 −10.1 C0.365H4.713N2O3 [3,4] 85.1 −1.6 Ammonium Nitrate/ Fuel Oil (ANFO) C0.336H4.656N2O3 [5] 84.7 0 Copyright © 2011 SciRes. OJSST 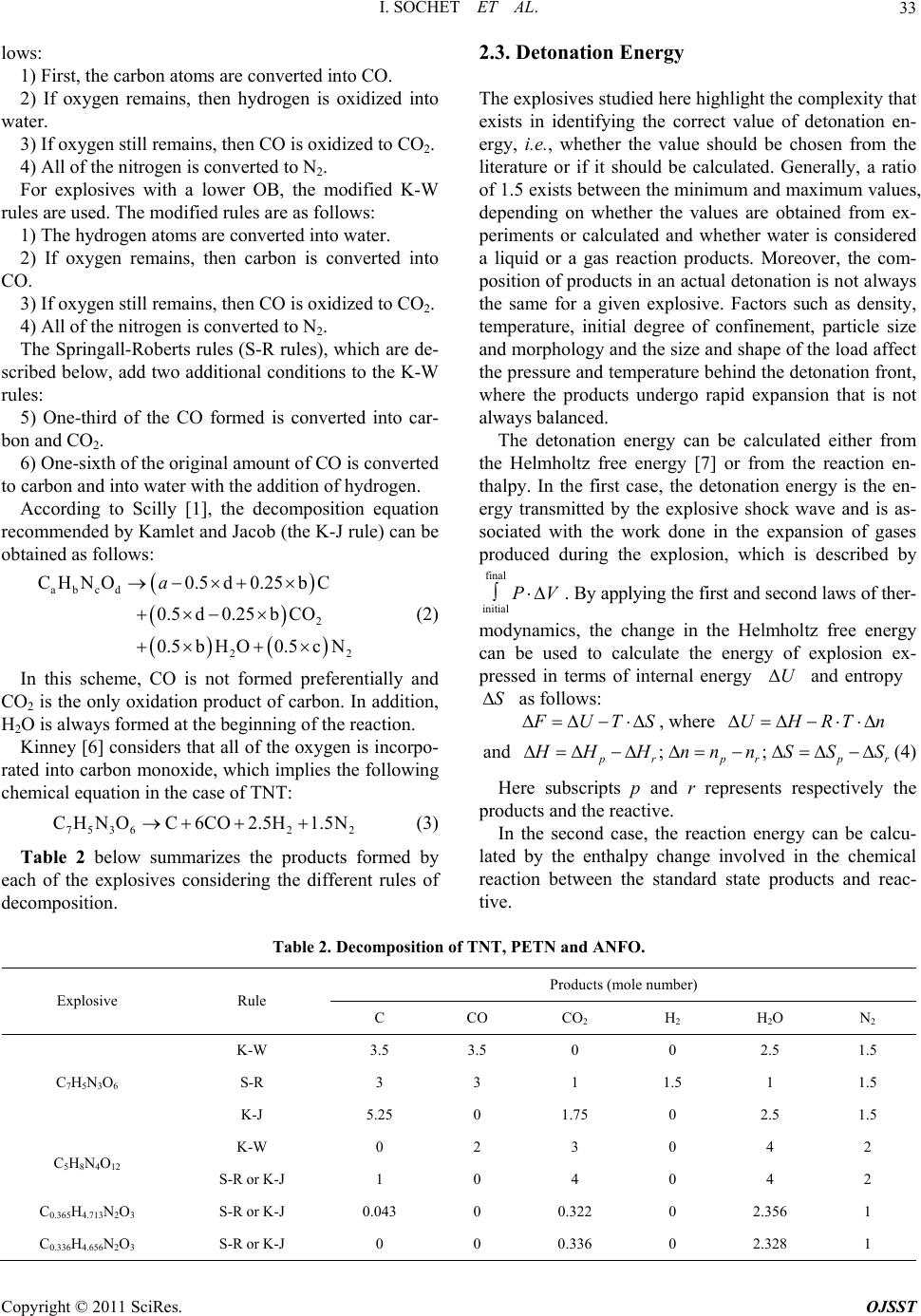 I. SOCHET ET AL. Copyright © 2011 SciRes. OJSST 33 2.3. Detonation Energy lows: 1) First, the carbon atoms are converted into CO. 2) If oxygen remains, then hydrogen is oxidized into water. The explosives studied here highlight the complexity that exists in identifying the correct value of detonation en- ergy, i.e., whether the value should be chosen from the literature or if it should be calculated. Generally, a ratio of 1.5 exists between the minimum and maximum values, depending on whether the values are obtained from ex- periments or calculated and whether water is considered a liquid or a gas reaction products. Moreover, the com- position of products in an actual detonation is not always the same for a given explosive. Factors such as density, temperature, initial degree of confinement, particle size and morphology and the size and shape of the load affect the pressure and temperature behind the detonation front, where the products undergo rapid expansion that is not always balanced. 3) If oxygen still remains, then CO is oxidized to CO2. 4) All of the nitrogen is converted to N2. For explosives with a lower OB, the modified K-W rules are used. The modified rules are as follows: 1) The hydrogen atoms are converted into water. 2) If oxygen remains, then carbon is converted into CO. 3) If oxygen still remains, then CO is oxidized to CO2. 4) All of the nitrogen is converted to N2. The Springall-Roberts rules (S-R rules), which are de- scribed below, add two additional conditions to the K-W rules: 5) One-third of the CO formed is converted into car- bon and CO2. The detonation energy can be calculated either from the Helmholtz free energy [7] or from the reaction en- thalpy. In the first case, the detonation energy is the en- ergy transmitted by the explosive shock wave and is as- sociated with the work done in the expansion of gases produced during the explosion, which is described by 6) One-sixth of the original amount of CO is converted to carbon and into water with the addition of hydrogen. According to Scilly [1], the decomposition equation recommended by Kamlet and Jacob (the K-J rule) can be obtained as follows: abcd 2 22 C HN O0.5d0.25bC 0.5d0.25bCO 0.5bHO0.5cN a (2) final initial PV . By applying the first and second laws of ther- modynamics, the change in the Helmholtz free energy can be used to calculate the energy of explosion ex- pressed in terms of internal energy and entropy U S as follows: In this scheme, CO is not formed preferentially and CO2 is the only oxidation product of carbon. In addition, H2O is always formed at the beginning of the reaction. Kinney [6] considers that all of the oxygen is incorpo- rated into carbon monoxide, which implies the following chemical equation in the case of TNT: 75362 2 CHNOC 6CO 2.5H1.5N (3) Table 2 below summarizes the products formed by each of the explosives considering the different rules of decomposition. UT S , where UHRTn and ; ; rpr pr HHnnnSSS (4) Here subscripts p and r represents respectively the products and the reactive. In the second case, the reaction energy can be calcu- lated by the enthalpy change involved in the chemical reaction between the standard state products and reac- tive. Table 2. Decomposition of TNT, PETN and ANFO. Products (mole number) Explosive Rule C CO CO2 H 2 H 2O N2 K-W 3.5 3.5 0 0 2.5 1.5 S-R 3 3 1 1.5 1 1.5 C7H5N3O6 K-J 5.25 0 1.75 0 2.5 1.5 K-W 0 2 3 0 4 2 C5H8N4O12 S-R or K-J 1 0 4 0 4 2 C0.365H4.713N2O3 S-R or K-J 0.043 0 0.322 0 2.356 1 C0.336H4.656N2O3 S-R or K-J 0 0 0.336 0 2.328 1  I. SOCHET ET AL. Copyright © 2011 SciRes. OJSST 34 Thus, calculation of the detonation energy was con- ducted for different patterns of decomposition in TNT and PETN by considering 1) water molecules in the gaseous and liquid states, and 2) the data available in the literature for enthalpies and entropies of formation of explosives and detonation products. Only the enthalpies and entropies of formation for TNT and PETN are re- ported in Table 3 below. The results of the calculations for each configuration show that the detonation energy calculated using the free energy is higher than that obtained using the reaction enthalpy regardless of the explosive studied, and both of these calculations are higher than the average value in the literature. Moreover, the energies are greater if water is considered in the liquid phase. However, regardless of the enthalpy of formation selected, the results are always in the same energy range for a given decomposition rule. Tongchang et al. [8] have conducted experimental de- terminations for TNT using a calorimeter in which the cylindrical bomb had an internal volume of 5 L and could support a pressure of 200 MPa. The experiments were performed on maximum loads of 50 g. The explo- sive force was measured as a function of the nature of the cartridge (porcelain, brass) and its thickness. All tests resulted in a value between −4.31 MJ·kg−1 and −4.40 MJ·kg−1 Finally, it should be emphasized that these val- ues correlate with the values used by Gelfand (−4.517 MJ·kg−1) [9], Baker (−4.520 MJ·kg−1) [10], Pförtner (−4.686 MJ·kg−1) [11] and Lannoy (−4.690 MJ·kg−1) [3], in addition to the value proposed by Trelat [12] (−4.600 MJ·kg−1) that was obtained using the average of the Baker [10] and Lannoy [3] results. Other detonation en- ergy values are quoted by Filler [4], including the energy calculated in the Encyclopedia of Chemical Technology (−3.87 MJ·kg−1) [4], the measurement results from To- negutti [4] that assume a 2 g charge with a conventional calorimeter (detonation energy −3.21 MJ·kg−1) and the measurements by the Armaments Research Establish- ment for a load of 100 g (−4.535 MJ·kg−1). Omang et al. [13] used a detonation energy value of −4.26 MJ kg−1 to characterize the propagation of shock waves following detonation of a spherical and hemispherical charge of 1 kg TNT. Thus, all energies of detonation reported here range from a minimum value of −3.21 MJ·kg−1 [4] to a maximum value of −4.832 MJ·kg−1 [14]. The average value of the detonation energy in the literature is −4.4 MJ·kg−1 In the case of TNT, the detonation enthalpy of de- composition calculated by Kinney [6] underestimated the energy by approximately 40%, whereas the decomposi- tion calculated by K-J rule [1] overestimated the energies by a factor of 1.3 compared to the average value of −4.4 MJ·kg−1. Using calculations based on liquid water, the S-R rule led to deviations from the mean value between 1.4% and 5.5%. The difference of 1.4% is obtained by taking the enthalpy of formation given by Akhavan [2]. The modified K-W rules show differences on the order of 3.2% to 7.6%. Greater differences are obtained by considering water as a gas in the calculations. Finally, for a reference enthalpy, the range of differences between calculations based on the Gibbs free enthalpy and the enthalpy of the reaction varies by a factor of 2 between the lower and upper bounds. In the case of PETN, considering water as gas, the en- ergy values ranged from −5.667 MJ·kg−1 [1] to −6.359 MJ·kg−1 [15], resulting in an average of −6.06 MJ·kg−1 (Table 4). Ornellas [16] reported a significantly higher value that was not taken into account in calculating the average. Scilly [1] obtained experimentally a detonation energy value of −5.73 MJ·kg−1. The S-R (or K-J) rules applied to the calculation of free energies correlates very well (<1%) with this value for the enthalpies of forma- tion reported in previous work [17-19]. For other data [2,6,14], the differences range from 1.4% to 3%. The differences in energy values calculated from the enthal- pies of reaction are of the order of 3% to 7%. However, Table 3. Enthalpy of formation and entropy for TNT and PETN. TNT-H (kJ·mol−1) −26.00 [2]; −41.13 [20]; −54.40 [6]; −54.49 [21]; −59.47 [17] TNT-S (J·mol−1·K−1) 272.00 [6]; 271.96 [21]; 554.00 [20] PETN-H (kJ·mol−1) −477.05 [2]; −502.66 [14]; −514.63 [6]; −532.06 [17]; −538.50 [18,22,23] PETN-S (J·mol−1·K−1) 129.36 [18] Table 4. Detonation energy of PETN. Detonation Energy (MJ·kg−1) Experimental Value Theoretical Value H2O Gas −5.73 [1]; −8.137 [16] −5.949 [17]; −6.359 [15]; −6.276 [24]; −5.9 [14]; −5.8 [9]; −5.667 [1] H2O Liquid −6.234 [1]; −6.322 [17]; −6.19, −6.24, −6.30 [8] −6.404 [17]; −6.347[22]; −5.792 [2] 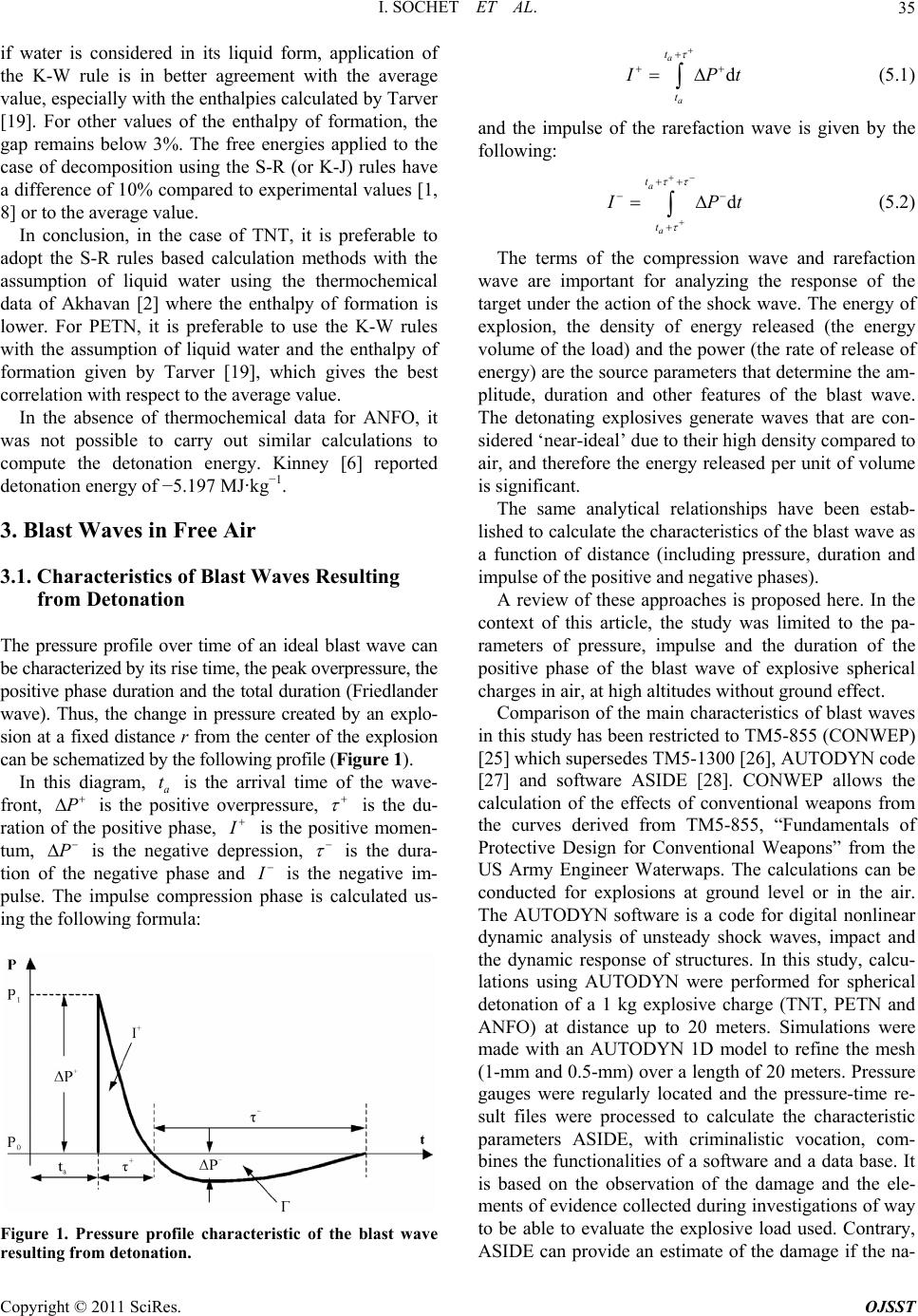 I. SOCHET ET AL. Copyright © 2011 SciRes. OJSST 35 if water is considered in its liquid form, application of the K-W rule is in better agreement with the average value, especially with the enthalpies calculated by Tarver [19]. For other values of the enthalpy of formation, the gap remains below 3%. The free energies applied to the case of decomposition using the S-R (or K-J) rules have a difference of 10% compared to experimental values [1, 8] or to the average value. In conclusion, in the case of TNT, it is preferable to adopt the S-R rules based calculation methods with the assumption of liquid water using the thermochemical data of Akhavan [2] where the enthalpy of formation is lower. For PETN, it is preferable to use the K-W rules with the assumption of liquid water and the enthalpy of formation given by Tarver [19], which gives the best correlation with respect to the average value. In the absence of thermochemical data for ANFO, it was not possible to carry out similar calculations to compute the detonation energy. Kinney [6] reported detonation energy of −5.197 MJ·kg−1. 3. Blast Waves in Free Air 3.1. Characteristics of Blast Waves Resulting from Detonation The pressure profile over time of an ideal blast wave can be characterized by its rise time, the peak overpressure, the positive phase duration and the total duration (Friedlander wave). Thus, the change in pressure created by an explo- sion at a fixed distance r from the center of the explosion can be schematized by the following profile (Figure 1). d a a t t Pt (5.1) and the impulse of the rarefaction wave is given by the following: d a a t t Pt (5.2) The terms of the compression wave and rarefaction wave are important for analyzing the response of the target under the action of the shock wave. The energy of explosion, the density of energy released (the energy volume of the load) and the power (the rate of release of energy) are the source parameters that determine the am- plitude, duration and other features of the blast wave. The detonating explosives generate waves that are con- sidered ‘near-ideal’ due to their high density compared to air, and therefore the energy released per unit of volume is significant. The same analytical relationships have been estab- lished to calculate the characteristics of the blast wave as a function of distance (including pressure, duration and impulse of the positive and negative phases). A review of these approaches is proposed here. In the context of this article, the study was limited to the pa- rameters of pressure, impulse and the duration of the positive phase of the blast wave of explosive spherical charges in air, at high altitudes without ground effect. Comparison of the main characteristics of blast waves in this study has been restricted to TM5-855 (CONWEP) [25] which supersedes TM5-1300 [26], AUTODYN code [27] and software ASIDE [28]. CONWEP allows the calculation of the effects of conventional weapons from the curves derived from TM5-855, “Fundamentals of Protective Design for Conventional Weapons” from the US Army Engineer Waterwaps. The calculations can be conducted for explosions at ground level or in the air. The AUTODYN software is a code for digital nonlinear dynamic analysis of unsteady shock waves, impact and the dynamic response of structures. In this study, calcu- lations using AUTODYN were performed for spherical detonation of a 1 kg explosive charge (TNT, PETN and ANFO) at distance up to 20 meters. Simulations were made with an AUTODYN 1D model to refine the mesh (1-mm and 0.5-mm) over a length of 20 meters. Pressure gauges were regularly located and the pressure-time re- sult files were processed to calculate the characteristic parameters ASIDE, with criminalistic vocation, com- bines the functionalities of a software and a data base. It is based on the observation of the damage and the ele- ments of evidence collected during investigations of way to be able to evaluate the explosive load used. Contrary, ASIDE can provide an estimate of the damage if the na- In this diagram, a is the arrival time of the wave- front, is the positive overpressure, t P is the du- ration of the positive phase, is the positive momen- tum, is the negative depression, P is the dura- tion of the negative phase and is the negative im- pulse. The impulse compression phase is calculated us- ing the following formula: Figure 1. Pressure profile characteristic of the blast wave resulting from detonation. 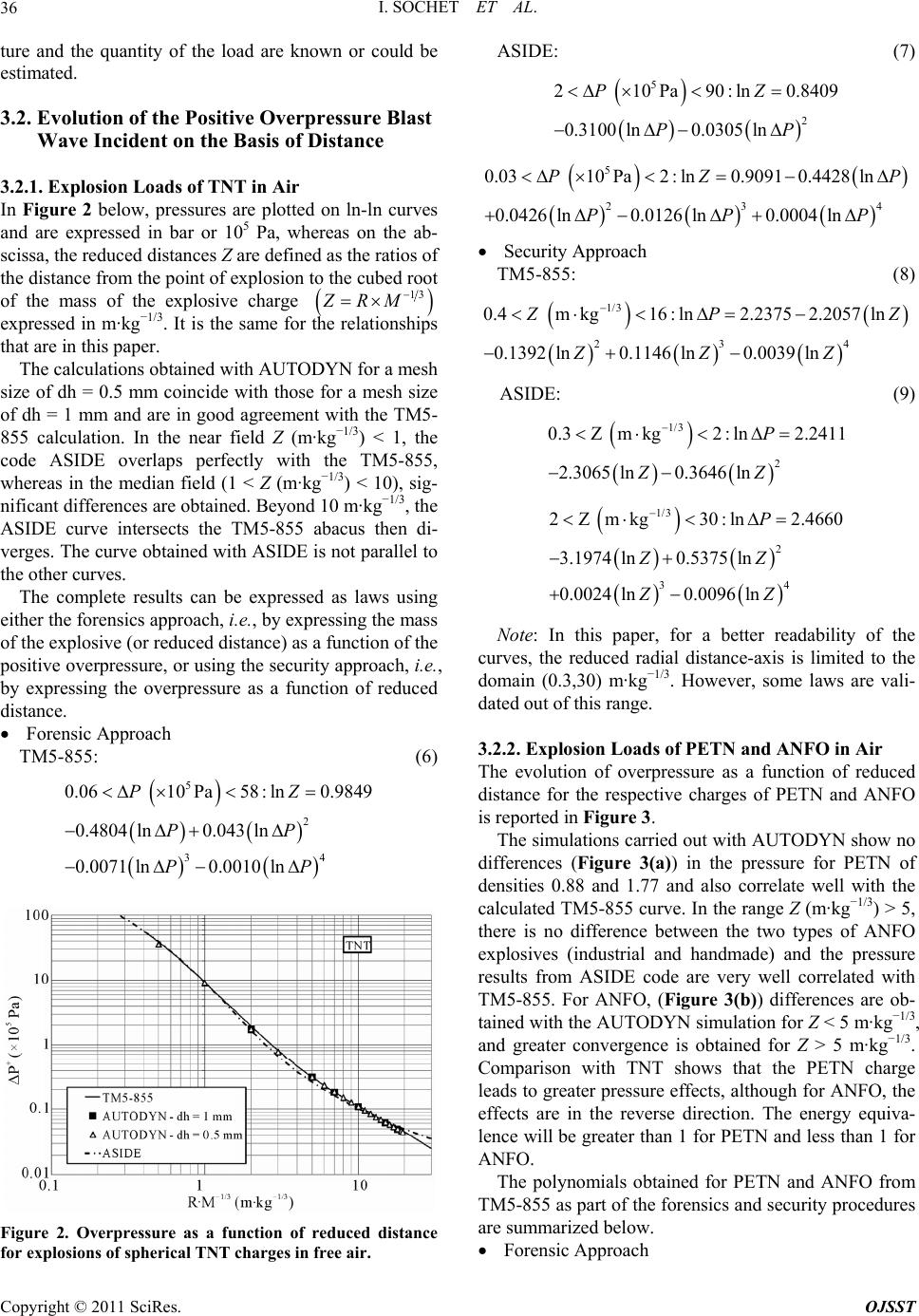 I. SOCHET ET AL. 36 ture and the quantity of the load are known or could be estimated. 3.2. Evolution of the Positive Overpressure Blast Wave Incident on the Basis of Distance 3.2.1. Explosion Loads of TNT in Air In Figure 2 below, pressures are plotted on ln-ln curves and are expressed in bar or 105 Pa, whereas on the ab- scissa, the reduced distances Z are defined as the ratios of the distance from the point of explosion to the cubed root of the mass of the explosive charge 13 ZRM expressed in m·kg−1/3. It is the same for the relationships that are in this paper. The calculations obtained with AUTODYN for a mesh size of dh = 0.5 mm coincide with those for a mesh size of dh = 1 mm and are in good agreement with the TM5- 855 calculation. In the near field Z (m·kg−1/3) < 1, the code ASIDE overlaps perfectly with the TM5-855, whereas in the median field (1 < Z (m·kg−1/3) < 10), sig- nificant differences are obtained. Beyond 10 m·kg−1/3, the ASIDE curve intersects the TM5-855 abacus then di- verges. The curve obtained with ASIDE is not parallel to the other curves. The complete results can be expressed as laws using either the forensics approach, i.e., by expressing the mass of the explosive (or reduced distance) as a function of the positive overpressure, or using the security approach, i.e., by expressing the overpressure as a function of reduced distance. Forensic Approach TM5-855: (6) ASIDE: (7) 5 2 2 10Pa90:ln0.8409 0.3100 ln0.0305 ln PZ PP 5 23 0.03 10Pa2:ln0.90910.4428ln 0.0426 ln0.0126 ln0.0004 ln PZ PP 4 P P Security Approach TM5-855: (8) 1/3 234 0.4 mkg16:ln2.23752.2057ln 0.1392 ln0.1146 ln0.0039 ln PZ ZZZ ASIDE: (9) 1/3 2 0.3Z mkg2:ln2.2411 2.3065 ln0.3646 ln P ZZ 1/3 2 34 2Z mkg30:ln2.4660 3.1974 ln0.5375 ln 0.0024 ln0.0096 ln P ZZ ZZ Note: In this paper, for a better readability of the curves, the reduced radial distance-axis is limited to the domain (0.3,30) m·kg−1/3. However, some laws are vali- dated out of this range. 3.2.2. Explosion Loads of PETN and ANFO in Air The evolution of overpressure as a function of reduced distance for the respective charges of PETN and ANFO is reported in Figure 3. 5 2 34 0.06 10Pa58:ln0.9849 0.4804ln0.043 ln 0.0071 ln0.0010ln PZ PP PP The simulations carried out with AUTODYN show no differences (Figure 3(a)) in the pressure for PETN of densities 0.88 and 1.77 and also correlate well with the calculated TM5-855 curve. In the range Z (m·kg−1/3) > 5, there is no difference between the two types of ANFO explosives (industrial and handmade) and the pressure results from ASIDE code are very well correlated with TM5-855. For ANFO, (Figure 3(b)) differences are ob- tained with the AUTODYN simulation for Z < 5 m·kg−1/3, and greater convergence is obtained for Z > 5 m·kg−1/3. Comparison with TNT shows that the PETN charge leads to greater pressure effects, although for ANFO, the effects are in the reverse direction. The energy equiva- lence will be greater than 1 for PETN and less than 1 for ANFO. The polynomials obtained for PETN and ANFO from TM5-855 as part of the forensics and security procedures are summarized below. Figure 2. Overpressure as a function of reduced distance for explosions of spherical TNT charges in free air. Forensic Approach Copyright © 2011 SciRes. OJSST  I. SOCHET ET AL. Copyright © 2011 SciRes. OJSST 37 (a) (b) Figure 3. Overpressistance in free air. PETN-TM5-855: (10) A NFO-TM5-855: (11) P Security Approach (12) ure spherical charges of PETN and ANFO as a function of reduced d 1/3 23 10 mkg30:ln1.6259 2.4095 ln0.4477ln0.0605 ln ZP ZZ 5 2 11 10Pa65:ln0.8156 0.2371 ln0.0421 ln PZ PP 5 2 34 0.02 10Pa11:ln1.0755 0.4397 ln0.0557 ln 0.0163 ln0.0043 ln PZ PP PP 5 23 0.1 10Pa5:ln0.9303 0.4768 ln0.0344 ln0.0085 ln PZ PP 3.3. Evolution of the Duration of the Incident Blast Wave as a Function of Distance in the Positive Phase The positive phase duration is typically reported as the bed root of the mass of the explosive. The graph in altho ve ith reduced distance. The duration cu shown below in Figure 4 indicates that developments positive phase durations are more difficult to characterize, ugh the overall trend shows that the duration posi- phase increases wti also approaches an asymptote in the far and near field. The simulations with AUTODYN show that the posi- tive phase durations are underestimated compared to the references in TM5-855. In addition, there is a visibly large gap for short distances less than 3 m·kg−1/3. Regardless of the explosive (i.e., PETN or ANFO), changes in the duration of the positive phase of the blast 5 2 0.02 10Pa1.7:ln0.7899 0.6296 ln0.0175 ln PZ PP P ETN-TM5-855: 1/3 2 0.4 mkg2:ln 2.41472.2208 ln0.3184 ln ZP Z 1/3 2 34 2 mkg30:ln2.4038 2.1387 ln0.3354 ln 0.2848 ln0.0452 ln ZP ZZ ZZ ANFO-TM5-855: (13) 1/3 2 34 0.4 mkg10:ln2.0892 2.2328 ln0.1259 ln 0.1206 ln0.0030 ln ZP ZZ ZZ Figure 4. Duration of the reduced distance function for explosions of spherical TNT charges in free air. 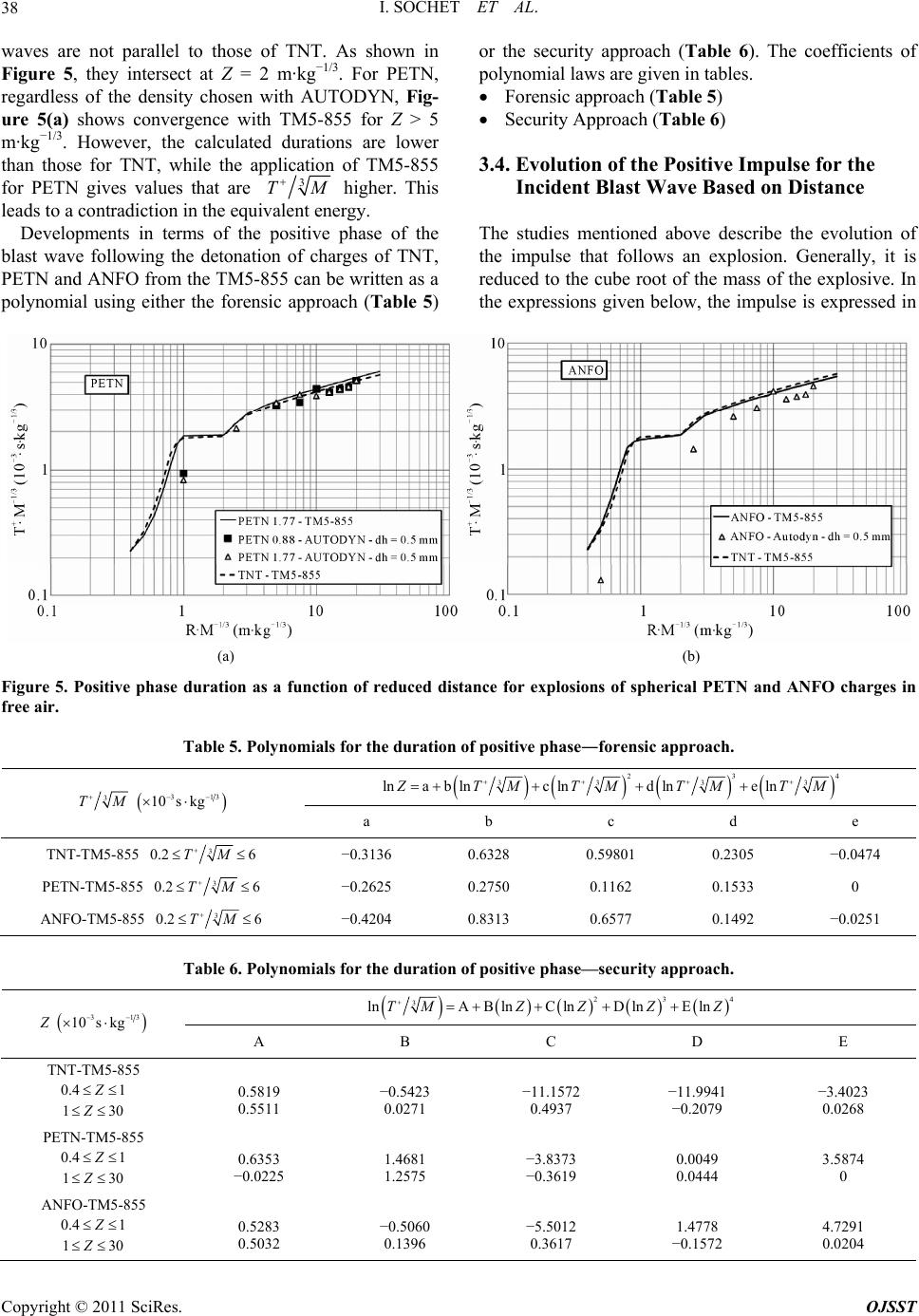 I. SOCHET ET AL. 38 waves are not parallel to those of TNT. As shown in Figure 5, they intersect at Z = 2 m·kg−1/3. For PETN, regardless of the density chosen with AUTODYN, Fig- ure 5(a) shows convergence with TM5-855 for Z > 5 m·kg−1/3. However, the calculated durations are lower than those for TNT, while the application of TM5-855 for PETN gives values that are 3 TM valent ener higher. This leads to a contradiction in the equigy. Developments in terms of the positive phase of the blast wave following the detonation of charges of T PETN and ANFO from the TM5-855 can be written as a polynomial using either the forensic approach (Table 5) 6). The c scribe the evolution of losion. Generally, it is duced to the cube root of the mass of the explosive. In in or the security approach (Table oefficients of polynomial laws are given in tables. T Forensic approach (Table 5) Security Approach (Table 6) 3.4. Evolution of the Positive Impulse for the Incident Blast Wave Based on Distance NT, the impulse that follows an exp he studies mentioned above de re the expressions given below, the impulse is expressed (a) (b) Figure 5. Positive phase duration as a function of reduced distance for explosions of spherical PETN and ANFO charges in free air. Table 5. Polynomials for the duration of positive phase―forensic approach. 23 33 3 lnab lnclnd lneln 4 3 TM TMTMTM 313 3 10skgTM a b c d e 3 0.2 6M −0.3136 0.6328 0.59801 T 0.2305 −0.0474 TNT-TM5-855 PETN-TM5-855 3 0.2 6TM −0.2625 0.2750 0.1162 0.1533 0 ANFO-T 3 0.2 6TM −0.4204 0.8313 0.6577 0.1492 −0.0251 M5-855 Table 6. Polynomials for the duration of positive phase—security approach. 23 3 lnAB lnC lnD lnElnTMZ ZZZ 4 31 10skgZ 3 A B C D E TNT-TM5-855 0.5819 0.5511 5423 271 −11.1572 0.4937 −11.99 −0.20 −3.4023 0 0.4 1Z 130Z −0. 0.0 41 79 .0268 PETN-TM5- 0.4 1Z 855 0 3.5874 0 ANFO-TM5-855 0 0.5283 0.2 −0.5060 0.6 −5.5012 0.7 1.4778 −0.2 4.7291 0.4 13Z 0.6353 −0.0225 1.4681 1.2575 −3.8373 −0.3619 0.0049 0.0444 0.4 1Z 13Z 503139361157 020 Copyright © 2011 SciRes. OJSST 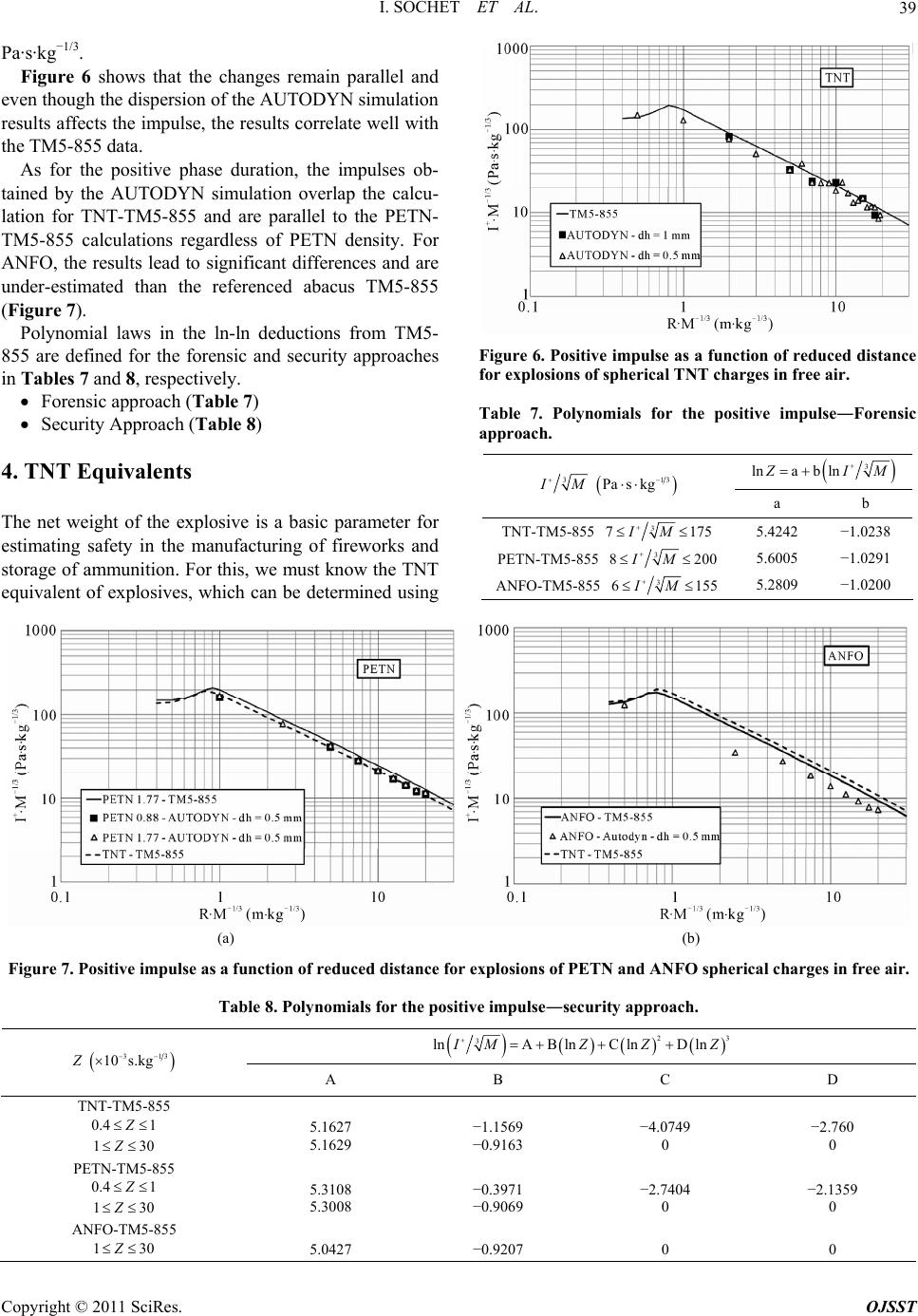 I. SOCHET ET AL.39 Pa·s·k Figure 6 shows that the chremain parallel and even though the dispersion of TODYN sim resulate well with the TM5-855 da As for the positive phase duration, the impulses ob- taineDYN simuloverlap the calcu- lation for TNT-855 and allel to the PETN- ulations regardless of PETN density. For in n be determined using g−1/3. anges the AUulation ts affects the impulse, the results correl ta. d by the AUTOation TM5- are par TM5-855 calc ANFO, e thresults lead to significant differences and are under-estimated than the referenced abacus TM5-855 (Figure 7). Polynomial laws in the ln-ln deductions from TM5- 855 are defined for the forensic and security approaches Tables 7 and 8, respectively. Forensic approach (Table 7) Security Approach (Table 8) 4. TNT Equivalents The net weig Figure 6. Positive impulse as a function of reduced distance for explosions of spherical TNT charges in free air. Table 7. Polynomials for the positive impulse―Forensic approach. ht of the explosive is a basic parameter for estimating safety in the manufacturing of fireworks and storage of ammunition. For this, we must know the TNT equivalent of explosives, which ca 3 lnabln IM 13 3 PaskgIM a b 3 71IM 75 5.4242 −1.0238 TNT-TM5-855 PETN-TM5-855 3 8 200IM 5.6005 −1.0291 ANFO-TM5-855 3 6155IM 5.2809 −1.0200 (a) (b) Figure 7. Positive impulse as a function of reduced distance for explosions of PETN and ANFO spherical charges in free air. Table 8. Polynomials for the positive impulse―security approach. 23 3 lnAB lnClnD ln MZZ Z 313 10s.kgZ A B C D TNT-TM5-855 0 −2.760 0 0.4 1Z 13Z 5.1627 5.1629 −1.1569 −0.9163 −4.0749 0 PETN-TM5-855 0.4 1Z 0 5.3108 −0.3971 −2.7404 −2.1359 0 ANFO-TM5-855 0 5.0427 −0.9207 0 0 13Z 5.3008 −0.9069 0 13Z Copyright © 2011 SciRes. OJSST  I. SOCHET ET AL. 40 several approaches. Generally, the TNT equivalent is the mass of TNT that proides an equal amount of energy during the explosion as the unit mass of tplosive in question. the TNT equivalen the ratio of the mass of TNT to the mas explosive that leads to tmplitude of a ter of the blast wav impulse) at the samdial dis- tance for each charge assuming the scals of Sachs and Hopivalent mass of prsure [29] for n explosive is then given as follows: every unknown explosive in accordance with the Allied v he ex Specifically, t is defined as s of the he same a e (pressurer parame oe ra ing law kinson. The eques Ammunition Storage and Transport Publication [32]. rmby and Wharton [33] and Wharton al. [34] based their calculation o the TNT equivalent used in the a approach fotypes of conplo- nd identified lin trends of TNT eq pressure anse as a functioduced e. egarding the explosives studied in this work, the de- ent of overpressure and impulse as a function of a 3 TNT PTNT cst MZ ETNT MZ where Z is the reduced distance. A similar approach is conducted for the equivalent mass of impulse as follows: 3 TNT ITNT cst MZ ETNTMZ However, when the impulses are reported as a cubed root of the mass, the equivalent impulses can be obtained by sliding the curves along the first diagonal. Esparza [29] conducted a study of several condensexplosives d e (Composition B, PBX-9404, PETN, TNT, PBX 9501, PBX 9502) and presented the average values of an equivalent TNT pressure range of 0.9 to 1.7 for these explosives, whereas the impulses, in terms of TNT equivalent, range from 0.6 to 1.2. This is robably the p easiest method, but it only applies for explosives with the same geometry. However, Gelfand [9] defines the energy equivalent of TNT as the ratio of the detonation energy of an explosive and the detonation energy of TNT. Taking into account the equation of detonation prod- ucts, it can be shown that the effects of detonation are influenced by the basic parameters of detonation speed, pressure, detonation energy and the number of moles of gaseous detonation products. These values can be ob- tained from thermochemical calculations and it is possi- ble to obtain an average value. 12 34 EHEHEHE TNT TNT nEPD ETNT kkkk nEPD TNT TNT where k1, k2, k3 and k4 are empirical coefficients obtained experimentally [30]. The difficulty of estimating a single value for TNT equivalents is confirmed by Peugeot et al. [31], who de- scribe the parameters influencing the equivalent value, namely the composition of the material energy, the dis- tance and the geometry of the load. If the energy equiva- lent cannot be measured or estimated, then a TNT equivalent energy factor of 1.4 leads to a reasonable and Fo et n Esparzr other densed ex sives aearuivalents in terms of d impuln f reo distanc R velopm reduced distance for PETN and ANFO are parallel to those of TNT. Therefore, TNT equivalent means can eas- ily be deduced for each of the characteristic parameters of the blast wave with the exception of the positive duration. For overpressure, TNT energy equivalents of 1.14 and 0.90 are obtained for PETN and ANFO, respect- tively, by sliding the PETN or ANFO pressure curves along the abscissa Z. For duration and impulse positive phase, the parame- ters are reduced to the cubed root of mass, and thus, the curves of PETN and ANFO are slid along the di- agonal. Impulse energy equivalents of 1.15 and 0.90 are obtained for PETN and ANFO, respectively. However, in case of reduced duration an average equivalent TNT cannot be obtained because the curve of the explosive is either above or below the curve corresponding to TNT (Figure 5). There are two emerging areas for which it is possible to define a TNT equivalent. In the case of PETN, for Z < 0.9 m·k g−1/3 the equivalent is 0.95 (Figure 5(a)) and for Z > 5 m·kg−1/3 it is 1.06. In the case of ANFO (Figure 5(b)), the equivalent is 1.1 if Z ≤0.9 m·kg−1/3. The TNT equivalent energies obtained in this study are in good agreement with values reported in the lit- erature (Table 9) in terms of pressure and impulse for ANFO. In case of PETN, the calculated TNT equiva- lent is smaller than the reported values (Table 9). 5. Conclusions First, the computation of the detonation energy reported in this study has permitted us to compare different de- composition rules and thermodynamic data to underline the energy discrepancy for the three explosives studied (TNT, PETN, ANFO). In addition, the computation demonstrates the importance of clarifying the choice between the theoretical and experimental conditions. Second, this study has identified the analytical solu- tions and the available abacus to estimate the effects of exploding pyrotechnic charges in terms of the overpres- sure, duration and impulse of the positive phase. Com- parison of the main characteristics of blast waves in this conservative estimate of the equivalent TNT mass for study is focused on the TM5-855 (CONWEP), AUTO Copyright © 2011 SciRes. OJSST  I. SOCHET ET AL.41 Table 9. TNT equivalent energ TN ies obtained in this study. T Equivalent Explosive Pressure Impulse Global PETN 1.27 [15,25,32,35]; 1.33, 1.45 - 1.73 [9] 1.28 [9] ANFO 0.82 [15,25,35]; 0.83 [32]; 0.83 for DP = 2.07 at 13.8 bar and 0.59 for DP > 13.8 bar [36] 0.82 [1] 0.70 [36] 0.75 [9] DYN and ASIDE codes. Comparison with TNT shows that PETN charges lead to higher pressures than TNT, whereas ANFO shows lower pressures. AUTODYN simulations show that the effects of Acorrelate well in terms of the duration and NFO do not imherefore, the simulation of ANFOs pulse. T charge detonation must be examined with caution, and for ex- ample in a next step it is possible to consider a refined mehoish, the cce of the state equation and the complex composition of ANFO. Polynomial laws have been established based on two approaches, one forensic-based and the other security- based. In the first, the reduced distance is expressed in terms of characteristic quantities of the explosive and in the second, the quantities of the explosive are expressed in terms of reduced distance. 6. References [1] N. F. Scilly, “Measurement of the Explosive Performance of High Explosives,” Journal of Loss Prevention in the Process Industries, Vol. 8, No. 5, 1995, pp. 265-273. doi:10.1016/0950-4230(95)00031-U [2] J. Akhavan, “The Chemistry of Explosive,” 2nd Edition, Royal Society of Chemistry (Great Britain), 2004. [3] A. Lannoy, “Effet des Explosions sur les Structures, Extrapolation de l’Abaque du TM5-1300,” EDF, Dir. Etudes et Recherches, 1979. “Post Detonation and Thermal Studies of plosives in a Closed Chamber,” 6th Int national Symposium on Coal Combustion, Yale Univer- [4] W. S. Filler, Solid HIgh Exer- sity, New Haven, 1956, pp. 648-657. [5] DoD standard TNTe, 1998. http://www.fas.org/man/dod,101/navy/docs/es310/chemis try/chemistry.htm [6] G. F. Kinney and K. J. Graham, “Explosives Shocks in Air,” 2nd Edition, Springer-Verlag, Berlin, 1985. [7] F. P. Lees, “Loss Prevention in the Process Industries, Hazard Identification, Assessment and Control, Vol. 2,” 2nd Edition, Butterworth Heinemann, Oxford, 1996. Analysis, Vo doi:10.1007/BF02549223 [8] Y. Tongchang, Y. Menchao and W. Jianling, “Determi- nation of Heats of Detonation and Influence of Compo- nents of Composite Explosives on Heats of Detonation of High Explosives,” Journal of Thermal l. 44, No. 6, 1995, pp. 1347-1356. [10] W. E. Baker, “Explosions in Air,” University of Texas [11] H. Pförtner, “Gas Cloud Explosions and Resulting Blast ,” Nuclear Engineering and Design, Vol. 41, No. 1, 1977, pp. 59-67. doi:10.1016/0 (77)900 [9] B. Gelfand and M. Silnikov, “Blast Effects Caused by Explosions” United States Army, European Research Of- fice of the US Army, London, 2004. Press, Austin, 1973. Effects 029-5493 94-2 [12] S. Trélat, “Impact de Fortes Explosions sur les Bâtiments ’Une Installation dustrielle,”e d’O 006 [13] M. Omang, S.O. Christensen, S. Borve and J. Trulsen, plosions: A Comparative Study of Représentatifs dIn Thèse d Doctorat, l’Université rléans, Orléans, 2. “Height of Burst Ex Numerical and Experimental Results,” Journal Shock Waves, Vol. 19, No. 2, 2009, pp. 135-143. doi:10.1007/s00193-009-0196-8 [14] N. Kubota, “Propellants and Explosives,” 2nd Edition, Wiley-VCH, Weinheim, 2007. [15] G. Parmentier, “Synthèse des Résultats Expérimentaux Relatifs aux Détonations d’Explosifs Sphériques,”Institut Franco-Allemand de Recherche de Saint-Louis, 1993. [16] J. D. Ornellas, “The Heat and Products of Detonation of Cyclotat ramethyle netetra nitramine, 2,4,6-Trinitrotoluene, Nitromethane, and Bis[2,2-dinitro-2-fluoroethyl]formal,” Journal Physical Chemistry, Vol. 72, No. 7, 1968, pp. 2390-2394. doi:10.1021/j100853a019 [17] R. Meyer, “Explosives,” 3rd Edition, VCH-Verlag, Wein- heim, 1987. [18] National Institute of Standards and Technology, National Institute of Standards and Technology. www.nist.gov/ [19] C. M. Tarver, T. D. Tran and R. E. Whipple, “Thermal Decomposition of Pentaerythritol Tetranitrate,” Propel- lants Explosives Pyrotechnics, Vol. 28, No. 4, 2003, pp. 189-193. doi:10.1002/prep.200300004 [20] American Society for testing and Materials (ASTM), The ASTM Computer Program for Chemical Thermodynamic and Energy Release Evaluation CHETAH 7.3, 2001. [21] NASA Lewis Research Center. http://www.me.berkeley.edu/gri_mech/data/thermo_table. html [22] P. W. Cooper, “Explosives Engineering,” Wiley-VCH, f [24] J. Yinon and S. Zitrin, “Modern Methods and Applica- Weinheim, 1996. [23] M. Finger, E. Lee, F. H. Helm, B. Hayes, H. Horning, R. McGuine, M. Kahara and M. Guidry, “The Effects o Elemental Composition on the Detonation Behavior of Explosives,” Sixth Symposium (International) on Detona- tion, ACR-221, 1976, p. 710. Copyright © 2011 SciRes. OJSST  I. SOCHET ET AL. 42 ctive Design for DC, 1987. S y for led Distances,” Interna- 0. doi:10.1016/0734-743X(86)90025-4 tions in Analysis of Explosive,” Wiley, Hoboken, 1996. [25] TM5-855-1, “Fundamentals of Prote Conventional Weapons,” US Department of the Army, Washington [26] TM5-1300, “The Design of Structures to Resist the Ef- fects of Accidental Explosions,” Technical Manual, U Department of the Army, the Navy and the Air Force, Washington DC, 1990, [27] AUTODYN, Software for Non-Linear Dynamics, Pro- prietary to ANSYS. [28] ASIDE, Forensic Software Database for Crime Scene Investigation, Proprietary of IRCGN. [29] E. D. Esparza, “Blast Measurements and Equivalenc Spherical Charges at Small Sca tional Journal of Impact Engineering, Vol. 4, No. 1, 1986, pp. 23-4 lent of High Explosives,” Scien eristics 98. [30] R. Jeremié and Z. Bajié, “An Approach to Determining the TNT Equivatific Technical Review, Vol. 56, No.1, 2006, pp. 58-62. [31] F. Peugeot, E. Deschalbault and P. F. Péron, “TNT Equivalency: Misconceptions and Reality, Munitions Safety Information Analysis Center,” Munitions Safety Information Analysis Center (MSIAC) Unclassified, Re- port L-132, October 2006. [32] Allied Ammunition Storage and Transport Publication (AASTP), 4, Explosives Safety Risks Analysis, Part II, AC/258(ST)WP/221, 2003. [33] S. A. Formby and R. K. Wharton, “Blast Charact and TNT Equivalence Values for Some Commercial Ex- plosives Detonated at Ground Level,” Journal of Haz- ardous Materials, Vol. 50, No. 2-3, 1996, pp. 183-1 doi:10.1016/0304-3894(96)01791-8 [34] R. K. Wharton, S. A. Formby and R. Merrifield, “Airblast TNT Equivalence for a Range of Commercial Blasting Explosives,” Journal of Hazardous Materials, Vol. 79, No. 1-2, 2000, pp. 31-39. doi:10.1016/S0304-3894(00)00168-0 [35] P. Naz, “Etude de Souffle des Têtes Explosives. Etude Bibliographique,” Institut Franco-Allemand de Recherche , Saint-Malo, 23-27 de Saint-Louis, Saint-Louis, 2005. [36] J. Mory, R. Branka, “Les Equivalents TNT des Explosifs Intentionnels et des Produits Chimiques Condensés Solides ou Liquides à Risque d’Explosion,”Europyro 8th Congrès International de Pyrotechnie June 2003, pp. 407-440. Copyright © 2011 SciRes. OJSST
|