 International Journal of Organic Chemistry, 2011, 1, 78-86 doi:10.4236/ijoc.2011.13013 Published Online September 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC Synthesis and Antimicrobial Activity of a New Class of Sulfone Linked Bisheterocycles Venkatapuram Padmavathi*, Thunga Radha Lakshmi, Bhumireddy Chinnachennaiahgari Venkatesh, Konda Mahesh Department of Chemistry, Sri Venkateswara University, Tirupati, India E-mail: *vkpuram2001@yahoo.com Received June 1, 2011; revised July 20, 2011; accepted August 1, 2011 Abstract A new and novel class of bis(heterocycles) viz., bis pyrroles, pyrrolyl pyrazoles and pyrrolyl isoxazoles are prepared from 1-aroyl-2-styrylsulfonylethenes by 1,3-dipolar cycloaddition of tosylmethyl isocyanide, dia- zomethane, nitrile imines and nitrile oxides. The lead compounds are screened for antimicrobial activity. Keywords: 1-Aroyl-2-Styrylsulfonylethene, 1,3-Dipolar Cycloaddition, Chloramine-T, Antimicrobial Activity 1. Introduction In the last few decades the chemistry of five-membered heterocycles particularly pyrroles, pyrazolines and iso- xazolines has received considerable attention owing to their synthetic and effective biological importance. In- creasing evidence suggests that pyrazoline derivatives possess a broad spectrum of biological activities such as antidepressant, anticonvulsant, psychoanalytic, antihy- potensive and monamine oxidase inhibitory activities [1, 2]. In fact, Celecoxib, a pyrazole derivative and Valde- coxib an isoxazole derivative are extensively used as anti-inflammatory drugs [3]. Besides, pyrrole carboxy- lates exhibit antibiotic, antiviral and oncolytic properties [4-8]. Hence, it is thought that a worthwhile programme would be to prepare molecules having both pyrrole and pyrazole/isoxazole units. Literature evidenced the syn- thesis of 3,4-disubstituted pyrroles by cyclocondensation of Michael acceptors with tosylmethyl isocyanide (Tos- MIC) [9]. Pyrroles have also been prepared by Paal- Knorr condensation of alkyl and aryl amines with 1,4- diketones [10-13]. Similarly, among different methods for the synthesis of pyrazolines and isoxazolines, 1,3- dipolar cycloaddition of an ylide onto an alkene in a 3 + 2 manner is a facile one [14,15]. Indeed, diazomethane, nitrile imines and nitrile oxides have been used exten- sively as reactive intermediates. The nitrile imines and nitrile oxides can be generated by dehydrogenation of araldehyde phenylhydrazones and araldoximes with lead tetraacetate [16], mercuric acetate [17], 1-chloroben- zotriazole [18], chloramine-T [19-22] etc. The present communication deals with the synthesis of sulfone linked bis heterocycles having a pyrrole in combination with a pyrazole or an isoxazole unit. 2. Results and Discussion The synthetic scheme is based on the reactivity of 1-aroyl-2-styrylsulfonylethene (1) towards 1,3-dipolar reagents viz., TosMIC, diazomethane, nitrile imines, ni- trile oxides. When compound (1) is treated with TosMIC in the presence of sodium hydride in a mixture of ether and DMSO, the reaction took place regioselectively re- sulting in a mixture of compounds in 3:1 ratio. They are identified as 4-aroyl-3-(phenylethenesulfonyl)-1H-pyr- role (2) and 4-aroyl-3-(4’-phenyl-1’H-pyrrol-3’-ylsulfon- yl)-1H-pyrrole (3) in major and minor amounts, respec- tively (Scheme 1, Table 1). However, repetition of this reaction with excess TosMIC resulted in 3 only. The latter compound is also obtained by treating 2 with one equivalent of TosMIC. The 1H NMR spectrum of 2a showed two singlets at δ 7.01 and 8.02 ppm for C2-H, and C5-H of pyrrole ring protons. Two doublets are ob- served at 6.79 and 7.48 ppm corresponding to olefin protons in addition to the signals of the aromatic protons. The coupling constants value (J = 17.8 Hz) indicates that they are in trans geometry. Compound 3a exhibited three singlets at δ 6.89 (C2-H & C2’-H), 6.96 (C5’-H) and 8.04 (C5-H) ppm apart from signals due to aromatic protons (Table 2). The olefin moiety in 2 is used to develop different het- 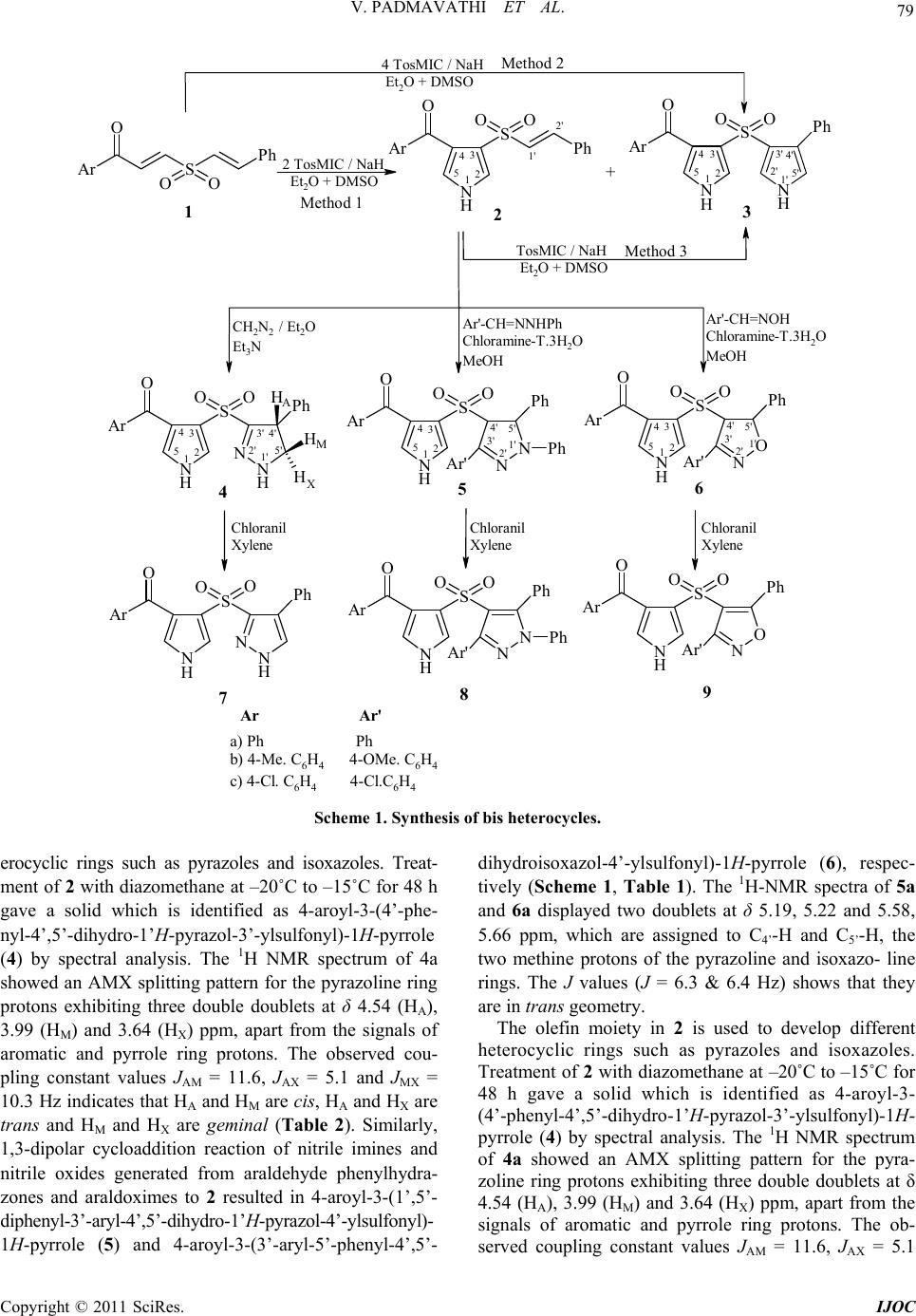 V. PADMAVATHI ET AL. 79 Ar S OO Ph O N N N H S Ar OO Ar' Ph Ph O N O N H S Ar OO Ar' Ph O N N N H S Ar O O Ar' Ph Ph O N O N H S Ar OO Ar' Ph O N H N N H S Ar OOPh OH H H N H N N H S Ar OOPh O N H N H S Ar O O Ph O 1' 2' 3' 4' 5' 12 34 5 N H S Ar OO Ph O 12 3 4 5 1' 2' + 123 56 7 Method 2 Method 3 4 89 A M X Ar Ar' a) Ph Ph b) 4-Me. C6H4 4-OMe. C6H4 c) 4-Cl. C6H4 4-Cl.C6H4 Method 1 2 TosMIC / NaH Et O + DMSO 1' 2' 3' 4' 5' 12 34 5 1' 2' 3' 4' 5' 1' 2' 3' 4' 5' 12 3 4 512 3 4 5 TosMIC / NaH Et O + DMSO 4 TosMIC / NaH Et O + DMSO CH2N2 / Et2O Et3N Ar'-CH=NNHPh Chloramine-T.3H2O MeOH Ar' -C H =N OH Chloramine-T.3H2O MeOH Chloranil Xylene Chloranil Xylene Chloranil Xylene Scheme 1. Synthesis of bis heterocycles. erocyclic rings such as pyrazoles and isoxazoles. Treat- ment of 2 with diazomethane at –20˚C to –15˚C for 48 h gave a solid which is identified as 4-aroyl-3-(4’-phe- nyl-4’,5’- dihydro-1’ H-pyrazol-3’-ylsulfonyl)-1H-pyrrole (4) by spectral analysis. The 1H NMR spectrum of 4a showed an AMX splitting pattern for the pyrazoline ring protons exhibiting three double doublets at δ 4.54 (HA), 3.99 (HM) and 3.64 (HX) ppm, apart from the signals of aromatic and pyrrole ring protons. The observed cou- pling constant values JAM = 11.6, JAX = 5.1 and JMX = 10.3 Hz indicates that HA and HM are cis, HA and HX are trans and HM and HX are geminal (Table 2). Similarly, 1,3-dipolar cycloaddition reaction of nitrile imines and nitrile oxides generated from araldehyde phenylhydra- zones and araldoximes to 2 resulted in 4-aroyl-3-(1’,5’- diphenyl-3’-aryl-4’,5’-dihydro-1’H-pyrazol-4’-ylsulfonyl)- 1H-pyrrole (5) and 4-aroyl-3-(3’-aryl-5’-phenyl-4’,5’- dihydroisoxazol-4’-ylsulfonyl)-1H-pyrrole (6), respec- tively (Scheme 1, Table 1). The 1H-NMR spectra of 5a and 6a displayed two doublets at δ 5.19, 5.22 and 5.58, 5.66 ppm, which are assigned to C4’-H and C5’-H, the two methine protons of the pyrazoline and isoxazo- line rings. The J values (J = 6.3 & 6.4 Hz) shows that they are in trans geometry. The olefin moiety in 2 is used to develop different heterocyclic rings such as pyrazoles and isoxazoles. Treatment of 2 with diazomethane at –20˚C to –15˚C for 48 h gave a solid which is identified as 4-aroyl-3- (4’-phenyl-4’,5’-dihydro-1’H-pyrazol-3’-ylsulfonyl)-1H- pyrrole (4) by spectral analysis. The 1H NMR spectrum of 4a showed an AMX splitting pattern for the pyra- zoline ring protons exhibiting three double doublets at δ 4.54 (HA), 3.99 (HM) and 3.64 (HX) ppm, apart from the signals of aromatic and pyrrole ring protons. The ob- erved coupling constant vales JAM = 11.6, JAX = 5.1 s u Copyright © 2011 SciRes. IJOC 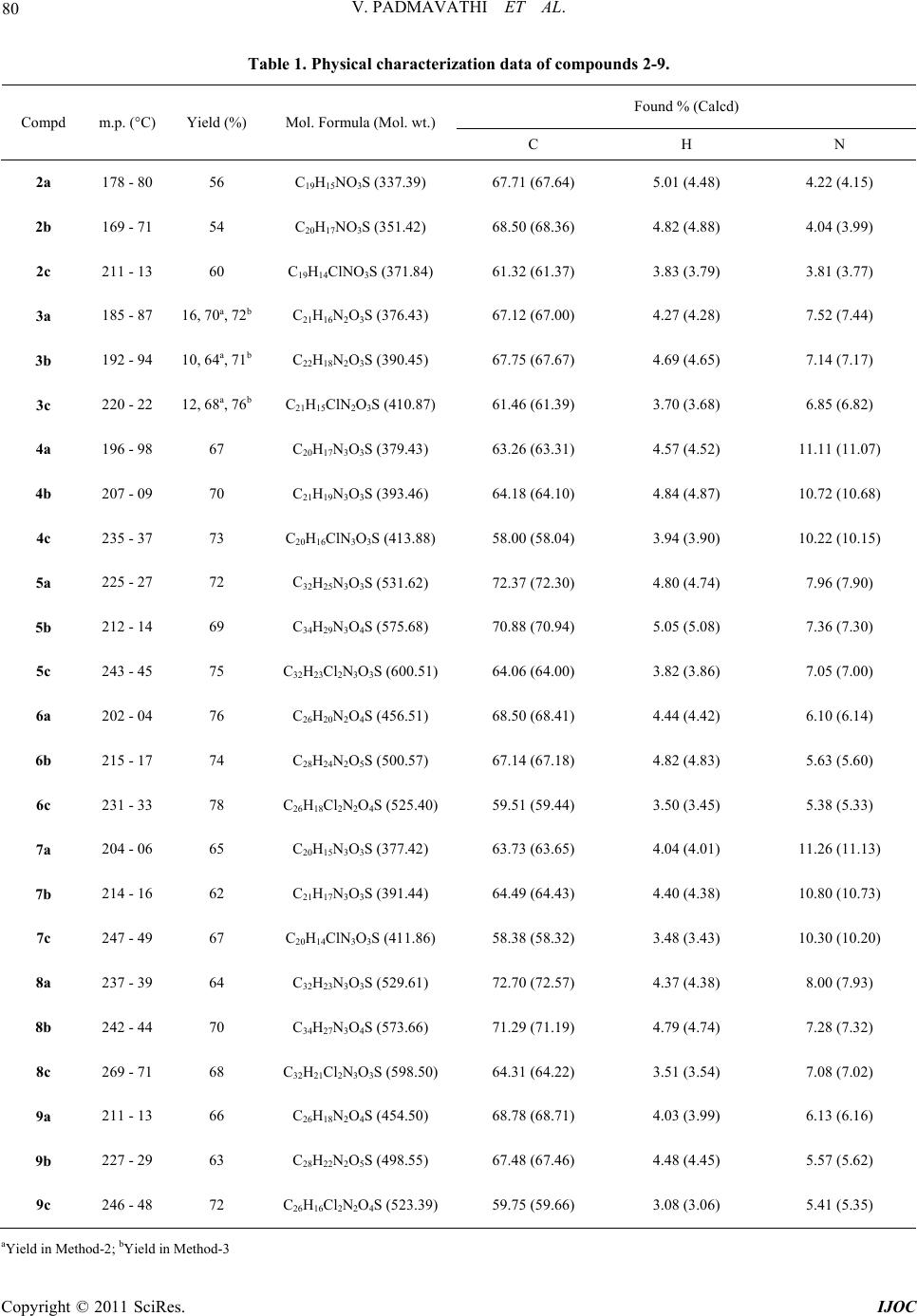 V. PADMAVATHI ET AL. 80 Table 1. Physical characterization data of compounds 2-9. Found % (Calcd) Compd m.p. (C) Yield (%) Mol. Formula (Mol. wt.) C H N 2a 178 - 80 56 C19H15NO3S (337.39) 67.71 (67.64) 5.01 (4.48) 4.22 (4.15) 2b 169 - 71 54 C20H17NO3S (351.42) 68.50 (68.36) 4.82 (4.88) 4.04 (3.99) 2c 211 - 13 60 C19H14ClNO3S (371.84) 61.32 (61.37) 3.83 (3.79) 3.81 (3.77) 3a 185 - 87 16, 70a, 72b C 21H16N2O3S (376.43) 67.12 (67.00) 4.27 (4.28) 7.52 (7.44) 3b 192 - 94 10, 64a, 71b C 22H18N2O3S (390.45) 67.75 (67.67) 4.69 (4.65) 7.14 (7.17) 3c 220 - 22 12, 68a, 76b C 21H15ClN2O3S (410.87) 61.46 (61.39) 3.70 (3.68) 6.85 (6.82) 4a 196 - 98 67 C20H17N3O3S (379.43) 63.26 (63.31) 4.57 (4.52) 11.11 (11.07) 4b 207 - 09 70 C21H19N3O3S (393.46) 64.18 (64.10) 4.84 (4.87) 10.72 (10.68) 4c 235 - 37 73 C20H16ClN3O3S (413.88) 58.00 (58.04) 3.94 (3.90) 10.22 (10.15) 5a 225 - 27 72 C32H25N3O3S (531.62) 72.37 (72.30) 4.80 (4.74) 7.96 (7.90) 5b 212 - 14 69 C34H29N3O4S (575.68) 70.88 (70.94) 5.05 (5.08) 7.36 (7.30) 5c 243 - 45 75 C32H23Cl2N3O3S (600.51) 64.06 (64.00) 3.82 (3.86) 7.05 (7.00) 6a 202 - 04 76 C26H20N2O4S (456.51) 68.50 (68.41) 4.44 (4.42) 6.10 (6.14) 6b 215 - 17 74 C28H24N2O5S (500.57) 67.14 (67.18) 4.82 (4.83) 5.63 (5.60) 6c 231 - 33 78 C26H18Cl2N2O4S (525.40) 59.51 (59.44) 3.50 (3.45) 5.38 (5.33) 7a 204 - 06 65 C20H15N3O3S (377.42) 63.73 (63.65) 4.04 (4.01) 11.26 (11.13) 7b 214 - 16 62 C21H17N3O3S (391.44) 64.49 (64.43) 4.40 (4.38) 10.80 (10.73) 7c 247 - 49 67 C20H14ClN3O3S (411.86) 58.38 (58.32) 3.48 (3.43) 10.30 (10.20) 8a 237 - 39 64 C32H23N3O3S (529.61) 72.70 (72.57) 4.37 (4.38) 8.00 (7.93) 8b 242 - 44 70 C34H27N3O4S (573.66) 71.29 (71.19) 4.79 (4.74) 7.28 (7.32) 8c 269 - 71 68 C32H21Cl2N3O3S (598.50) 64.31 (64.22) 3.51 (3.54) 7.08 (7.02) 9a 211 - 13 66 C26H18N2O4S (454.50) 68.78 (68.71) 4.03 (3.99) 6.13 (6.16) 9b 227 - 29 63 C28H22N2O5S (498.55) 67.48 (67.46) 4.48 (4.45) 5.57 (5.62) 9c 246 - 48 72 C26H16Cl2N2O4S (523.39) 59.75 (59.66) 3.08 (3.06) 5.41 (5.35) aYield in Method-2; bYield in Method-3 Copyright © 2011 SciRes. IJOC  V. PADMAVATHI ET AL. 81 Table 2. IR, 1H and 13C NMR spectral characterization data of compounds 2-9. Compd IR (KBr) cm-1 1H NMR (, ppm) (J in Hz) 13C NMR ( , ppm) 2a 3326 (NH), 1660 (C=O), 1641 (C=C), 1334, 1131 (SO2) 6.79 (d, 1H, C1’-H, J = 17.8 Hz), 7.01 (s, 1H, C2-H), 7.48 (d, 1H, C2’-H, J = 17.8 Hz), 7.14 - 7.85 (m, 10H, Ar-H), 8.02 (s, 1H, C5-H), 10.79 (bs, 1H, NH) 108.6 (C-3), 117.3 (C-4), 120.0 (C-1’), 121.4 (C-2), 136.9 (C-5), 137.7 (C-2’), 189.4 (C=O), 128.4, 129.6, 130.9, 131.5, 132.9, 133.7, 134.4 (aromatic carbons) 2b 3330 (NH), 1667 (C=O), 1634 (C=C), 1328, 1139 (SO2) 2.28 (s, 3H, Ar-CH3), 6.73 (d, 1H, C1’-H, J = 17.5 Hz), 7.05 (s, 1H, C2-H), 7.42 (d, 1H, C2’-H, J = 17.5 Hz), 7.17 - 7.82 (m, 9H, Ar-H), 8.05 (s, 1H, C5-H), 10.72 (bs, 1H, NH) 21.7 (Ar-CH3), 109.1 (C-3), 117.9 (C-4), 120.8 (C-1’), 121.6 (C-2), 136.4 (C-5), 137.1 (C-2’), 188.7 (C=O), 129.5, 130.6, 131.9, 132.5, 133.4, 134.0, 134.8 (aromatic carbons) 2c 3335 (NH), 1664 (C=O), 1635 (C=C), 1337, 1130 (SO2) 6.77 (d, 1H, C1’-H, J = 17.7 Hz), 7.07 (s, 1H, C2-H), 7.46 (d, 1H, C2’-H, J = 17.7 Hz), 7.21 - 7.89 (m, 9H, Ar-H), 8.01 (s, 1H, C5-H), 10.81 (bs, 1H, NH) 108.8 (C-3), 118.3 (C-4), 121.2 (C-1’), 121.3 (C-2), 136.2 (C-5), 137.4 (C-2’), 189.6 (C=O), 128.2, 129.6, 130.3, 131.7, 133.8, 134.9, 135.6 (aromatic carbons) 3a 3329 (NH), 1662 (C=O), 1331, 1129 (SO2) 6.89 (s, 2H, C2-H and C2’-H), 6.96 (s, 1H, C5’-H), 7.25 - 7.78 (m, 10H, Ar-H), 8.04 (s, 1H, C5-H), 10.42 (bs, 2H, NH) 105.3 (C-4’), 109.6 (C-3 and C-3’), 115.3 (C-4), 118.5 (C-2 and C-2’), 119.7 (C-5’), 138.2 (C-5), 188.4 (C=O), 128.2, 129.5, 130.6, 131.3, 132.9, 133.7, 134.6, 135.2 (aromatic carbons) 3b 3324 (NH), 1668 (C=O), 1335, 1139 (SO2) 2.25 (s, 3H, Ar-CH3), 6.85 (s, 2H, C2-H and C2’-H), 6.99 (s, 1H, C5’-H), 7.19-7.74 (m, 9H, Ar-H), 8.02 (s, 1H, C5-H), 10.32 (bs, 2H, NH) 22.4 (Ar-CH3), 105.7 (C-4’), 109.2 (C-3 and 3’), 116.0 (C-4), 118.9 (C-2 and 2’), 119.4 (C-5’), 137.9 (C-5), 187.0 (C=O), 129.8, 131.2, 131.7, 132.3, 132.9, 133.4, 133.9 (aromatic carbons) 3c 3330 (NH), 1666 (C=O), 1329, 1126 (SO2) 7.13 (s, 2H, C2-H and C2’-H), 7.35 (s, 1H, C5’-H), 7.26 - 7.99 (m, 9H, Ar-H), 8.12 (s, 1H, C5-H), 10.55 (bs, 2H, NH) 104.8 (C-4’), 108.3 (C-3 and C-3’), 115.3 (C-4), 118.5 (C-2 and C-2’), 121.8 (C-5’), 138.3 (C-5), 187.5 (C=O), 128.3, 129.1, 129.8, 130.6, 132.4, 133.0, 137.4, 138.3 (aromatic carbons) 4a 3323 (NH), 1664 (C=O), 1570 (C=N), 1325, 1138 (SO2) 3.64 (dd, 1H, HX), 3.99 (dd, 1H, HM, JMX = 10.3 Hz), 4.54 (dd, 1H, HA, JAM = 11.6 Hz, JAX = 5.1 Hz), 6.18 (s, 1H, C2-H), 7.22 - 7.40 (m, 10H, Ar-H), 7.80 (s, 1H, C5-H), 8.94 (bs, 1H, NH), 10.41 (bs, 1H, NH) 48.7 (C-5’), 57.5 (C-4’), 109.8 (C-3), 116.4 (C-4), 121.8 (C-2), 138.3 (C-5), 151.8 (C-3’), 188.7 (C=O), 127.1, 128.5, 128.8, 129.4, 130.7, 132.9, 134.1, 137.3 (aromatic carbons) 4b 3342 (NH), 1662 (C=O), 1576 (C=N), 1317, 1140 (SO2) 2.28 (s, 3H, Ar-CH3), 3.85 (dd, 1H, HX), 4.22 (dd, 1H, HM, JMX = 8.2 Hz), 4.54 (dd, 1H, HA, JAM = 12.0 Hz, JAX = 4.3 Hz), 6.94 (s, 1H, C2-H), 7.22 - 7.64 (m, 9H, Ar-H), 7.95 (s, 1H, C5-H), 8.89 (bs, 1H, NH), 10.36 (bs, 1H, NH) 22.4 (Ar-CH3), 48.1 (C-5’), 58.5 (C-4’), 108.9 (C-3), 116.6 (C-4), 121.9 (C-2), 136.3 (C-5), 152.8 (C-3’), 188.6 (C=O), 128.3, 129.2, 130.4, 131.6, 132.7, 133.8, 134.2, 135.3 (aromatic carbons) 4c 3320 (NH), 1669 (C=O), 1569 (C=N), 1321, 1131 (SO2) 3.83 (dd, 1H, HX), 4.27 (dd, 1H, HM, JMX = 8.0 Hz), 4.56 (dd, 1H, HA, JAM = 12.1 Hz, JAX = 4.5 Hz), 6.97 (s, 1H, C2-H), 7.29 - 7.74 (m, 9H, Ar-H), 7.93 (s, 1H, C5-H), 8.94 (bs, 1H, NH), 10.39 (bs, 1H, NH) 48.7 (C-5’), 59.2 (C-4’), 108.1 (C-3), 116.9 (C-4), 122.6 (C-2), 136.9 (C-5), 153.2 (C-3’), 187.1 (C=O), 128.6, 129.4, 130.8, 131.7, 132.6, 133.2, 135.6, 138.1 (aromatic carbons) 5a 3332 (NH), 1682 (C=O), 1572 (C=N), 1328, 1123 (SO2) 5.19 (d, 1H, C4’-H, J = 6.3 Hz), 5.58 (d, 1H, C5’-H, J = 6.3 Hz), 6.99 (s, 1H, C2-H), 7.21 - 7.74 (m, 20H, Ar & Ar’-H), 8.01 (s, 1H, C5-H), 10.71 (bs, 1H, NH) 61.9 (C-4’), 82.8 (C-5’), 108.9 (C-3), 117.4 (C-4), 121.9 (C-2), 136.2 (C-5), 155.2 (C-3’), 188.4 (C=O), 127.3, 128.1, 128.6, 129.2, 130.2, 131.2, 132.7, 133.4, 134.6, 136.3 (aromatic carbons) 5b 3336 (NH), 1678 (C=O), 1564 (C=N), 1335, 1133 (SO2) 2.25 (s, 3H, Ar-CH3), 3.72 (s, 3H, OCH3), 5.23 (d, 1H, C4’-H, J = 6.5 Hz), 5.54 (d, 1H, C5’-H, J = 6.5 Hz), 6.94 (s, 1H, C2-H), 7.29 - 7.71 (m, 18H, Ar & Ar’-H), 7.98 (s, 1H, C5-H), 10.67 (bs, 1H, NH) 22.6 (Ar-CH3), 55.6 (-OCH3), 62.5 (C-4’), 84.8 (C-5’), 108.2 (C-3), 117.9 (C-4), 121.2 (C-2), 135.2 (C-5), 154.9 (C-3’), 187.7 (C=O), 126.2, 127.6, 128.7, 129.4, 130.9, 131.2, 132.7, 133.2, 134.8, 135.6 (aromatic carbons) 5c 3347 (NH), 1684 (C=O), 1573 (C=N), 1331, 1135 (SO2) 5.27 (d, 1H, C4’-H, J = 6.8 Hz), 5.64 (d, 1H, C5’-H, J = 6.8 Hz), 6.98 (s, 1H, C2-H), 7.25 - 7.88 (m, 18H, Ar & Ar’-H), 8.03 (s, 1H, C5-H), 10.64 (bs, 1H, NH) 63.1 (C-4’), 83.2 (C-5’), 108.5 (C-3), 117.5 (C-4), 121.8 (C-2), 135.6 (C-5), 155.5 (C-3’), 188.6 (C=O), 127.2, 128.6, 129.2, 129.9, 130.5, 131.8, 132.3, 133.1, 135.2, 137.1 (aromatic carbons) Copyright © 2011 SciRes. IJOC  V. PADMAVATHI ET AL. 82 6a 3340 (NH), 1662 (C=O), 1577 (C=N), 1336, 1131 (SO2) 5.22 (d, 1H, C4’-H, J = 6.4 Hz), 5.66 (d, 1H, C5’-H, J = 6.4 Hz), 6.93 (s, 1H, C2-H), 7.19 - 7.71 (m, 15H, Ar & Ar’-H), 8.01 (s, 1H, C5-H), 10.61 (bs, 1H, NH) 62.4 (C-4’), 83.6 (C-5’), 108.7 (C-3), 116.9 (C-4), 122.5 (C-2), 136.8 (C-5), 155.0 (C-3’), 189.5 (C=O), 128.3, 129.1, 129.9, 130.2, 130.6, 131.2, 132.7, 133.4, 134.6, 136.3 (aromatic carbons) 6b 3337 (NH), 1669 (C=O), 1581 (C=N), 1329, 1130 (SO2) 2.23 (s, 3H, Ar-CH3), 3.69 (s, 3H, OCH3), 5.24 (d, 1H, C4’-H, J = 6.5 Hz), 5.71 (d, 1H, C5’-H, J = 6.5 Hz), 6.98 (s, 1H, C2-H), 7.21 - 7.68 (m, 13H, Ar & Ar’-H), 7.99 (s, 1H, C5-H), 10.52 (bs, 1H, NH) 21.7 (Ar-CH3), 56.1 (-OCH3), 63.1 (C-4’), 84.6 (C-5’), 108.3 (C-3), 117.6 (C-4), 122.1 (C-2), 135.3 (C-5), 155.6 (C-3’), 188.3 (C=O), 127.2, 128.3, 129.6, 130.9, 131.4, 133.4, 133.9, 134.0, 134.5 (aro- matic carbons) 6c 3332 (NH), 1667 (C=O), 1570 (C=N), 1339, 1142, (SO2) 5.27 (d, 1H, C4’-H, J = 6.4 Hz), 5.76 (d, 1H, C5’-H, J = 6.4 Hz), 7.02 (s, 1H, C2-H), 7.25 - 7.78 (m, 13H, Ar & Ar’-H), 8.06 (s, 1H, C5-H), 10.47 (bs, 1H, NH) 62.7 (C-4’), 84.0 (C-5’), 108.9 (C-3), 116.5 (C-4), 122.9 (C-2), 134.9 (C-5), 154.2 (C-3’), 189.1 (C=O), 128.7, 129.2, 130.9, 131.4, 132.7, 133.2, 134.8, 135.3, 137.2 (aromatic carbons) 7a 3339 (NH), 1656 (C=O), 1632 (C=C), 1564 (C=N), 1337, 1121 (SO2) 6.38 (bs, 1H, NH), 6.98 (s, 1H, C2-H), 7.26 - 7.62 (m, 11H, C5’-H & Ar-H), 7.96 (s, 1H, C5-H), 8.84 (bs, 1H, NH) 110.1 (C-3), 115.9 (C-4), 122.1 (C-2), 135.2 (C-5), 137.3 (C-5’), 139.8 (C-4’), 153.4 (C-3’), 188.3 (C=O), 128.1, 129.7, 130.7, 131.1, 132.4, 133.9, 134.2, 135.2 (aromatic carbons) 7b 3328 (NH), 1668 (C=O), 1644 (C=C), 1574 (C=N), 1326, 1138 (SO2) 2.31 (s, 3H, Ar-CH3), 6.44 (bs, 1H, NH), 7.01 (s, 1H, C2-H), 7.28 - 7.71 (m, 10H, C5’-H & Ar-H), 7.99 (s, 1H, C5-H), 8.72 (bs, 1H, NH) 22.7 (Ar-CH3), 109.5 (C-3), 115.1 (C-4), 122.9 (C-2), 134.8 (C-5), 136.6 (C-5’), 138.3 (C-4’), 154.7 (C-3’), 189.4 (C=O), 128.6, 129.3, 130.1, 131.8, 132.7, 133.2, 134.0, 134.9 (aromatic carbons) 7c 3336 (NH), 1666 (C=O), 1640 (C=C), 1567 (C=N), 1330, 1122 (SO2) 6.39 (bs, 1H, NH), 6.97 (s, 1H, C2-H), 7.21 - 7.78 (m, 10H, C5’-H & Ar-H), 8.03 (s, 1H, C5-H), 8.79 (bs, 1H, NH) 110.4 (C-3), 114.7 (C-4), 122.2 (C-2), 135.0 (C-5), 135.1 (C-5’), 138.2 (C-4’), 155.2 (C-3’), 188.9 (C=O), 127.4, 128.7, 130.6, 131.2, 132.3, 133.5, 134.6, 135.0, 135.6 (aromatic carbons) 8a 3331 (NH), 1658 (C=O), 1637 (C=C), 1578 (C=N), 1335, 1126 (SO2) 7.04 (s, 1H, C2-H), 7.19 - 7.65 (m, 20H, Ar & Ar’-H), 7.97 (s, 1H, C5-H), 10.46 (bs, 1H, NH) 109.9 (C-3), 116.4 (C-4), 121.9 (C-2), 136.9 (C-5), 146.5 (C-3’), 147.8 (C-4’), 153.2 (C-5’), 187.8 (C=O), 127.0, 127.9, 128.7, 129.2, 129.9, 130.9, 131.8, 132.4, 133.9, 134.2, 135.3 (aromatic carbons) 8b 3338 (NH), 1669 (C=O), 1641 (C=C), 1569 (C=N), 1339, 1130 (SO2) 2.29 (s, 3H, Ar-CH3), 3.71 (s, 3H, OCH3), 6.98 (s, 1H, C2-H), 7.22 - 7.71 (m, 18H, Ar & Ar’-H), 7.99 (s, 1H, C5-H), 10.38 (bs, 1H, NH) 22.4 (Ar-CH3), 56.6 (-OCH3), 109.2 (C-3), 116.9 (C-4), 122.6 (C-2), 136.2 (C-5), 146.9 (C-3’), 148.2 (C-4’), 152.9 (C-5’), 188.5 (C=O), 128.2, 129.1, 129.7, 130.2, 131.5, 132.9, 133.5, 134.0, 134.7 (aromatic carbons) 8c 3335 (NH), 1671 (C=O), 1645 (C=C), 1565 (C=N), 1333, 1128 (SO2) 7.01 (s, 1H, C2-H), 7.27 - 7.83 (m, 18H, Ar & Ar’-H), 8.01 (s, 1H, C5-H), 10.42 (bs, 1H, NH) 109.5 (C-3), 116.1 (C-4), 121.8 (C-2), 136.4 (C-5), 146.1 (C-3’), 148.9 (C-4’), 152.2 (C-5’), 189.4 (C=O), 128.7, 129.4, 129.9, 130.5, 131.9, 132.2, 133.3, 134.6, 135.9 (aromatic carbons) 9a 3328 (NH), 1682 (C=O), 1656 (C=C), 1571 (C=N), 1327, 1138 (SO2) 6.96 (s, 1H, C2-H), 7.09 - 7.68 (m, 15H, Ar & Ar’-H), 8.01 (s, 1H, C5-H), 10.48 (bs, 1H, NH) 108.4 (C-3), 116.1 (C-4), 119.3 (C-2), 138.4 (C-5), 147.1 (C-4’), 148.3 (C-3’), 151.8 (C-5’), 187.7 (C=O), 130.0, 130.3, 130.4, 130.6, 130.8, 131.0, 132.7, 134.1 (aromatic carbons) 9b 3339 (NH), 1678 (C=O), 1651 (C=C), 1579 (C=N), 1321, 1135 (SO2) 2.26 (s, 3H, Ar-CH3), 3.67 (s, 3H, -OCH3), 6.92 (s, 1H, C2-H), 7.14 - 7.76 (m, 13H, Ar & Ar’-H), 8.03 (s, 1H, C5-H), 10.31 (bs, 1H, NH) 22.7 (Ar-CH3), 57.8 (-OCH3), 109.1 (C-3), 117.2 (C-4), 122.9 (C-2), 135.7 (C-5), 146.9 (C-4’), 148.8 (C-3’), 152.9 (C-5’), 189.9 (C=O), 127.6, 128.2, 128.7, 130.7, 131.3, 131.9, 132.8, 133.1, 134.8 (aromatic carbons) 9c 3332 (NH), 1684 (C=O), 1648 (C=C), 1583 (C=N), 1339, 1145 (SO2) 7.03 (s, 1H, C2-H), 7.21 - 7.82 (m, 13H, Ar & Ar’-H), 7.99 (s, 1H, C5-H), 10.43 (bs, 1H, NH) 109.6 (C-3), 117.9 (C-4), 122.4 (C-2), 135.9 (C-5), 146.2 (C-4’), 148.3 (C-3’), 153.4 (C-5’), 189.7 (C=O), 128.6, 129.5, 130.4, 131.2, 132.6, 133.1, 133.9, 134.7, 135.9 (aromatic carbons) Copyright © 2011 SciRes. IJOC 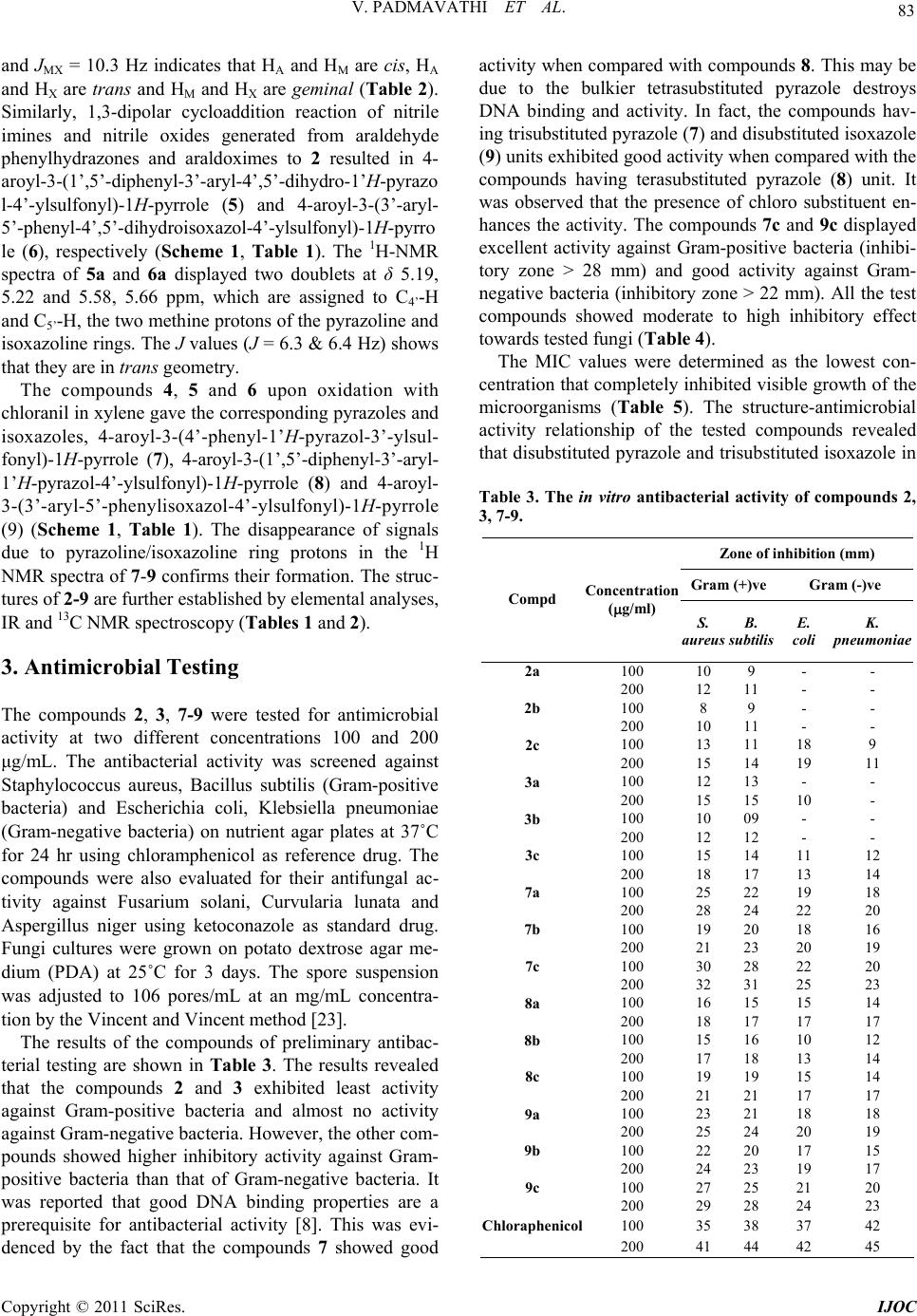 V. PADMAVATHI ET AL. Copyright © 2011 SciRes. IJOC 83 and JMX = 10.3 Hz indicates that HA and HM are cis, HA and HX are trans and HM and HX are geminal (Table 2). Similarly, 1,3-dipolar cycloaddition reaction of nitrile imines and nitrile oxides generated from araldehyde phenylhydrazones and araldoximes to 2 resulted in 4- aroyl-3-(1’,5’-diphenyl-3’-aryl-4’,5’-dihydro-1’H-pyrazo l-4’-ylsulfonyl)-1H-pyrrole (5) and 4-aroyl-3-(3’-aryl- 5’-phenyl-4’,5’-dihydroisoxazol-4’-ylsulfonyl)-1H-pyrro le (6), respectively (Scheme 1, Table 1). The 1H-NMR spectra of 5a and 6a displayed two doublets at δ 5.19, 5.22 and 5.58, 5.66 ppm, which are assigned to C4’-H and C5’-H, the two methine protons of the pyrazoline and isoxazoline rings. The J values (J = 6.3 & 6.4 Hz) shows that they are in trans geometry. The compounds 4, 5 and 6 upon oxidation with chloranil in xylene gave the corresponding pyrazoles and isoxazoles, 4-aroyl-3-(4’-phenyl-1’H-pyrazol-3’-ylsul- fonyl)-1H-pyrrole (7), 4-aroyl-3-(1’,5’-diphenyl-3’-aryl- 1’H-pyrazol-4’-ylsulfonyl)-1H-pyrrole (8) and 4-aroyl- 3-(3’-aryl-5’-phenylisoxazol-4’-ylsulfonyl)-1H-pyrrole (9) (Scheme 1, Table 1). The disappearance of signals due to pyrazoline/isoxazoline ring protons in the 1H NMR spectra of 7-9 confirms their formation. The struc- tures of 2-9 are further established by elemental analyses, IR and 13C NMR spectroscopy (Tables 1 and 2). 3. Antimicrobial Testing The compounds 2, 3, 7-9 were tested for antimicrobial activity at two different concentrations 100 and 200 μg/mL. The antibacterial activity was screened against Staphylococcus aureus, Bacillus subtilis (Gram-positive bacteria) and Escherichia coli, Klebsiella pneumoniae (Gram-negative bacteria) on nutrient agar plates at 37˚C for 24 hr using chloramphenicol as reference drug. The compounds were also evaluated for their antifungal ac- tivity against Fusarium solani, Curvularia lunata and Aspergillus niger using ketoconazole as standard drug. Fungi cultures were grown on potato dextrose agar me- dium (PDA) at 25˚C for 3 days. The spore suspension was adjusted to 106 pores/mL at an mg/mL concentra- tion by the Vincent and Vincent method [23]. The results of the compounds of preliminary antibac- terial testing are shown in Table 3. The results revealed that the compounds 2 and 3 exhibited least activity against Gram-positive bacteria and almost no activity against Gram-negative bacteria. However, the other com- pounds showed higher inhibitory activity against Gram- positive bacteria than that of Gram-negative bacteria. It was reported that good DNA binding properties are a prerequisite for antibacterial activity [8]. This was evi- denced by the fact that the compounds 7 showed good activity when compared with compounds 8. This may be due to the bulkier tetrasubstituted pyrazole destroys DNA binding and activity. In fact, the compounds hav- ing trisubstituted pyrazole (7) and disubstituted isoxazole (9) units exhibited good activity when compared with the compounds having terasubstituted pyrazole (8) unit. It was observed that the presence of chloro substituent en- hances the activity. The compounds 7c and 9c displayed excellent activity against Gram-positive bacteria (inhibi- tory zone > 28 mm) and good activity against Gram- negative bacteria (inhibitory zone > 22 mm). All the test compounds showed moderate to high inhibitory effect towards tested fungi (Table 4). The MIC values were determined as the lowest con- centration that completely inhibited visible growth of the microorganisms (Table 5). The structure-antimicrobial activity relationship of the tested compounds revealed that disubstituted pyrazole and trisubstituted isoxazole in Table 3. The in vitro antibacterial activity of compounds 2, 3, 7-9. Zone of inhibition (mm) Gram (+)ve Gram (-)ve Compd Concentration (g/ml) S. aureus B. subtilis E. coli K. pneumoniae 2a 100 10 9 - - 200 12 11 - - 2b 100 8 9 - - 200 10 11 - - 2c 100 13 11 18 9 200 15 14 19 11 3a 100 12 13 - - 200 15 15 10 - 3b 100 10 09 - - 200 12 12 - - 3c 100 15 14 11 12 200 18 17 13 14 7a 100 25 22 19 18 200 28 24 22 20 7b 100 19 20 18 16 200 21 23 20 19 7c 100 30 28 22 20 200 32 31 25 23 8a 100 16 15 15 14 200 18 17 17 17 8b 100 15 16 10 12 200 17 18 13 14 8c 100 19 19 15 14 200 21 21 17 17 9a 100 23 21 18 18 200 25 24 20 19 9b 100 22 20 17 15 200 24 23 19 17 9c 100 27 25 21 20 200 29 28 24 23 Chloraphenicol 100 35 38 37 42 200 41 44 42 45  V. PADMAVATHI ET AL. 84 Table 4. The in vitro antifungal activity of compounds 2, 3, 7-9. Zone of inhibition (mm) Compd Concentration (g/ml) F. solani C. lunata A. niger 2a 100 17 13 12 200 21 16 14 2b 100 15 12 10 200 18 14 13 2c 100 17 17 15 200 20 21 19 3a 100 16 13 10 200 18 14 13 3b 100 14 10 9 200 15 12 12 3c 100 18 16 14 200 20 19 17 7a 100 29 26 22 200 32 32 24 7b 100 26 26 23 200 30 31 26 7c 100 33 32 27 200 35 36 29 8a 100 17 17 14 200 20 21 17 8b 100 16 16 15 200 19 20 18 8c 100 17 18 17 200 21 21 20 9a 100 25 26 23 200 27 29 26 9b 100 22 23 19 200 26 25 23 9c 100 28 28 26 200 32 30 29 Ketoconazole 100 38 41 36 200 42 44 39 combination with pyrrole displayed greater activity. The compounds having tetrasubstituted pyrazole with pyrrole exhibited least activity. The maximum activity waso- bserved with the compounds 7c and 9c. 4. Experimental Section Melting points were determined in open glass capillaries on a Mel-Temp apparatus and are uncorrected. The pu- rity of the compounds was checked by TLC (silica gel H, BDH, ethyl acetate/hexane, 1:3). The IR spectra were recorded on a Thermo Nicolet IR 200 FT-IR spectrome- ter as KBr pellets and the wave numbers were given in cm-1. The 1H and 13C NMR spectra were run in CDCl3/ DMSO-d6 on a Jeol JNM spectrometer operating at 400 and 100 MHz. All chemical shifts were reported in δ ppm using TMS as an internal standard. The elemental analyses were determined on a Perkin-Elmer 24˚C ele- mental analyzer. The starting material 1-aroyl-2-styryl- sulfonylethene (1) was prepared by the literature proce- dure [24]. Method 1: General procedure for the synthesis of 4-aroyl-3-(phen- ylethenesulfonyl)-1H-pyrrole (2)/4-aroyl-3-(4’-phenyl- 1’H -pyrrol-3’-ylsulfonyl)-1H-pyrrole (3) A mixture of 1 (0.5 mmol) and TosMIC (1 mmol) in Et2O-DMSO (2:1) was added dropwise under stirring to a suspension of NaH (2 mmol) in Et2O (10 mL) at room temperature and stirring was continued for 5 - 6 hr. Then, water was added and the reaction mass was extracted with Et2O. The ethereal fraction was dried over anhy- drous Na2SO4. The solvent was removed in vacuo. The resulting mixture was separated by column chromatog- raphy (hexane-ethyl acetate; 4:1) and identified as 4-aroyl-3-(phenylethenesulfonyl)-1H-pyrrole (2) (major) and 4-aroyl-3-(4’-phenyl-1’H-pyrrole-3’-ylsulfonyl)-1H- pyrrole (3) (minor). Method 2: General procedure for the synthesis of 4-aroyl-3-(4’- phenyl-1’H-pyrrol-3’-ylsulfonyl)-1H-pyrrole (3) A solution of 1 (1 mmol) and TosMIC (4 mmol) in Et2O-DMSO (2:1) was added dropwise under stirring to a suspension of NaH (4 mmol) in Et2O (20 mL) at RT and stirring was continued for about 3 - 4 hr. Then, water was added and the reaction mass extracted with Et2O. The ethereal layer was dried (an. Na2SO4) and the sol- vent was removed in vacuo. The solid obtained was puri- fied by column chromatography (ethyl acetate/hexane, 1:4). Method 3: The compound 3 was also obtained by adding an equimolar (5 mmol) mixture of 2 and TosMIC in Et2O-DMSO (2:1) dropwise under stirring to a suspension of NaH (1 mmol) in Et2O (6 mL) at RT. Stirring was con- tinued for 4 - 5 hr. Then, the contents were diluted with wa- ter and extracted with Et2O. The ethereal layer was dried over anhydrous Na2SO4. Evaporation of the solvent Table 5. Minimum inhibitory concentration of compounds 7c and 9c. Minimum inhibitory concentration (MIC), g/ml Compd S. aureus B. subtilis E. Coli K. pneumoniae F. solani C. lunata A. niger 7c 50 50 100 50 25 12.5 50 9c 100 100 100 100 100 50 100 Chloramphenicol 6.25 6.25 6.25 12.5 - - - Ketoconazole - - - - 12.5 6.25 6.25 Copyright © 2011 SciRes. IJOC  V. PADMAVATHI ET AL. 85 under vacuum resulted in a solid which was purified by column chromatography (ethyl acetate/hexane, 1:4). General procedure for the synthesis of 4-aroyl-3-(4’- phenyl-4’,5’-dihydro-1’H-pyrazol-3’-ylsulfonyl)-1H-pyr role (4) To a cooled solution of 2 (5 mmol) in dichloromethane (20 mL), an ethereal solution of diazomethane (40 ml, 0.4 M) and triethylamine (0.12 g) were added. The reac- tion mixture was kept at –20 to –15 ˚C for 40 - 48 hr. The solvent was removed under reduced pressure and the resultant solid was recrystallized from 2-pro- panol. General procedure for the synthesis of 4-aroyl-3- (1’,5’-diphenyl-3’-aryl-4’,5’-dihydro-1’H-pyrazol-4-yl- sulfonyl)-1H-pyrrole (5) A mixture of 2 (1 mmol), araldehyde phenylhydrazone (2 mmol) and chloramine-T (2 mmol) in methanol (15 mL) was refluxed for 16 - 18 hr on a water bath. The precipitated inorganic salts were filtered off. The filtrate was concentrated and the residue was extracted with di- chloromethane. The organic layer was washed with wa- ter, saturated brine and dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure af- forded a crude product which was recrystallized from ethanol. General procedure for the synthesis of 4-aroyl-3- (3’-aryl-5’-phenyl-4’,5’-dihydroisoxazol-4’-ylsulfonyl)- 1H-pyrrole (6) The compound 2 (1 mmol), araldoxime (2 mmol) and chloramine-T (2 mmol) in methanol (20 mL) was re- fluxed for 14-16 hr on a water bath. The precipitated inorganic salts were filtered off. The filtrate was concen- trated and the residue was extracted with dichloro- methane. The organic layer was washed with water, sa- turated brine and dried over anhydrous Na2SO4. The sol- vent was removed in vacuo. The solid obtained was puri- fied by recrystallization from ethanol. General procedure for the synthesis of 4-aroyl-3- (4’-phenyl-1’H-pyrazol-3’-ylsulfonyl)-1H-pyrrole (7)/4- aroyl-3-(1’,5’-diphenyl-3’-aryl-1’H-pyrazol-4’-yl-sulfon yl)-1H-pyrrole (8)/4-aroyl-3-(3’-aryl-5’-phenylisoxazol- 4’-ylsulfonyl)-1H-pyrrole (9) A solution of 4-6 (1 mmol) and chloranil (1.4 mmol) in xylene (10 mL) was refluxed for 25 - 30 hr. Then, the reaction mixture was treated with a 5% NaOH solution. The organic layer was separated and repeatedly washed with water. It was then dried over anhydrous Na2SO4 and the solvent was removed on a rotary evaporator. The resultant solid was purified by recrystallization from me- thanol. 5. Conclusions A new class of bis heterocycles 4-aroyl-3-(4’-phenyl- 1’H-pyrazol-3’-ylsulfonyl)-1H-pyrrole (7), 4-aro-yl-3- (1’,5’-diphenyl-3’-aryl-1’H-pyrazol-4’-ylsulfonyl)-1H- pyrrole (8) and 4-aroyl-3-(3’-aryl-5’-phenylisoxazol- 4’-ylsulfonyl)-1H-pyrrole (9) were prepared by the re- gioselective reaction of tosylmethyl isocyanide and 1,3- dipolar cycloaddtion reaction of diazomethane, nitrile imines and nitrile oxides with 1-aroyl-2-styrylsulfonyle- thene (1). The antimicrobial testing showed that the com- pounds 7c and 9c exhibited greater antimicrobial acti- vity. 6. Acknowledgements The authors are thankful to UGC, New Delhi, India for financial assistance under major research project. 7. References [1] S. S. Parmar, B. R. Pandey, C. Dwivedi and R. D. Harbi- son, “Anticonvulsant Activity and Monoamine Oxidase Inhibitory Properties of 1,3,5-Trisubstituted Pyrazolines,” Journal of Pharmaceutical Sciences, Vol. 63, No. 7, 1974, pp. 1152-1155.doi:10.1002/jps.2600630730 [2] N. Soni, K. Pande, R. Kalsi, T. K. Gupta, S. S. Parmar and J. P. Barthwal, “Evaluation of Anti-Depressant-Like Effect of 2-Pyrazoline,” Research Communications in Chemical Pathology and Pharmacology, Vol. 56, 1987, pp. 129-132. [3] G. Dannahardt, W. Kiefer, G. Kramer, S. Maehrlein, U. Nowe and B. Fiebich, “The Pyrrole Moiety as a Template for COX-1/COX-2 Inhibitors,” European Journal of Me- dicinal Chemistry, Vol. 35, No. 5, 2000, pp. 499-510. doi:10.1016/S0223-5234(00)00150-1 [4] C. C. C. Wang and P. B. Derven, “Sequence-Specific Trapping of Topoisomerase I by DNA Binding Polyam- ide-Camptothecin Conjugates,” Journal of the American Chemical Society, Vol. 123, No. 36, 2001, pp. 8657-8661. doi:10.1021/ja010392p [5] B. Wellenzohn, W. Flader, R. H. Winger, A. Hallabruker, E. Mayer and K. R. Liedl, “Complex of B-DNA with Polyamides Freezes DNA Backbone Flexibility,” Journal of the American Chemical Society, Vol. 123, 2001, pp. 5044-5049. doi:10.1021/ja003639b [6] S. K. Sharma, M. Tandon and J. W. Lown, “A General Solution and Solid-Phase Synthetic Procedure for Incor- porating Three Contiguous Imidazole Moieties into DNA Sequence Reading Polyamides,” Journal of Organic Chemistry, Vol. 66, No. 3, 2001, pp. 1030-1034. doi:10.1021/jo001034s [7] N. R. Wurtz, J. M. Turner, E. E. Baird and P. B. Dervan, “Solid phase synthesis of polyamides containing pyrrole and imidazole amino acids,” Organic Letters, Vol. 3, No. 8, 2001, pp. 1201-1203. doi:10.1021/ol0156796 [8] N. B. Dyatkina, C. D. Roberts, J. D. Keicher, Y. Dai, J. P. Nadherny, W. Zhang, U. Schmitz, A. Kongpachith, K. Fung, A. A. Nokikov, L. Lou, M. Velligan, A. A. Khor- Copyright © 2011 SciRes. IJOC  V. PADMAVATHI ET AL. 86 lin and M. S. Chen, “Minor Groove DNA Binders as An- timicrobial Agents. 1. Pyrrole Tetraamides Are Potent Antibacterials against Vancomycin Resistant Enteroccoci and Methicillin Resistant,” Journal of Medicinal Chemis- try, Vol. 45, No. 4, 2002, pp. 805-817. doi:10.1021/jm010375a [9] M. Curini, F. Montanari, O. Rosati, E. Lioy and R. Margarita, “Layered Zirconium Phosphate and Phospho- nate as Heterogeneous Catalyst in the Preparation of Pyrroles,” Tetrahedron Letters, Vol. 44, No. 20, 2003, pp. 3923-3925.doi:10.1016/S0040-4039(03)00810-4 [10] A. M. Van Leusen, H. Siderius, B. E. Hoogenboom and D. van Leusen, “A New and Simple Synthesis of the Pyrrole Ring System from Michael Acceptors and To- sylmethylisocyanides,” Tetrahedron Letters, Vol. 13, No. 52, 1972, pp. 5337-5340. doi:10.1016/S0040-4039(01)85244-8 [11] N. P. Pavri and M. L. Trudell, “An Efficient Method for the Synthesis of 3-Arylpyrroles,” Journal of Organic Chemistry, Vol. 62, No. 8, 1997, pp. 2649-2651. doi:10.1021/jo961981u [12] T. N. Danks, “Microwave Assisted Synthesis of Pyr- roles,” Tetrahedron Letters, Vol. 40, No. 20, 1999, pp. 3957- 3960. doi:10.1016/S0040-4039(99)00620-6 [13] G. Minetto, L. F. Raveglia and M. Taddei, “Micro- wave-Assisted Paal-Knorr Reaction. A Rapid Approach to Substituted Pyrroles and Furans,” Organic Letters, Vol. 6, No. 3, 2004, pp. 389-392. doi:10.1021/ol0362820 [14] A. G. Lee, “A Simplified Synthesis of Unsaturated Ni- trogen-Heterocycles Using Nitrile Betaines,” Synthesis, 1982, pp. 508-509. doi:10.1055/s-1982-29860 [15] Z. Bao-Xiang, Yu Yang and E. Shoji, “Synthesis of Sta- ble Δ4-Isoxazolines by 1,3-Dipolar Cycloaddition of 3,4-Dihydroisoquinoline N-Oxides with Alkynes and Their Rearrangement to Isoquinoline-Fused Pyrroles,” Tetrahedron, Vol. 52, No. 37, 1996, pp. 12049-12060. doi:10.1016/0040-4020(96)00698-9 [16] G. Just and K. Dhal, “Lead Tetraacetate Oxidation of Aldoximes,” Tetrahedron, Vol. 24, No. 15, 1968, pp. 5251-5269. doi:10.1016/S0040-4020(01)96322-7 [17] K. M. L. Rai, N. Linganna, A. Hassner and C. A. Murthy, “A Convenient Method for the Generation of Nitrile Ox- ide and Its Application to the Synthesis of 2-Isoxazo- lines,” Organic Preparations and Procedures Interna- tional, Vol. 24, 1992, pp. 91-94. doi:10.1080/00304949209356711 [18] J. N. Kim and E. K. Ryu, “A Convenient Synthesis of Nitrile Oxides from Aldoximes by 1-Chloroben Zotria- zole,” Synthetic Communications, Vol. 20, No. 9, 1990, pp. 1373-1377. doi:10.1080/00397919008052851 [19] K. M. L. Rai and A. Hassner, “Intramolecular 1,3-Diploar cycoaddition of Nitrile Oxides with Vinyl Acetate and Acrylonitrile,” Indian Journal of Chemistry, Vol. 36B, 1997, pp. 242-245. [20] K. M. L. Rai and A. Hassner, “Intermolecular 1,3-Dipolar cycloadditions of Preformed Nitrile Oxides with Phenyl Vinyl Sulphone,” Synthetic Communications, Vol. 27, 1997, pp. 467-472. doi:10.1080/00397919708006048 [21] A. Hassner and K. M. Lokanath Rai, “A New Method for the Generation of Nitrile Oxides and Its Application to the Synthesis of 2-Isoxazolines,” Synthesis, Vol. 1, 1989, pp. 57-59. doi:10.1055/s-1989-27152 [22] K. M. L. Rai and A. Hassner, “Chlorahine-T in Hetero- cyclic Synthesis; a Simple Procedure for the Genedation of Nitrilihines and Its Application to the Synthesis of Pyrazolines,” Synthetic Communications, Vol. 19, No. 16, 1989, pp. 2799-2807. doi:10.1080/00397918908052667 [23] J. G. Vincent and H. W. Vincent, “Filter Paper Disc Modification of the Oxford Cup Penicillin Determina- tion,” Proceedings of the Society for Experimental Biol- ogy and Medicine, Vol. 55, No. 3, 1944, pp. 162-164. [24] D. B. Reddy, N. C. Babu, K. V. Reddy and V. Padmavathi, “A New Route for the Synthesis of Bisun- saturated Oxosulfones and Bis Sulfones under Friedel- Crafts Conditions,” Indian Journal of Chemistry, Vol. 40B, 2001, pp. 416-418. Copyright © 2011 SciRes. IJOC
|