 International Journal of Organic Chemistry, 2011, 1, 71-77 doi:10.4236/ijoc.2011.13012 Published Online September 2011 (http://www.SciRP.org/journal/ijoc) Copyright © 2011 SciRes. IJOC Synthesis, Characterization and Antibacterial Activity of Biologically Important Vanillin Related Hydrazone Derivatives Thiyagarajan Govindasami1, Anjana Pandey2, Nithya Palanivelu3, Ashutosh Pandey1* 1Department of Chemistry, Motilal Nehru National Institute of Technology, Allahabad, India 2Department of Biotechnology, University of Allahabad, Allahabad, India 3Department of Chemistry, BharathiarUniversity, Coimbatore, India E-mail: *apandey70@yahoo.com Received June 3, 2011; revised July 27, 2011; accepted August 5, 2011 Abstract Hydrazone derivatives of vanillin are found to possess anti-bacterial activities. Based on higher bio-activity of hydrazones, new hydrazone derivatives were synthesized from Piperdine-4-carboxylicacid methyl ester (1). The compounds 1-pyrimidine-2-yl piperidine-4-carboxylicacid(4-hydroxy-3-methoxy benzylidine)-hy- drazide (10), 1-pyrimidine-2-yl piperidine-4-carboxylicacid (3,4-dimethoxy benzylidine) hydrazide (11), 1-pyrimidine-2-yl piperidine-4-carboxylicacid(4-butoxy-3-methoxy benzylidine)-hydrazide (12), 1-pyrimi- dine-2-yl piperidine-4-carboxylicacid(3-methoxy-4(2-methoxy ethoxy) benzylidine)-hydrazide (13) were synthesized, purified and characterized by 1HNMR, 13CNMR, LCMS, FT-IR and HPLC techniques. The synthesized hydrazone derivatives were further checked for anti-bacterial activities by paper disc diffusion method against Pseudomonas aeruginosa and Staphylococcus aureus bacterial strains. Keywords: Antibiotics, Fractional Crystallization, Hydrazones, Coupling Reaction 1. Introduction Earlier, by Quantitative Structure Activity Relationship (QSAR) studies, most of the rifamycin derivatives were found to be biologically active compared to other com- pounds [1]. For example, the hydrazones obtained from 3-formyl rifamycin and N-amino-N-methyl piperazine derivatives were found to be biologically active and tested for oral treatment of infections in animals [2]. Re- cently, a lot of biologically important hydrazone deriva- tives with a number of functional groups have been syn- thesized from aromatic and aliphatic compounds [3]. Hydrazone derivatives are molecules containing highly reactive azomethine group (CO-NH-N=CH) and thus useful in new drug development [4]. Also, these are found to possess anti-microbial [5-7], anti-mycobacterial [8], anti-convulsant [9], analgesic [10], anti-inflamma- tory [11,12], anti-platelet [13], anti-tubercular [14-16] and anti-tumoral [17-19] activities. Diflunisal hydrazones were also prepared as possible dual acting antimicrobial and anti-tuberculosis agents with anti-inflammatory properties [20]. Moreover, hydrazones has been recently established as a good precursor for one-pot synthesis of C-4 functionalized 1,2,3,4-tetrahydro quinolones con- taining a quaternary stereo center [21]. Due to the growth of population and changes in climatic conditions several new diseases are likely to affect the human beings. So, there is a continuous need for the synthesis of new bio- logically active organic compounds by using a fast and efficient approach which may act as potential antimicro- bial agents. Based on the higher bio-reactivity of hydra- zones, we have synthesized novel hydrazones (10-13) from Piperdine-4-carboxylic acid methyl ester (1) cou- pled with 2-chloro pyrimidine (2) along with other vanil- lin derivatives (6-9). The anti-bacterial studies were ef- fectively done for newly synthesized hydrazones by standard disc diffusion method [22] with different con- centrations. 2. Results and Discussion 2.1 Synthesis Earlier studies on pyrimidine shows, that heterocyclic 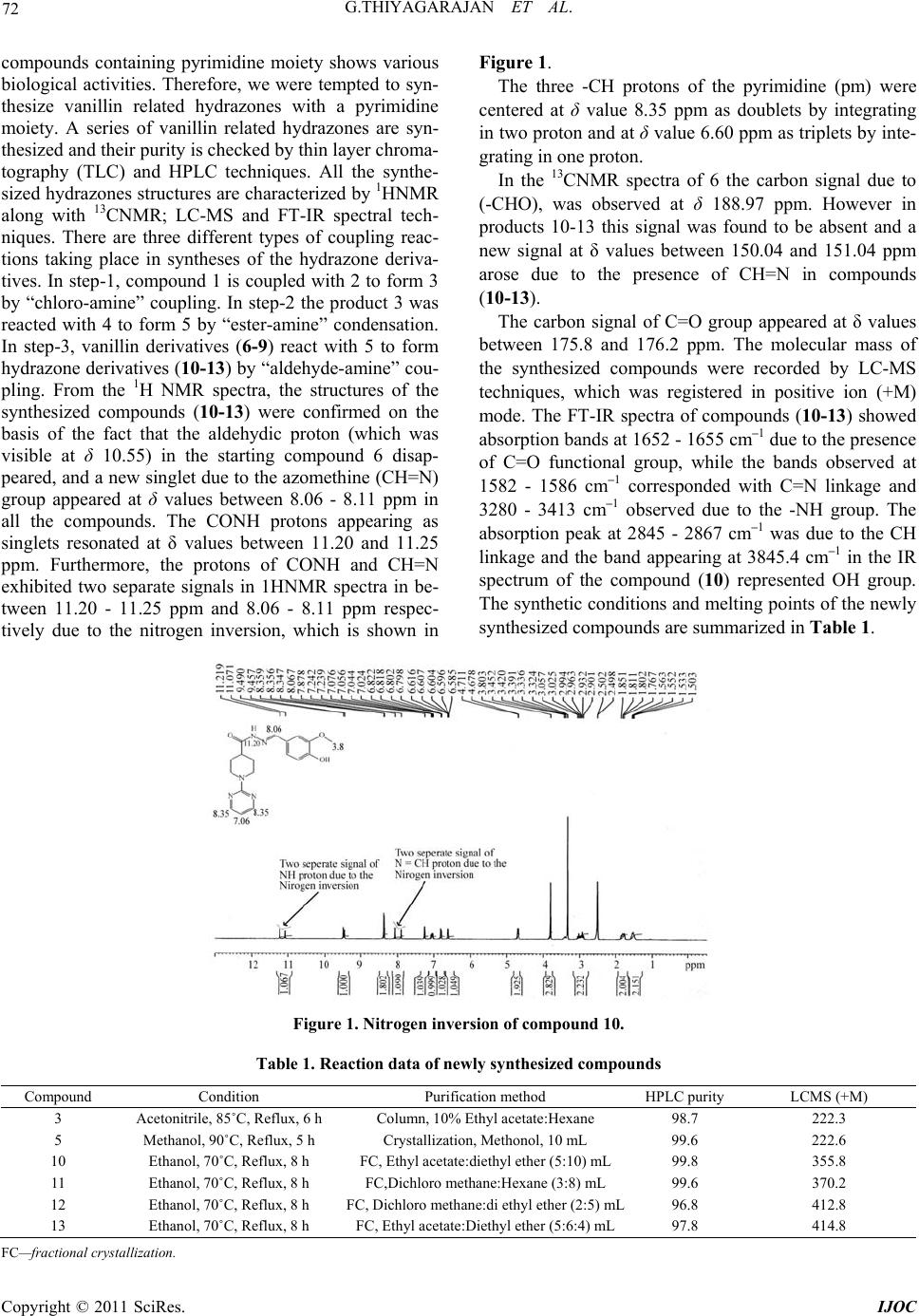 G.THIYAGARAJAN ET AL. 72 compounds containing pyrimidine moiety shows various biological activities. Therefore, we were tempted to syn- thesize vanillin related hydrazones with a pyrimidine moiety. A series of vanillin related hydrazones are syn- thesized and their purity is checked by thin layer chroma- tography (TLC) and HPLC techniques. All the synthe- sized hydrazones structures are characterized by 1HNMR along with 13CNMR; LC-MS and FT-IR spectral tech- niques. There are three different types of coupling reac- tions taking place in syntheses of the hydrazone deriva- tives. In step-1, compound 1 is coupled with 2 to form 3 by “chloro-amine” coupling. In step-2 the product 3 was reacted with 4 to form 5 by “ester-amine” condensation. In step-3, vanillin derivatives (6-9) react with 5 to form hydrazone derivatives (10-13) by “aldehyde-amine” cou- pling. From the 1H NMR spectra, the structures of the synthesized compounds (10-13) were confirmed on the basis of the fact that the aldehydic proton (which was visible at δ 10.55) in the starting compound 6 disap- peared, and a new singlet due to the azomethine (CH=N) group appeared at δ values between 8.06 - 8.11 ppm in all the compounds. The CONH protons appearing as singlets resonated at δ values between 11.20 and 11.25 ppm. Furthermore, the protons of CONH and CH=N exhibited two separate signals in 1HNMR spectra in be- tween 11.20 - 11.25 ppm and 8.06 - 8.11 ppm respec- tively due to the nitrogen inversion, which is shown in Figure 1. The three -CH protons of the pyrimidine (pm) were centered at δ value 8.35 ppm as doublets by integrating in two proton and at δ value 6.60 ppm as triplets by inte- grating in one proton. In the 13CNMR spectra of 6 the carbon signal due to (-CHO), was observed at δ 188.97 ppm. However in products 10-13 this signal was found to be absent and a new signal at δ values between 150.04 and 151.04 ppm arose due to the presence of CH=N in compounds (10-13). The carbon signal of C=O group appeared at δ values between 175.8 and 176.2 ppm. The molecular mass of the synthesized compounds were recorded by LC-MS techniques, which was registered in positive ion (+M) mode. The FT-IR spectra of compounds (10-13) showed absorption bands at 1652 - 1655 cm–1 due to the presence of C=O functional group, while the bands observed at 1582 - 1586 cm–1 corresponded with C=N linkage and 3280 - 3413 cm–1 observed due to the -NH group. The absorption peak at 2845 - 2867 cm–1 was due to the CH linkage and the band appearing at 3845.4 cm–1 in the IR spectrum of the compound (10) represented OH group. The synthetic conditions and melting points of the newly synthesized compounds are summarized in Table 1. Figure 1. Nitrogen inversion of compound 10. Table 1. Reaction data of newly synthesized compounds Compound Condition Purification method HPLC purity LCMS (+M) 3 Acetonitrile, 85˚C, Reflux, 6 h Column, 10% Ethyl acetate:Hexane 98.7 222.3 5 Methanol, 90˚C, Reflux, 5 h Crystallization, Methonol, 10 mL 99.6 222.6 10 Ethanol, 70˚C, Reflux, 8 h FC, Ethyl acetate:diethyl ether (5:10) mL 99.8 355.8 11 Ethanol, 70˚C, Reflux, 8 h FC,Dichloro methane:Hexane (3:8) mL 99.6 370.2 12 Ethanol, 70˚C, Reflux, 8 h FC, Dichloro methane:di ethyl ether (2:5) mL96.8 412.8 13 Ethanol, 70˚C, Reflux, 8 h FC, Ethyl acetate:Diethyl ether (5:6:4) mL97.8 414.8 FC—fractional crystallization. Copyright © 2011 SciRes. IJOC 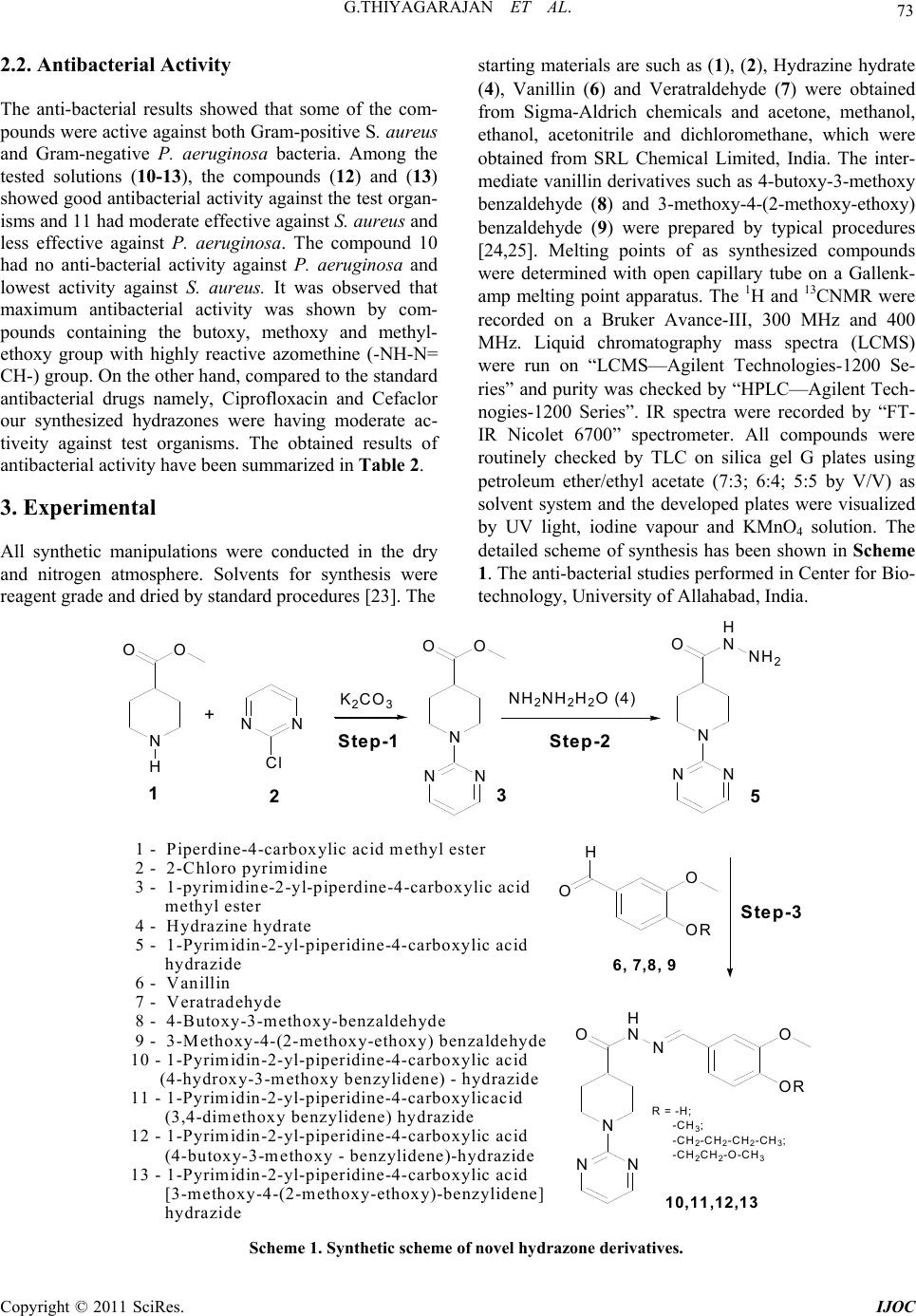 73 G.THIYAGARAJAN ET AL. 2.2. Antibacterial Activity The anti-bacterial results showed that some of the com- pounds were active against both Gram-positive S. aureus and Gram-negative P. aeruginosa bacteria. Among the tested solutions (10-13), the compounds (12) and (13) showed good antibacterial activity against the test organ- isms and 11 had moderate effective against S. aureus and less effective against P. aeruginosa. The compound 10 had no anti-bacterial activity against P. aeruginosa and lowest activity against S. aureus. It was observed that maximum antibacterial activity was shown by com- pounds containing the butoxy, methoxy and methyl- ethoxy group with highly reactive azomethine (-NH-N= CH-) group. On the other hand, compared to the standard antibacterial drugs namely, Ciprofloxacin and Cefaclor our synthesized hydrazones were having moderate ac- tiveity against test organisms. The obtained results of antibacterial activity have been summarized in Table 2. 3. Experimental All synthetic manipulations were conducted in the dry and nitrogen atmosphere. Solvents for synthesis were reagent grade and dried by standard procedures [23]. The starting materials are such as (1), (2), Hydrazine hydrate (4), Vanillin (6) and Veratraldehyde (7) were obtained from Sigma-Aldrich chemicals and acetone, methanol, ethanol, acetonitrile and dichloromethane, which were obtained from SRL Chemical Limited, India. The inter- mediate vanillin derivatives such as 4-butoxy-3-methoxy benzaldehyde (8) and 3-methoxy-4-(2-methoxy-ethoxy) benzaldehyde (9) were prepared by typical procedures [24,25]. Melting points of as synthesized compounds were determined with open capillary tube on a Gallenk- amp melting point apparatus. The 1H and 13CNMR were recorded on a Bruker Avance-III, 300 MHz and 400 MHz. Liquid chromatography mass spectra (LCMS) were run on “LCMS—Agilent Technologies-1200 Se- ries” and purity was checked by “HPLC—Agilent Tech- nogies-1200 Series”. IR spectra were recorded by “FT- IR Nicolet 6700” spectrometer. All compounds were routinely checked by TLC on silica gel G plates using petroleum ether/ethyl acetate (7:3; 6:4; 5:5 by V/V) as solvent system and the developed plates were visualized by UV light, iodine vapour and KMnO4 solution. The detailed scheme of synthesis has been shown in Scheme 1. The anti-bacterial studies performed in Center for Bio- technology, University of Allahabad, India. N OO H NN Cl K2CO3 N NN OO NH2NH2H2O (4) O OR O H N NN OH NNO OR N NN OH NNH2 + Step-1 Step-2 1235 10,11,12,13 6, 7,8, 9 R = -H; -C H3; -C H2-CH2-CH2-CH3; -C H2CH2-O -C H 3 Step-3 1 - Piperdine-4-carboxylic acid methyl ester 2 - 2-Chloro pyrimidine 3 - 1-pyrimidine-2-yl-piperdine-4-carboxylic acid methyl ester 4 - Hydrazine hydrate 5 - 1-Pyrimidin-2-yl-piperidine-4-carboxylic acid hydrazide 6 - Vanillin 7 - Veratradehyde 8 - 4-Butoxy-3-methoxy-benzaldehyde 9 - 3-Methoxy-4-(2-methoxy-ethoxy) benzaldehyde 10 - 1-Pyrimidin-2-yl-piperidine-4-carboxylic acid (4-hydroxy-3-methoxy benzylidene) - hydrazide 11 - 1-Pyrimidin-2-yl-piperidine-4-carboxylicacid (3,4-dimethoxy benzylidene) hydrazide 12 - 1-Pyrimidin-2-yl-piperidine-4-carboxylic acid (4-butoxy-3-methoxy - benzylidene)-hydrazide 13 - 1-Pyrimidin-2-yl-piperidine-4-carboxylic acid [3-methoxy-4-(2-methoxy-ethoxy)-benzylidene] hydrazide Scheme 1. Synthetic sche me of novel hy drazone derivatives. Copyright © 2011 SciRes. IJOC 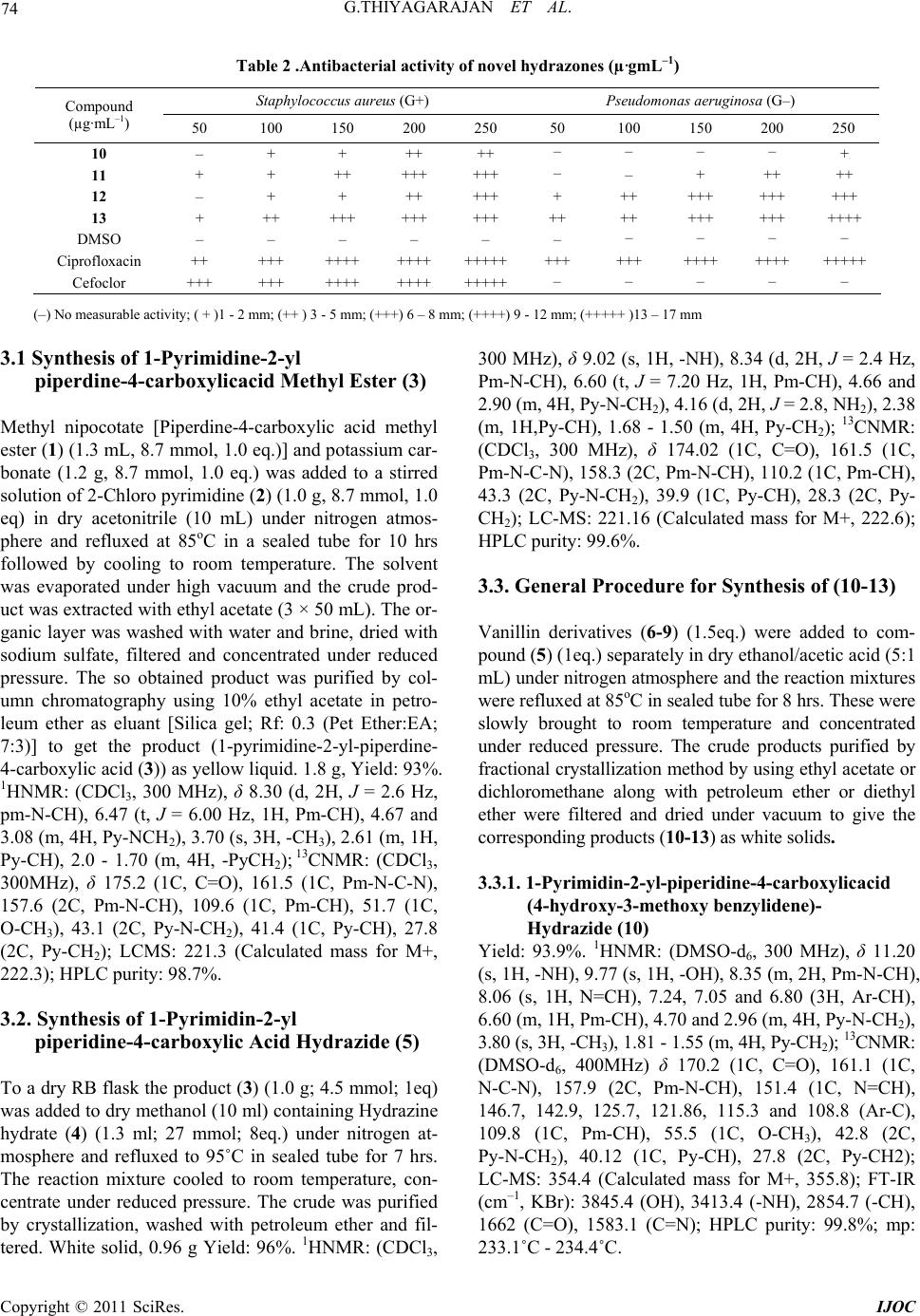 G.THIYAGARAJAN ET AL. 74 Table 2 .Antibacterial activity of novel hydrazones (µ·gmL–1) Staphylococcus aureus (G+) Pseudomonas aeruginosa (G–) Compound (µg·mL–1) 50 100 150 200 250 50 100 150 200 250 10 – + + ++ ++ – – – – + 11 + + ++ +++ +++ – – + ++ ++ 12 – + + ++ +++ + ++ +++ +++ +++ 13 + ++ +++ +++ +++ ++ ++ +++ +++ ++++ DMSO – – – – – – – – – – Ciprofloxacin ++ +++ ++++ ++++ ++++++++ +++ ++++ ++++ +++++ Cefoclor +++ +++ ++++ ++++ +++++– – – – – (–) No measurable activity; ( + )1 - 2 mm; (++ ) 3 - 5 mm; (+++) 6 – 8 mm; (++++) 9 - 12 mm; (+++++ )13 – 17 mm 3.1 Synthesis of 1-Pyrimidine-2-yl piperdine-4-carboxylicacid Methyl Ester (3) Methyl nipocotate [Piperdine-4-carboxylic acid methyl ester (1) (1.3 mL, 8.7 mmol, 1.0 eq.)] and potassium car- bonate (1.2 g, 8.7 mmol, 1.0 eq.) was added to a stirred solution of 2-Chloro pyrimidine (2) (1.0 g, 8.7 mmol, 1.0 eq) in dry acetonitrile (10 mL) under nitrogen atmos- phere and refluxed at 85oC in a sealed tube for 10 hrs followed by cooling to room temperature. The solvent was evaporated under high vacuum and the crude prod- uct was extracted with ethyl acetate (3 × 50 mL). The or- ganic layer was washed with water and brine, dried with sodium sulfate, filtered and concentrated under reduced pressure. The so obtained product was purified by col- umn chromatography using 10% ethyl acetate in petro- leum ether as eluant [Silica gel; Rf: 0.3 (Pet Ether:EA; 7:3)] to get the product (1-pyrimidine-2-yl-piperdine- 4-carboxylic acid (3)) as yellow liquid. 1.8 g, Yield: 93%. 1HNMR: (CDCl3, 300 MHz), δ 8.30 (d, 2H, J = 2.6 Hz, pm-N-CH), 6.47 (t, J = 6.00 Hz, 1H, Pm-CH), 4.67 and 3.08 (m, 4H, Py-NCH2), 3.70 (s, 3H, -CH3), 2.61 (m, 1H, Py-CH), 2.0 - 1.70 (m, 4H, -PyCH2); 13CNMR: (CDCl3, 300MHz), δ 175.2 (1C, C=O), 161.5 (1C, Pm-N-C-N), 157.6 (2C, Pm-N-CH), 109.6 (1C, Pm-CH), 51.7 (1C, O-CH3), 43.1 (2C, Py-N-CH2), 41.4 (1C, Py-CH), 27.8 (2C, Py-CH2); LCMS: 221.3 (Calculated mass for M+, 222.3); HPLC purity: 98.7%. 3.2. Synthesis of 1-Pyrimidin-2-yl piperidine-4-carboxylic Acid Hydrazide (5) To a dry RB flask the product (3) (1.0 g; 4.5 mmol; 1eq) was added to dry methanol (10 ml) containing Hydrazine hydrate (4) (1.3 ml; 27 mmol; 8eq.) under nitrogen at- mosphere and refluxed to 95˚C in sealed tube for 7 hrs. The reaction mixture cooled to room temperature, con- centrate under reduced pressure. The crude was purified by crystallization, washed with petroleum ether and fil- tered. White solid, 0.96 g Yield: 96%. 1HNMR: (CDCl3, 300 MHz), δ 9.02 (s, 1H, -NH), 8.34 (d, 2H, J = 2.4 Hz, Pm-N-CH), 6.60 (t, J = 7.20 Hz, 1H, Pm-CH), 4.66 and 2.90 (m, 4H, Py-N-CH2), 4.16 (d, 2H, J = 2.8, NH2), 2.38 (m, 1H,Py-CH), 1.68 - 1.50 (m, 4H, Py-CH2); 13CNMR: (CDCl3, 300 MHz), δ 174.02 (1C, C=O), 161.5 (1C, Pm-N-C-N), 158.3 (2C, Pm-N-CH), 110.2 (1C, Pm-CH), 43.3 (2C, Py-N-CH2), 39.9 (1C, Py-CH), 28.3 (2C, Py- CH2); LC-MS: 221.16 (Calculated mass for M+, 222.6); HPLC purity: 99.6%. 3.3. General Procedure for Synthesis of (10-13) Vanillin derivatives (6-9) (1.5eq.) were added to com- pound (5) (1eq.) separately in dry ethanol/acetic acid (5:1 mL) under nitrogen atmosphere and the reaction mixtures were refluxed at 85oC in sealed tube for 8 hrs. These were slowly brought to room temperature and concentrated under reduced pressure. The crude products purified by fractional crystallization method by using ethyl acetate or dichloromethane along with petroleum ether or diethyl ether were filtered and dried under vacuum to give the corresponding products (10-13) as white solids. 3.3.1. 1-Pyrim i di n-2 -yl-piperidin e-4 -car boxylicacid (4-hydroxy-3-methoxy benzylidene)- Hydrazide (10) Yield: 93.9%. 1HNMR: (DMSO-d6, 300 MHz), δ 11.20 (s, 1H, -NH), 9.77 (s, 1H, -OH), 8.35 (m, 2H, Pm-N-CH), 8.06 (s, 1H, N=CH), 7.24, 7.05 and 6.80 (3H, Ar-CH), 6.60 (m, 1H, Pm-CH), 4.70 and 2.96 (m, 4H, Py-N-CH2), 3.80 (s, 3H, -CH3), 1.81 - 1.55 (m, 4H, Py-CH2); 13CNMR: (DMSO-d6, 400MHz) δ 170.2 (1C, C=O), 161.1 (1C, N-C-N), 157.9 (2C, Pm-N-CH), 151.4 (1C, N=CH), 146.7, 142.9, 125.7, 121.86, 115.3 and 108.8 (Ar-C), 109.8 (1C, Pm-CH), 55.5 (1C, O-CH3), 42.8 (2C, Py-N-CH2), 40.12 (1C, Py-CH), 27.8 (2C, Py-CH2); LC-MS: 354.4 (Calculated mass for M+, 355.8); FT-IR (cm–1, KBr): 3845.4 (OH), 3413.4 (-NH), 2854.7 (-CH), 1662 (C=O), 1583.1 (C=N); HPLC purity: 99.8%; mp: 233.1˚C - 234.4˚C. Copyright © 2011 SciRes. IJOC  75 G.THIYAGARAJAN ET AL. 3.3.2. 1-Pyrim i di n-2 -yl-piperidin e-4 -car boxylicacid (3,4-dimethoxy benzylidene) Hydrazide (11) Yield: 96%. 1HNMR: (DMSO-d6, 300 MHz), δ 11.29 (m, 1H, -NH), 8.35 (d, J = 3.00 Hz, 2H, Pm-N-CH), 8.11 (s, 1H, N=CH), 7.27, 7.16 and 7.00 (3H, Ar-CH), 6.60 (m, 1H, Pm-CH), 4.70 and 2.96 (m, 4H, Py-N-CH2), 3.80 (s, 6H, O-CH3), 1.80 - 1.55 (m, 4H, Py-CH2);13CNMR: (DM-SO-d6, 300 MHz), δ 176.2 (1C, C=O), 161.6 (1C, N-C-N), 158.4 (2C, Pm-N-CH), 151.0 (1C, N=CH), 150.8, 149.4, 127.5, 122.0 and 112.1 (Ar-C), 108.9 (1C, Pm-CH), 108.7 (Ar-C), 56.0 (2C, O-CH3), 43.4 (2C, Py-N- CH2), 38.57 (1C, Py-CH), 28.3 (2C,Py-CH2); LC-MS: 369.5 (Calculated mass for M+, 370.2); FT-IR (cm–1, KBr): 3211.7 (NH), 2845.0 (-CH), 1652 (C=O), 1582 (C=N); HPLC purity: 99. 6%; mp: 220.6˚C - 221.8˚C. 3.3.3. 1-Pyrim i di n-2 -yl-piperidin e-4 -car boxylicacid (4-Butoxy-3-methoxy) Benzylidene Hydrazide (12) Yield: 96.1%. 1HNMR:(DMSO-d6, 300 MHz), δ 11.27 (m, 1H, -NH), 8.34 (d, J = 4.50 Hz, 2H, Pm-N-CH), 8.11 (s, 1H, N=CH), 7.25, 7.12 and 6.97 (3H,Ar-CH), 6.58 (m, 1H, Pm-CH), 4.68 and 2.93 (m, 4H, Py-CH2), 3.97 (m, 2H, -OCH2), 3.77 (m, 3H, -OCH3), 1.71 (m, 4H, -CH2- CH2), 1.40 (m, 4H, Py-CH2), 0.92 (m, 3H, -CH3). 13CNMR: ((DMSO-d6, 300MHz), 176.0 (1C, C=O), 161.6 (1C, N-C-N), 158.4 (2C, Pm-N-CH), 150.4 (1C, N=CH), 150.2, 149.61, 127.4, 122.0, 113.1 and 112.1 (Ar-CH), 110.2 (1C, Pm-CH), 68.3 (1C, O-CH2), 55.9 (1C, O-CH3), 43.4 (2C, Py-N-CH2), 41.5 (1C, Py-CH), 31.21 (1C, CH2), 28.3 (2C, Py-CH2), 19.1 (1C, CH2), 14.1 ((1C, -CH3); LC-MS: 411.41 (Calculated mass for M+, 412.8); IR(cm–1, KBr): 3208.2 (NH), 2867.8 (-CH), 1655 (C=O), 1583.9 (C=N); HPLC purity: 96.8%. mp: 213.8˚C - 215.1˚C. 3.3.4. 1-Pyrim i di n-2 -yl-piperidin e-4 -car boxylicacid [3-methoxy-4-(2-methoxy ethoxy)-benzylidene Hydrazide (13) Yield: 78.1%. 1HNMR: (DMSO-d6, 300MHz), δ 11.25 (m, 1H, -NH), 8.35 (d, J = 2.8Hz, 2H, Pm-N-CH), 7.90 (s, 1H, N=CH), 7.27, 7.13 and 6.99 (3H, Ar-CH), 6.60 (m, 1H, Pm-CH), 4.70 and 2.95 (m, 4H, Py-N-CH2), 4.10 (m, 2H, -O-CH2), 3.65 (m, 2H, -CH2), 3.31 (d, J=7.8 Hz, 6H, -O-CH3), 1.81 - 1.52 (m, 4H, Py-CH2); 13CNMR: (CDCl3, 400 MHz), δ 176.7 (1C, C=O), 161.5 (1C, N-C-N), 157.7 (2C, Pm-N-CH), 150.3 (1C, N=CH), 149.84, 143.1, 127.0, 121.4 and 112.8 (Ar-C), 109.6 (1C, Pm-CH), 108.7 (Ar-C), 70.8, 68.3 (CH2-O-CH2), 59.29 (1C, O-CH3), 55.8 (1C,O-CH3), 43.4 (2C, Py-N-CH2), 39.0 (1C, Py-CH), 27.6 (2C, Py-CH2); LC-MS: 413.5 (Calculated mass for +M, 414.8); FT-IR (cm–1, KBr): 3173.4 (NH), 2854.7 (-CH), 1659 (C=O), 1586.7 (C=N); HPLC purity: 97.8%; mp: 188.1˚C - 189.1˚C. 3.4. Anti-Bacterial Assay All the synthesized hydrazones were tested for their anti-bacterial activity against a set of bacterial strains, namely, Staphylococcus aureus, and Pseudomonas aeru- ginosa by paper disc diffusion method with different concentrations of the solutions prepared in Dimethyl sulfoxide (DMSO). The reason of choosing DMSO for antibacterial studies was that it has no effect on the above mentioned bacterial strains. Nutrient agar was used as the culture medium for the growth of bacterial colony that was prepared by using peptone (3.0 g), NaCl (3.0 g), Yeast (1.5 g), Agar (6.0 g) in 300 mL of distilled water with pH at 7.0 The as prepared medium is auto- claved at 15 pa for 20 minutes and kept at 85ºC for 30 minutes to sterilize the media. This media was then poured into petridishes slowly in laminar flow environ- ment, allowed to solidify and kept at 30˚C for 24 hrs. The bacterial strains were inoculated by spreading in peptidases and its temperature is maintained at 30˚C for 24 hr. Using paper disc (8 mm) in nutrient agar culture medium, different concentrations (50, 100, 150, 200, 250 µg/mL) of the newly synthesized hydrazones (10-13) were loaded through bacteria free micro pipettes. The anti-bacterial activity was determined by measuring the zone of inhibition in millimeters and compared with standard drug Ciprofloxacin and Cefaclor. 4. Conclusions We have developed the simple and crucial synthetic technique of vanillin related hydrazone derivatives and the reactions occurred very fast, under mild condition using reasonable reagents and solvents, yield is also higher. The anti-bacterial activity of synthesized novel hydrazones were effectively screened against Gram posi- tive S. aureus and Gram-negative P. aeruginosa bacterial strains. Most of these compounds show moderate anti- bacterial activity comparable with to marketable com- pounds. The zone of inhibition of tested compounds shows, the vanillin coupled hydrazone derivatives en- compass potent bio-activities against bacterial strains. Due to the strong bio-activity of our synthesized hydra- zones can be further allowed to attempt other bio-acti- vities against a number of diseases and this work will be precious for further studies in terms of toxicity effect and Quantity Structural Activity Relationship (QSAR) to improve their biological and pharmacological properties. 5. Acknowledgements We are appreciative to Dr. A. Thamaraichelvan and Dr. M.Ganesan, Department of Chemistry, Thiagarajar Col- Copyright © 2011 SciRes. IJOC  G.THIYAGARAJAN ET AL. 76 lege, Madurai, India for giving constant support to this research work. Funding support from the DST project SR/ME/S-3/0016/2008 is also acknowledged. 6. References [1] S. Riva and L. G. Silvestri, “Rifamycine: A General View,” Annual Review of Microbiology, Vol. 26, 1972, pp. 199-224. doi:10.1146/annurev.mi.26.100172.001215 [2] V. I. Yudelevich, V. V. Belakhov, E. V. Komarov, B. I. Ionin, G. L. Myasnikova, M. S. Polyak, M. A. Shneider and L. A. Rachkovskaya, “Synthesis and Biological Ac- tivity of New Derivatives of 1,2,3,4-Tetrahydroquina- zolin-4-one,” Pharmaceutical Chemistry Journal, Vol. 18, No. 10, 1984, pp. 704-707. doi:10.1007/BF00773018 [3] S. Rollas and S. G. Kucukguzel, “Biological Activities of Hydrazone Derivatives,” Molecules, Vol. 12, No. 8, 2007, pp. 1910-1939. [4] O. A. Olayinka, A. O. Craig, C. N. Obinna and A. A. David, “Microwave Assisted Synthesis and Antimicrobial Activity of 2-Quinoxalinone-3-hydra-zone Derivatives,” Bioorganic & Medicinal Chemistry, Vol. 18, No. 1, 2010, pp. 214-221. doi:10.1016/j.bmc.2009.10.064 [5] S. Rollas, N. Gulerman and H. Edeniz, “Synthesis and Antimicrobial Activity of Some New Hydrazones of 4-Fluorobenzoic Acid Hydrazide and 3-Acetyl-2,5-dis- ubstituted-1,3,4-oxadiazolines,” IL Farmaco, Vol. 57, No. 2,. 2002, pp. 171-174. [6] A. Cukurovali, B. Yilmaz, S. Gur and C. Kazaz, “Syn- thesis, Antibacterial and Antifungal Activity of Some New Thiazolylhydrazone Derivatives Containing 3-Sub- stituted Cyclobutane Ring,” European Journal of Medi- cinal Chemistry, Vol. 41, No. 2, 2006, pp. 201-207. doi:10.1016/j.ejmech.2005.01.013 [7] J. Capilla, C. Serena, F. Javier, T. Ortoneda and J. Guarro, “Efficacy of Voriconazole in Treatment of Systemic Sce- dosporiosis in Neutropenic Mice,” Antimicrobial Agents Chemother, Vol. 47, No. 12, 2003, pp. 3976-3978. doi:10.1128/AAC.47.12.3976-3978.2003 [8] M. G. Mamolo, V. Falagiani, D. Zampieri, U. Vio, E. Banfi and G. Scialino, “Synthesis and Antimycobacterial Activity of (3,4-Diaryl-3H-thiazol-2-ylidene)-hydrazide Derivatives,” IL Farmaco, Vol. 58, No. 9, 2003, pp. 631- 637. [9] J. R. Dimmock, S. C. Vasishtha and J. P. Stables, “Anti- convulsant Properties of Various Acetylhydrazones, Oxa- moylhydrazones and Semicarbazones Derived from Aro- matic and Unsaturated Carbonyl Compounds,” European Journal of Medicinal Chemistry, Vol. 35, No. 2, pp. 241- 248. [10] P. C. Lima, L. M. Lima, K. C. Silva, P. H. Leda, A. L. P. Miranda, C. A. M. Fraga and E. J. Barreiro, “Synthesis and Analgesic Activity of Novel N-Acylarylhydrazones and Isosters, Derived from Natural Safrole,” European Journal of Medicinal Chemistry, Vol. 35, No. 2, 2000, pp. 187-203. doi:10.1016/S0223-5234(00)00120-3 [11] G. U. Salgin, K. N. Gokham, O. Gostal, Y. Koysal, E. Kilici, S. Isik, G. Aktay and M. Ozalp, “1-Acylthiose- micarbazides, 1,2,4-Triazole-5(4H)-thiones, 1,3,4-Thia- diazoles and Hydrazones Containing 5-Methyl-2-Benzo- xazolinones: Synthesis, Analgesic-Anti-Inflammatory and Antimicrobial Activities,” Bioorganic & Medicinal Che- mistry, Vol. 15, No. 17, 2007, pp. 5738-5751. doi:10.1016/j.bmc.2007.06.006 [12] A. R. Todeschini, A. L. Miranda, C. M. Silva, S. C. Par- rini and E. J. Barreiro, “Synthesis and Evaluation of An- algesic, Anti-Inflammatory and Antiplatelet Properties of New 2-Pyridylarylhydrazone Derivatives,” European Jour- nal of Medicinal Chemistry, Vol. 33, No. 3, 1998, pp. 189-199. doi:10.1016/S0223-5234(98)80008-1 [13] G. A. Silva, L. M. M. Costa, F. C. B. Brito, A. L. P Miranda, E. J. Barreiro and C. A. M. Fraga, “New Class of Potent Antinociceptive and Antiplatelet 10H-Pheno- thiazine-1-Acylhydrazone Derivatives,” Bioorganic & Medicinal Chemistry, Vol. 12, No. 12, 2004, pp. 3149- 3158. doi:10.1016/j.bmc.2004.04.009 [14] A. Imramovsky, S. Polanc, J. Vinsova, M. Kocevar, J. Jampitek, Z. Reckova and J. A. Kaustova, “A New Modi- fication of Anti-Tubercular Active Molecules,” Bioor- ganic & Medicinal Chemistry, Vol. 15, No. 7, 2007, pp. 2551-2559. doi:10.1016/j.bmc.2007.01.051 [15] Y. Janin, “Antituberculosis Drugs: Ten Years of Research,” Bioorganic & Medicinal Chemistry, Vol. 15, No. 7, 2007, pp. 2479-2513. doi:10.1016/j.bmc.2007.01.030 [16] L. C. du Toit, V. Pillay and M. P. Danckwerts, “Tuber- culosis Chemotherapy: Current Drug Delivery Approa- ches,” Respiratory Research, Vol. 7, 2006, p. 118. doi:10.1186/1465-9921-7-118 [17] L. Savini, L. Chiasserini, V. Travagli, C. Pellerano, E. Novellino, S. Consentino and M. B. Pisano, “New α-(N)- Heterocyclichydrazones: Evaluation of Anticancer, Anti- HIV and Antimicrobial Activity,” European Journal of Medicinal Chemistry, Vol. 39, No. 2, 2004, pp. 113-122. doi:10.1016/j.ejmech.2003.09.012 [18] A. M. El-Hawash, W. A. E Abdel and M. A. El-Dewe- llawy, “Cyanoacetic Acid Hydrazones of 3-(and 4-)Acetyl- pyridine and Some Derived Ring Systems as Potential Antitumor and Anti-HCV Agents,” Archiv der Pharmazie, Vol. 339, No. 1, 2006, pp. 14-23. doi:10.1002/ardp.200500161 [19] M. T. Cocco, C. Congiu, V. Lilliu and V. Onnis, “Syn- thesis and in Vitro Antitumoral Activity of New Hy- drazinopyrimidine-5-carbonitrile Derivatives,” Bioorga- nic & Medicinal Chemistry, Vol. 14, No. 2, 2006, pp. 366-372. doi:10.1016/j.bmc.2005.08.012 [20] S. G. Kucukguzel, A. Mazi, F. Sahin, S. Ozturk and J. Stables, “Synthesis and Biological Activities of Diflunisal Hydrazide-Hydrazones,” European Journal of Medicinal Chemistry, Vol. 38, No. 11-12, 2003, pp. 1005-1013. doi:10.1016/j.ejmech.2003.08.004 [21] V. Sridharan, P. T. Perumal, C. Avendano and J. C. Menendez, “The First Aza Diels-Alder Reaction Involv- ing an α,β-Unsaturated Hydrazone as the Dienophile: Stereoselective Synthesis of C-4 Functionalized 1,2,3,4- Tetrahydroquinolines Containing a Quaternary Sterecen- Copyright © 2011 SciRes. IJOC  G.THIYAGARAJAN ET AL. Copyright © 2011 SciRes. IJOC 77 ter,” Organic & Biomolecular Chemistry, Vol. 5, 2007, pp. 1351-1353. doi:10.1039/b703083e [22] A. Espinel-Ingroff, “Standardized Disk Diffusion Method for Yeasts,” Clinical Microbiology Newsletter, Vol. 29, No. 13, 2007, pp. 97-100. doi:10.1016/j.clinmicnews.2007.06.001 [23] W. L. F Armarego and C. L. L. Chai, “Purification of Laboratory Chemicals,” 5th Edition, Elsevier, Amster- dam, 2003. [24] A. R. Katritzky, H.-Y. He, Q. H. Long and A. L. Wilcoxb, “Preparation of 2,6-Dialkoxybenzaldehydes,” ARKIVOC, Vol. 3, 2001, pp. 3-12. [25] F. S. Pashkovskii, E. M. Shchukina, M. G. Gribovskii and F. A. Lakhvich, “Heterocyclic Analogs of Prostaglandins: IV. Synthesis of 3,7-Interphenylene 3,10(11)-dioxa-13- azaprostanoids and 9-oxa-7-azaprostanoids Based on Te- tronic Acid and Aromatic Aldehydes,” Russian Journal of Organic Chemistry, Vol. 44, No. 5, 2008, pp. 657-670. doi:10.1134/S1070428008050047
|