 American Journal of Analytical Chemistry, 2011, 2, 605-611 doi:10.4236/ajac.2011.25068 Published Online September 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Determination of Mercuric Ion in Water Samples with a LED Exciting and CCD Based Portable Spectrofluorimeter Arsenio Muñoz de la Peña1*, María Isabel Rodríguez-Cáceres1, Diego Bohoyo Gil1, María del Carmen Mahedero1, María del Carmen Hurtado-Sánchez1, Reyes Babiano2 1Department of Analytical Chemistry, University of Extremadura, Badajoz, Spain 2Department of Organic and Inorganic Chemistry, University of Extremadura, Badajoz, Spain E-mail: *arsenio@unex.es Received March 29, 2011; revised June 3, 2011; accepted June 15, 2011 Abstract The fluorescent characteristics of a fluorimetric chemosensor for mercuric ion, Hg2+, employing a synthe- sized Rhodamine 6G derivative, have been analyzed. For that, a portable spectrofluorimeter composed of a 515 nm LED as excitation source, two fiber-optics and a CCD camera as detector, has been used, intended for “in situ” analysis. A highly selective Rhodamine based probe for Hg2+, that is water soluble and gives a positive response upon analyte binding, is reported. The reagent is bearing a monothiospirolactone group in a Rhodamine 6G architecture and the thiol atom served for the direct attack of thiophilic Hg2+. The fluores- cence enhancement is attributed to the spirolactone ring opening and the coordination of two sulphur atoms to Hg2+ giving a 2:1 reagent: Hg2+ stoichiometry complex. Keywords: Chemosensor, LED, Mercuric Ion, Water Analysis 1. Introduction Mercury is widely distributed in the air, water and soil, and is considered by the Environmental Protection Agency (EPA) to be a highly dangerous element because of its severe inmunotoxic, genotoxic and neurotoxic effects [1]. It is generated by volcanic emission and in several hu- man activities as gold mining, combustion of solid waste or burning of fossil fuels. The great time of residence of mercury in vapour state and his easy oxidation to inor- ganic species of Hg2+ soluble in water have caused envi- ronmental Hg2+ levels. Mercury easily passes through biological membranes such as skin, respiratory and gas- trointestinal tissues. When absorbed in human body, mercury causes damage to the central nervous and endo- crine systems as well as neurological irreversible dam- ages. The toxicity of mercury is known to be highly de- pendent on its chemical form: organomercury is gener- ally more toxic than inorganic mercury salts [2]. Recently, a variety of selective and sensitive fluores- cent Hg2+ chemosensors have been developed based on fluorescein derivatives [3-6], NO2S2-donor macrocyle [7], naphthalimide [8,9], BODIPY [10-12] or Rhodamine derivatives [13-23]. BODIPY (boron dipyrromethene) fluorophores have been widely used for the determination of Hg(II). Those fluorophores have high absorption coefficient (ε > 50000 M–1 cm–1), high fluorescence quantum yield (Φ > 0.5), and high photostability [24]. Polyamide receptor photo- electron transfer (PET) based fluorescent sensor mole- cules could be used to detect Hg2+ ions with either fluo- rescence off-on response or fluorescence colour change [8]. J. Wang et al. used a series of polyamide receptors incorporating two, three and four amide arms to the BODIPY fluorophore. They observed that a clear emis- sion turn-on response when as low as 2 ppb (content limit in drinking water set by EPA) of Hg(II) are present. Thus, the sensor is practical as Hg2+ ion “annunciator” for drinking water [10]. On the other hand, J. Du et al. synthesized a highly selective PET fluorescent sensor for Hg2+ containing a BODIPY fluorophore and a NS2O2 pentachelating receptor. With this sensor the Hg2+ could be detected in a wide pH range [11]. Also, thiacrown and crown ethers have been appended to BODIPY fluoro- phores [12]. Rhodamines are classic dyes/fluorophores whose photo- chemical properties have already been well studied. Be- cause of their low cost, long-wavelength (> 500 nm) ab- sorption/emission and high molar absorption coefficient and quantum yield, these fluorophores are usually util-  606 A. M. de la PEÑA ET AL. ized as reporting groups in routine optical analysis. The metal ion sensing behaviour of these rhodamine-based optical sensors is very interesting. Typically, in the ab- sence of metal ions, the sensor molecules prefer their spirolactam ring-closed state, which shows little absorp- tion or fluorescence in the visible range (400 - 700 nm). However, upon the addition of specific metal ions, the chelating or reaction of metal ions with sensor molecules will simultaneously open the spirolactam ring and make the sensors converted into their ring-opened state, which is highly absorbent and fluorescent above 500 nm [25]. Y.-K. Yang et al. have developed a rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg ions in aqueous media. The selec- tivity of the system for mercuric ion over other metal ions is remarkably high, and its sensitivity is below 2 ppb in aqueous solution [13]. On the other hand, a rhodamine thiospirolactone chemosensor was developed by X.-Q. Zhan et al. [14] for the selective and sensitive reversible sensing of Hg2+. W. Shi and H. Ma develop a rhodamine B thiolactone for Hg2+ in neutral solution with good linearity between 0.5 and 5 μM and detection limit 20 nM [15]. S.-K. Ko et al. develop a rhodamine-based mo- lecular probe for in vivo monitor mercury ions in living cells and vertebrate organisms in aqueous media. The results of the experiments shown that the chemosensor responds selectively to mercury ions over other metal ions and it is possible to detect the accumulation of mer- cury ions in zebrafish tissue and organs [16]. A rhoda- mine B thiohydrazide assay has been developed by H. Zheng et al. in which Hg(II) could be detected fluorimet- rically at least down to 5.0 × 10–8 M [17]. Finally, H.N. Kim et al. developed a method to determine mercury in vivo in the nematode Caenorhabditis elegans, previously incubated with Hg(ClO4)2 in concentrations of Hg2+ comprised between 1 nm and 1 µM [19]. Moreover, Rhodamine has been employed to deter- mine organic derivatives of mercury such as methylmer- cury. This compound is produced by aquatic microor- ganism from inorganic mercury and is one of the most toxic species of mercury. Methylmercury passes through biological membranes causing severe damage to various tissues and organs humans. So, Yang et al. [20] have been developed a method to determine this compound with a rhodamine hydrazide derivative. The analysis is based in the desulfurization of the derivative in presence of methylmercury and formation of the cyclic fluorescent compound, and the method was applied in zebrafish. The same chemosensor was used by Muñoz de la Peña et al. for the determination of Hg2+ in water and fish samples [21,22]. Recently, derivatives for determination of mercury have been developed from rhodamine in combination with pyrene [23]. The use of pyrene provided a deriva- tive with excellent spectroscopic properties such as high molar absorption coefficients and high fluorescence quan- tum yields. The semiconductor light sources, as laser diodes (LDs) and ultrabright light emitting diodes (LEDs), are a huge improvement in fluorescence applications. LED is a semiconductor p-n junction device, which emits light in a narrow spectrum, produced by a form of electrolumines- cence [26]. LEDs are cheap and commercially available from 350 nm to the near infrared. LEDs with different colours and white light have been developed. High- brightness LEDs are becoming increasingly important because of their potential applications. Light is generated from the active region of LED spontaneously in all di- rections and escape from LED die. Commercially manu- factured LEDs consists of a small point like light emit- ting active area typically ≈ 1 mm2 surrounded by high refractive-index semiconductor surface shape [27]. A general overview of LDs and LEDs is given by Landgraf [28]. Among other applications, different LEDs and filter combinations have been tested for the analysis of crude oil fluorescence. In the work described here, a LED based spectro- fluorimeter combined with fiber-optics and a CCD de- tector has been tested, in order to check its selectivity and sensitivity for the determination of mercuric ion in aqueous samples, using a highly selective synthesized monothiospirolactone Rhodamine 6G derivative. 2. Experimental Procedures 2.1. Synthesis and Characterization of Rhodamine-Derivative I Rhodamine derivative I was synthesized by a similar procedure, which has been already reported [29]. Rho- damine 6 G (R6G) (3 g, 6.3 mmol) was added to the so- lution of NaOH (4.8 g, 0.12 mol) in ethanol (30 mL) and water (60 mL). After refluxed for 12 h, the reaction mix- ture was cooled and ethanol was evaporated in vacuo, then adjusted pH to 6 - 7 using hydrochloric acid (2 M). The formed precipitate was filtered, washed several times with water and dried to give 2.88 g Rhodamine- derivative II as a red solid (yield 96%). To a stirred solution of II (2 g, 4.8 mmol) in 1,2-di- chloroethane (15 mL), phosphorus oxychloride (3 mL) was added dropwise. After refluxed for 4 h, the reaction mixture was cooled and evaporated in vacuo. The crude acid chloride was dissolved in THF (6 mL), and the re- sulting solution was then added dropwise to a mixed so- lution of thiourea (1.52 g, 20 mmol) and triethylamine (12 mL) in THF (50 mL)/water (10 mL) at room tem- perature. After stirring over night, the solvent was re- Copyright © 2011 SciRes. AJAC  A. M. de la PEÑA ET AL. 607 moved under reduced pressure. Then, 50 mL of water was added, and the formed precipitate was filtered. The precipitate was washed several times with water and dried. The crude product was purified by silica-gel col- umn chromatography with CH2Cl2 as eluent, affording 1.0 g of Rhodamine-derivative I (yield 50%). The structure of Rhodamine-derivative I was con- firmed by 1H NMR and 13C NMR. 1H NMR’s study of Rhodamine-derivative I structure agrees with the as- signed structure. At low fields there are four protons (7.89, 7.55, 7.49 and 7.18 ppm) of the ring of ben- zothiolane-2-one with their expected multiplicity, fol- lowed by two singlets (6.55 and 6.36 ppm), correspond- ing to the protons of the xanthene ring, and the reso- nances of the groups N-Et (3.23 ppm and 1.34 ppm) and Me (1.98 ppm). A second set of signals appears in the 1H NMR spectrum, probably due to the balance with the opened structure, which appears in approximately 20% in chloroform. The spectrum of 13C NMR shows also relevant signals, as the resonance at 62.8 ppm, that is assigned to the quaternary carbon of the spiranic bridge, and at 197.90 ppm, assigned to CO-S. 2.2. Chemicals and Reagents All chemical and solvents used in this study were of analytical grade. Ultra pure water was provided by use of a Millipore Milli-Q system (Millipore, Bedford, MA, USA). Mercury chloride (II) was obtained from Panreac. The standard stock solution of mercury (II) (1 mM) and the HEPES buffer (Sigma) 0.02 M (pH 7.4) were prepared in ultra pure water. The Rhodamine-derivative I (10–3 M) was prepared in acetonitrile. Diluted solutions of the stock solutions were prepared by appropriate dilution. Standard Reference Material 1641d was purchased from NIST, containing 1.557 ± 0.020 mg/kg of Hg2+. 2.3. Apparatus UV-Vis measurements were performed using a Spectro- photometer Varian Model Cary 50 Bio, equipped with a Xenon lamp. For the studies of the influence of physical and chemi- cal variables on the reaction, fluorescence measurements were performed using a Fluorescence Spectrophotometer Varian Model Cary Eclipse, equipped with a Xenon flash lamp. Excitation and emission bandwidths of 2.5 nm were used in these studies. Intensity was measured at 554 nm (excitation at 527 nm), which is the emission maxi- mum of the complex Rhodamine-derivative I-Hg2+. All measurements were performed in 10 mm quartz cells at 10˚C, by use of a thermostatically controlled cell holder and a Selecta Model 382 thermostatically controlled wa- ter-bath. On the other hand, a portable instrument AvaSpec- 2048-USB2-SPU was used. The instrument is equipped with a Cerny-Turner symmetric monocromator, with 75 mm of focal distance, and a CCD detector of 2048 pixels connected to an USB2 interface. A Prizmatix Black- LED-515, exciting at 515 nm (spectrum half width 36 nm), equipped with a filter Omega Optical 525AF45 (XF1074), to suppress the emission of LED further than 525 nm, was employed. The AvaSoft Full Software was used for data acquisition and analysis. All measurements were carried out after 5 minutes of turning on the LED to ensure a constant operating temperature. 1H and 13C NMR spectra were recorded on a Bruker 400 AC/PC instrument at 400 and 100 MHz, respectively in CDCl3. Assignments were confirmed by homo- and hetero-nuclear double-resonance, DEPT (distortionless enhancement by polarization transfer). TMS was used as the internal standard (δ = 0.00 ppm) and all J values are given in Hz. A Crison MicropH 501 meter was used for pH measurements. 2.4. General Procedure for Fluorescence Measurements In a fluorescence cell, 30 μL of Rhodamine-derivative I in acetonitrile (10–3 M) were placed. Later, an aliquot of the mercuric ion stock solution (1 mM) to give the final concentration desired is added, followed by 1.5 mL of HEPES buffer (0.02 M, pH 7.4). Then, the solution was diluted with ultra pure water to complete 3 mL, and the fluorescence was measured at 554 nm (excitation at 527 nm) in the Varian spectrofluorimeter or at 555 nm (exci- tation at 515 nm) in the portable instrument. For the determination of mercury (II) at low concen- trations, a mercury (II) stock solution of 10 µM was pre- pared, by diluting the original stock solution, and proper amounts of this solution were used in the analytical pro- cedure. In those cases, fluorescence measurements were carried out after addiction of Hg2+ for 5 min. 2.5. Procedure for Determination of Mercury(II) in Certified Samples In a fluorescence cell, 30 μL of Rhodamine-derivative I in acetonitrile (10–3 M) was placed. Later, an aliquot of the certified sample (Standard Reference Material 1641d) to give the final concentration desired is added (155 μL), followed by 1.5 mL of HEPES buffer (0.02 M, pH 7.4), and ultra pure water to complete 3 mL. The fluorescence was measured at 555 nm (excitation at 515 nm) in the portable instrument. Copyright © 2011 SciRes. AJAC 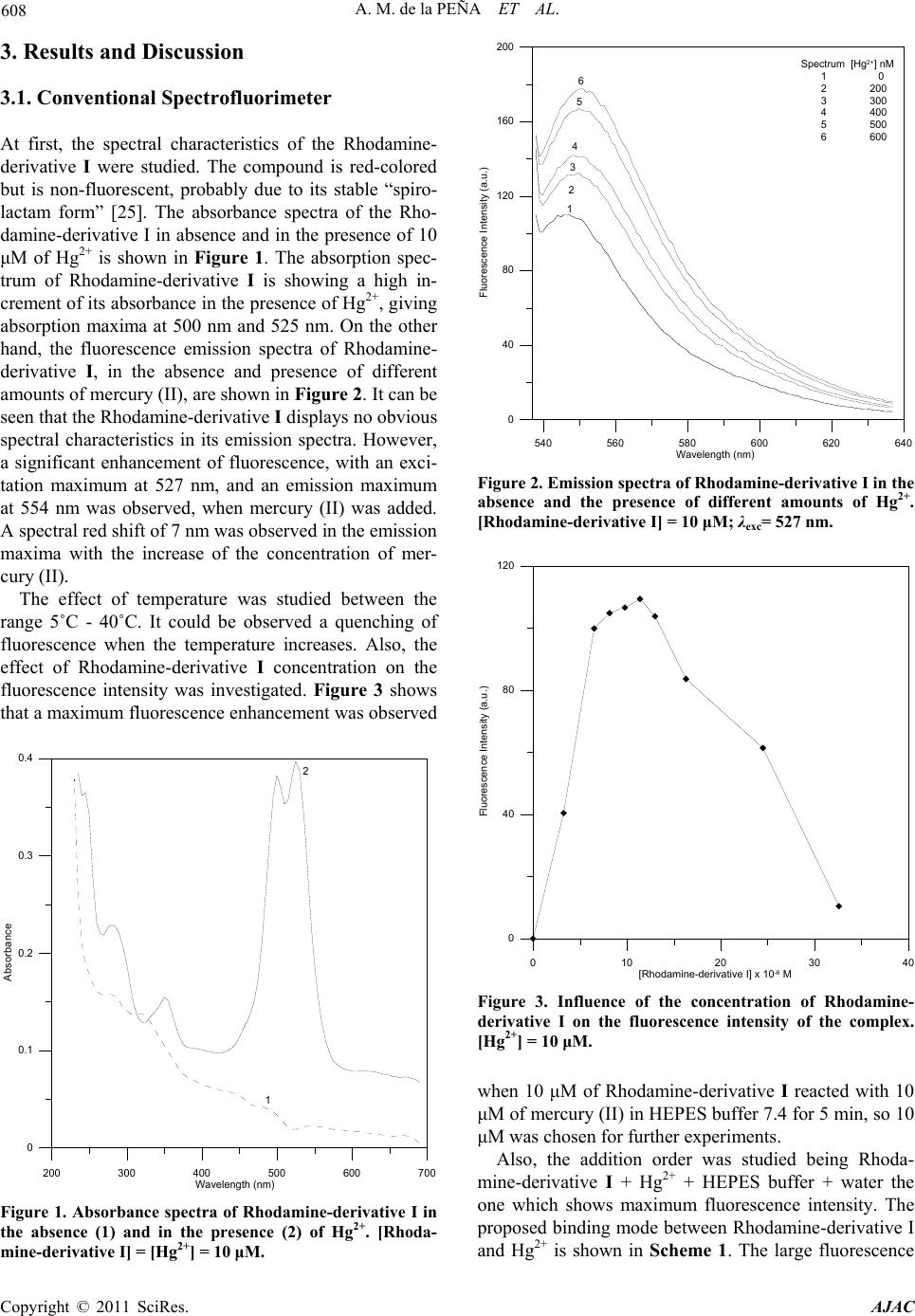 608 A. M. de la PEÑA ET AL. 3. Results and Discussion 3.1. Conventional Spectrofluorimeter At first, the spectral characteristics of the Rhodamine- derivative I were studied. The compound is red-colored but is non-fluorescent, probably due to its stable “spiro- lactam form” [25]. The absorbance spectra of the Rho- damine-derivative I in absence and in the presence of 10 μM of Hg2+ is shown in Figure 1. The absorption spec- trum of Rhodamine-derivative I is showing a high in- crement of its absorbance in the presence of Hg2+, giving absorption maxima at 500 nm and 525 nm. On the other hand, the fluorescence emission spectra of Rhodamine- derivative I, in the absence and presence of different amounts of mercury (II), are shown in Figure 2. It can be seen that the Rhodamine-derivative I displays no obvious spectral characteristics in its emission spectra. However, a significant enhancement of fluorescence, with an exci- tation maximum at 527 nm, and an emission maximum at 554 nm was observed, when mercury (II) was added. A spectral red shift of 7 nm was observed in the emission maxima with the increase of the concentration of mer- cury (II). The effect of temperature was studied between the range 5˚C - 40˚C. It could be observed a quenching of fluorescence when the temperature increases. Also, the effect of Rhodamine-derivative I concentration on the fluorescence intensity was investigated. Figure 3 shows that a maximum fluorescence enhancement was observed 200 300 400 500 600 700 Wavelength (nm) 0 0.1 0.2 0.3 0.4 Absorbance 1 2 Figure 1. Absorbance spectra of Rhodamine-derivative I in the absence (1) and in the presence (2) of Hg2+. [Rhoda- mine-derivative I] = [Hg2+] = 10 μM. 540 560 580 600 620 640 Wavelength (nm) 0 40 80 120 160 200 Fluo escence Intensity (a.u.) Spectrum [Hg 2+ ] nM 1 0 2 200 3 300 4 400 5 500 6 600 1 2 3 4 5 6 Figure 2. Emission spectra of Rhodamine-derivative I in the absence and the presence of different amounts of Hg2+. [Rhodamine-derivative I] = 10 μM; λexc= 527 nm. 0 40 80 120 Fluo 0 10203040 escence Int ensity (a.u.) [Rhodamine-der i vative I] x 10 -6 M Figure 3. Influence of the concentration of Rhodamine- derivative I on the fluorescence intensity of the complex. [Hg2+] = 10 μM. when 10 μM of Rhodamine-derivative I reacted with 10 μM of mercury (II) in HEPES buffer 7.4 for 5 min, so 10 μM was chosen for further experiments. Also, the addition order was studied being Rhoda- mine-derivative I + Hg2+ + HEPES buffer + water the one which shows maximum fluorescence intensity. The proposed binding mode between Rhodamine-derivative I and Hg2+ is shown in Scheme 1. The large fluorescence Copyright © 2011 SciRes. AJAC 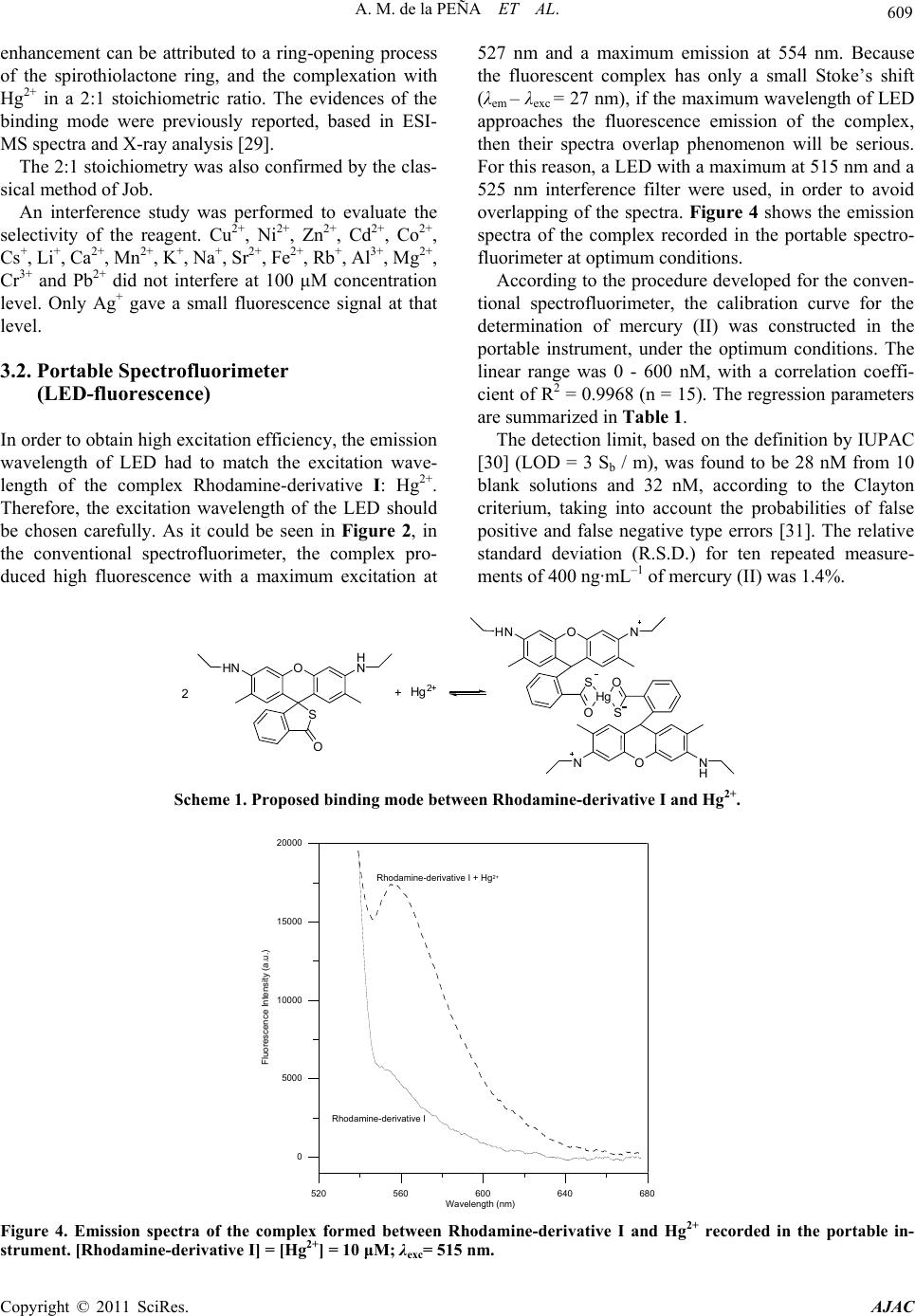 A. M. de la PEÑA ET AL. Copyright © 2011 SciRes. AJAC 609 enhancement can be attributed to a ring-opening process of the spirothiolactone ring, and the complexation with Hg2+ in a 2:1 stoichiometric ratio. The evidences of the binding mode were previously reported, based in ESI- MS spectra and X-ray analysis [29]. The 2:1 stoichiometry was also confirmed by the clas- sical method of Job. An interference study was performed to evaluate the selectivity of the reagent. Cu2+, Ni2+, Zn2+, Cd2+, Co2+, Cs+, Li+, Ca2+, Mn2+, K+, Na+, Sr2+, Fe2+, Rb+, Al3+, Mg2+, Cr3+ and Pb2+ did not interfere at 100 μM concentration level. Only Ag+ gave a small fluorescence signal at that level. 3.2. Portable Spectrofluorimeter (LED-fluorescence) In order to obtain high excitation efficiency, the emission wavelength of LED had to match the excitation wave- length of the complex Rhodamine-derivative I: Hg2+. Therefore, the excitation wavelength of the LED should be chosen carefully. As it could be seen in Figure 2, in the conventional spectrofluorimeter, the complex pro- duced high fluorescence with a maximum excitation at 527 nm and a maximum emission at 554 nm. Because the fluorescent complex has only a small Stoke’s shift (λem – λexc = 27 nm), if the maximum wavelength of LED approaches the fluorescence emission of the complex, then their spectra overlap phenomenon will be serious. For this reason, a LED with a maximum at 515 nm and a 525 nm interference filter were used, in order to avoid overlapping of the spectra. Figure 4 shows the emission spectra of the complex recorded in the portable spectro- fluorimeter at optimum conditions. According to the procedure developed for the conven- tional spectrofluorimeter, the calibration curve for the determination of mercury (II) was constructed in the portable instrument, under the optimum conditions. The linear range was 0 - 600 nM, with a correlation coeffi- cient of R2 = 0.9968 (n = 15). The regression parameters are summarized in Table 1. The detection limit, based on the definition by IUPAC [30] (LOD = 3 Sb / m), was found to be 28 nM from 10 blank solutions and 32 nM, according to the Clayton criterium, taking into account the probabilities of false positive and false negative type errors [31]. The relative standard deviation (R.S.D.) for ten repeated measure- ments of 400 ng·mL–1 of mercury (II) was 1.4%. OHN S O H N OHN S O N HgO S O N H N Hg 2+ 2+ Scheme 1. Proposed binding mode between Rhodamine-derivative I and Hg2+. 520 560 600 640 680 Wavelength (nm ) 0 5000 10000 15000 20000 Fluorescence Intensity (a.u. ) Rhodamine-derivative I Rhodamine-derivative I + Hg 2+ Figure 4. Emission spectra of the complex formed between Rhodamine-derivative I and Hg2+ recorded in the portable in- strument. [Rhodamine-derivative I] = [Hg2+] = 10 μM; λexc= 515 nm.  610 A. M. de la PEÑA ET AL. Table 1. Regression parameters for the determination of mercury (II) in the portable instrument. Portable Linear range (nM) 0 - 600 Slope (nM) 5561 Intercept 16.3 St. Dev. of slope 87 St. Dev. of Intercept 0.3 Linearity (%) 98.4 RSD (%) (n = 10) 1.4 R2 0.9968 LOQ (IUPAC), nM 93 LOD (IUPAC), nM 28 LOD* (Clayton, = = 0.05), nM 32 *LOD according to the criterium of Clayton et al. (Ref. 31) The proposed method was validated by application to the certified sample Standard Reference Material 1641d. Three replicates were analyzed and satisfactory results were found with the portable instrument, suggesting the potential application of the reagent for the better analysis of environmental water samples. 4. Conclusions In conclusion, the Rhodamine 6G monothiolactone de- rivative was synthesized and characterized. Its absorp- tiometric and fluorimetric properties, in absence and in the presence of Hg2+, were established, and a calibration study was performed and validated by using of a certified sample reference material. A suitable method for “in situ” analysis of Hg2+ was developed, using a portable instrument, composed of a 515 nm LED as excitation source, two fiber optics and a CCD camera as detector. The high selectivity of the method towards other metal ions was corroborated by an interference study. 5. Acknowledgements This work was supported by the Ministerio de Ciencia e Innovación of Spain (Project CTQ2011-25388) and the Junta de Extremadura (Consolidation Project GR10033 of Research Group FQM003 co-financed by European FEDER Funds). 6. References [1] J. E. Sánchez Uria and A. Sanz-Medel, “Inorganic and Methylmercury Speciation in Environmental Samples,” Talanta, Vol. 47, No. 3, 2008, pp. 509-524. [2] M. Morita, J. Yoshinaga and J. S. Edmonds, “The Deter- mination of Mercury Species in Environmental and Bio- logical Samples,” Pure & Applied Chemistyr, Vol. 70, No. 8, 1998, pp. 1585-1615. doi:org/10.1351/pac199870081585 [3] E. M. Nolan and S. J. Lippard, “A “Turn-On” Fluorescent Sensor for the Selective Detection of Mercuric Ion in Aqueous Media,” Journal of the American Chemical So- ciety, Vol. 125, No. 47, 2003, pp. 14270-14271. doi:org/10.1021/ja037995g [4] E. M. Nolan and S. J. Lippard, “Turn-On and Ratiometric Mercury Sensing in Water with a Red-Emmiting Probe,” Journal of the American Chemical Society, Vol. 129, No. 18, 2007, pp. 5910-5918. doi:org/10.1021/ja068879r [5] E. M. Nolan, M. E. Racine and S. J. Lippard, “Selective Hg (II) Detection in Aqueous Solution with Thiol Deri- vatized Fluoresceins,” Inorganic Chemistry, Vol. 45, No. 6, 2006, pp. 2742-2749. doi:org/10.1021/ic052083w [6] H. J. Kim, J. E. Park, M. G. Choi, S. Ahn and S. K. Chang, “Selective Chromogenic and Fluorogenic Signal- ling, of Hg2+ Ions Using a Fluorescein-Coumarin Conju- gate,” Dyes and Pigments, Vol. 84, No. 1, 2010, pp. 54-58. doi:org/10.1016/j.dyepig.2009.06.009 [7] Y. Jin, I. Yoon, J. Seo, J. E. Lee, S. T. Moon, J. Kim, S. W. Han, K. M. Park, L. F. Lindoy and S. S. Lee, “Cad- mium(II) and Mercury(Ii) Complexes of an NO2S2-Donor Macrocycle and Its Ditopic Xylyl-Bridged Analogue,” Dalton Transactions, Vol. 4, 2005, pp. 788-796. doi:org/10.1039/b415794j [8] J. Wang and X. Qian, “Two Regioisomeric and Exclu- sively Selective Hg(II) Sensor Molecules Composed of a Naphthalimide Fluorophore and an O-Phenylenediamine Derived Triamide Receptor,” Chemical Communication, Vol. 1, 2006, pp. 109-111. doi:org/10.1039/b511319a [9] Q. Meng, X. Zhang, C. He, P. Zhou, W. Su and C. Duan, “A Hybrid Mesoporous Material Functionalized by 1,8-Naphthalimide-Base Receptor and the Application as Chemosensor and Absorbent for Hg2+ in Water,” Talanta, Vol. 84, No. 1, 2011, pp. 53-59. doi:org/10.1016/j.talanta.2010.12.008 [10] J. Wang and X. Qian, “A Series of Polyamide Receptor Based PET Fluorescent Sensor Molecules: Positively Cooperative Hg Ion Binding with High Sensitivity,” Or- ganic Letters, Vol. 8, No. 17, 2006, pp. 3721-3724. Copyright © 2011 SciRes. AJAC  A. M. de la PEÑA ET AL. 611 doi:org/10.1021/ol061297u [11] J. Du, J. Fan, X. Peng, H. Li, J. Wang and S. Sun, “Highly Selective and Anions Controlled Fluorescent Sensor for Hg2+ in Aqueous Environment,” Journal of Fluorescence, Vol. 18, No. 5, 2008, pp. 919-924. doi:org/10.1007/s10895-008-0324-3 [12] H. J. Kim, S. H. Kim, J. H. Kim, E. H. Lee, K. W. Lim and J. S. Kim, “BODIPY Appended Crown Ethers: Se- lective Fluorescence Changes for Hg2+ Binding,” Bulletin of the Korean Chemical Society, Vol. 29, No. 9, 2008, pp. 1831-1834. doi:org/10.5012/bkcs.2008.29.9.1831 [13] Y. K. Yang, K. J. Yook and J. Tae. “A Rhodamine-Based Fluorescent and Colorimetic Chemodosimeter for the Rapid Detection of Hg Ions in Aqueous Media,” Journal of the American Chemical Society, Vol. 127, No. 48, 2005, pp. 16760-16761. doi:org/10.1021/ja054855t [14] X. Q. Zhan, Z. H. Qian, H. Zheng, B. Y. Su, Z. Lan and J. G. Xu, “Rhodamine Thiospirolactone. Highly Selective and Sensitive Reversible Sensing of Hg(II),” Chemical Communication, Vol. 16, 2008, pp. 1859-1861. doi:org/10.1039/b719473k [15] W. Shi and H. Ma, “Rhodamine B thiolactone: a Simple Chemosensor for Hg2+ in Aqueous Media,” Chemical Communication, Vol. 16, 2008, pp. 1856-1858. doi:org/10.1039/b717718f [16] S. K. Ko, Y. K. Yang, J. Tae and I. Shin, “In vivo Moni- toring of Mercury Ions Using a Rhodamine-Based Mo- lecular Probe,” Journal of the American Chemical Society, Vol. 128, No. 43, 2006, pp. 14150-14155. doi:org/10.1021/ja065114a [17] H. Zheng, Z. H. Qian, L. Xu, F. F. Yuan, L. D. Lan and J. G. Xu, “Switching the Recognition Preference of Rho- Damine B Spirolactam by Replacing One Atom: Design of Rhodamine B Thiohydrazide for Recognition of Hg(II) in Aqueous Solution,” Organic Letters, Vol. 8, No. 5, 2006, pp. 859-861. doi:org/10.1021/ol0529086 [18] X. Q. Zhan, Z. H. Qian, H. Zheng, B. Y. Su, Z. Lan and J. G. Xu, “Rhodamine Thiospirolactone. Highly Sensitive Reversible Sensing of Hg2+,” Chemical Communication, 2008, pp. 1859-1861. [19] H. N. Kim, S. W. Nam, K. M. K. Swamy, J. Yan, X. Chen, Y. Kim, S. J. Kim, S. Park and J. Yoon, “Rhoda- mine Hydrazone Derivatives as Hg2+ Selective Fluores- cent and Colorimetric Chemosensors and Their Applica- tions to iBoimaging and Microfluidic System,” Analyst, Vol. 136, No. 7, 2011, pp. 1339, 1343. [20] Y. K. Yang, S. K. Ko, I. Shin and J. Tae, “Fluorescent Detection of Methylmercury by Desulfurization Reaction of Rhodamine Hydrazide Derivatives,” Organic and Biomolecular Chemistry, Vol. 7, No. 22, 2009, pp. 4590-4593. doi:org/10.1039/b915723a [21] D. Bohoyo-Gil, M. I. Rodríguez-Cáceres, M. C. Hurtado- Sánchez and A. Muñoz de La Peña, “Fluorescent Determination of Hg2+ in Water and Fish Samples Using a Chemo-Dosimeter Based in a Rhodamine 6G Derivative and a Portable-Optic Spectrofluorimeter,” Applied Specifications, Vol. 64, No. 5, 2010, pp. 520-527. [22] A. Muñoz de la Peña, M. I. Rodríguez-Cáceres, M. C. Hurtado-Sánchez and D. Bohoyo Gil, “A Novel Application of Hg2+ Based in a Spirocyclic Rhodamine 6G Phenyl-Thiosemicarbazide Derivative,” Luminiscence, Vol. 25, 2010, pp. 229-230. [23] B. N. Ahamed and P. Ghosh, “An Integrated System of Pyrene and Rhodamine-6G for Selective Colorimetric and Fluorometric Sensing of Mercury(II),” Inorganica Chimicia Acta, 2011, doi:10.1016/j.ica.2011.01.071. [24] Y. Gabe, Y. Urano, K. Kikuchi, H. Kojima and T. Na- gano, “Highly Sensitive Fluorescence Probes for Nitric oxide Based on Boron Dipyrromethene Chromophore Rational Design of Potentially Useful Bioimaging Fluo- rescence Probe,” Journal of the American Chemical So- ciety, Vol. 126, No. 10, 2004, pp. 3357-3367. doi:org/10.1021/ja037944j [25] T. Nguyen and M. B. Francis, “Practical Synthetic Route to Functionalized Rhodamine Dyes,” Organic Letters, Vol. 5, No. 18, 2003, pp. 3245-3248. doi:org/10.1021/ol035135z [26] M. A. Khan, “AlGaN Multiple Quantum Well Based Deep UV LEDs and Their Applications,” Solid State Physics, Vol. 203, No. 7, 2006, pp. 1764-1770. doi:org/10.1002/pssa.200565427 [27] E. F. Schubert, “Light-Emitting Diodes”, Cambridge University Press (UK), Cambridge, 2003 [28] S. Landgraf, “Application of Semiconductor Light Sou- rces for Investigations of Photochemical Reactions,” Spectrochimica Acta A, Vol. 57, No. 10, 2001, pp. 2029- 2048. doi:org/10.1016/S1386-1425(01)00502-9 [29] X. Chen, S. W. Nam, M. J. Jou, Y. Kim, S. J. Kim, S. Park and J. Yoon, “Hg2+ Selective Fluorescent and Col- orimetric Sensor: Its Crystal Structure and Application to Bioimaging,” Organic Letters, Vol. 10, No. 22, 2008, pp. 5235-5238. doi:org/10.1021/ol8022598 [30] H. M. Irving, H. Freiser and T. S. West, “IUPAC Com- pendium of Analytical Nomenclature, Definitive Rules,” Pergamon Press, Oxford, 2004. [31] C. A. Clayton, J. W. Hines and P. D. Elkins, “Detection limits with Specified Assurance Probabilities,” Analytical Chemistry, Vol. 59, No. 20, 1987, pp. 250. doi:org/10.1021/ac00147a014 Copyright © 2011 SciRes. AJAC
|