Paper Menu >>
Journal Menu >>
 American Journal of Anal yt ical Chemistry, 2011, 2, 582-588 doi:10.4236/ajac.2011.25066 Published Online September 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Selective Detection of Dopamine in the Presence of Ascorbic Acid at Poly (m-Aminobenzene Sulfonic Acid) Gamze Erdoğdu1,*, Mehmet Mutluhan Mutlu2 1Department of Chemistry, Faculty of Arts and Sciences, İnönü University, Malatya, Turkey 2Department of Chemistry, Faculty of Arts and Sciences, Harran University, Şanlıurfa, Turkey E-mail: gerdogdu@inonu.edu.tr Received August 4, 2010; revised October 26, 2010; accepted May 1, 2011 Abstract Poly (m-aminobenzene sulfonic acid, m-ABSA) films were electrochemically prepared by cyclic voltam- metry (CV) in 0.1 mol L–1 KCl solution. The dopamine (DA) selectivity of polymeric electrodes prepared at the different thicknesses was examined in the presence of ascorbic acid (AA). The results showed that the modified electrode showed an excellent electrocatalytical effect towards oxidation of dopamine (DA) and ascorbic acid (AA). Electrostatic interaction between the negatively charged poly(m-ABSA) film and either cationic DA species or anionic AA species favorably contributed to the redox response of DA and AA. Moreover, the regular and repetitive responses for dopamine were obtained even in the presence of the some interfering substances such as ascorbic acid, NaCl, NaClO4, Na2SO4 ,NaNO3 and KCl. Keywords: Poly (m-Aminobenzene Sulfonic Acid), Dopamine, Ascorbic Acid 1. Introduction Among the catecholamines, DA has attracted most inter- est, because the change in DA levels has proved to be a very effective route toward understanding brain func- tions, such as learning and memory formation, and physiological and pathological process of Parkinson’s disease [1,2]. All attempts to measure neurotransmitters, particularly DA, in the brain with voltammetric proce- dures require the use of several strategies to improve the qualitative and quantitative aspects of these measure- ments. The main problem associated with in vivo meas- uring of levels of this monoamine is the very low DA concentration (10–8 - 10–6 M) and the large excesses of interfering species such as ascorbic acid (AA) (about 0.1 M). As well known, a major problem encountered with the detection of DA is the interference from ascorbic acid (AA), which largely coexists with DA in brain issue and has an overlapping oxidation potential on the solid elec- trodes, so it is very difficult to determine DA directly [3]. In order to resolve this problem, many different strate- gies have been used to modify the electrode, which in- clude self-assembled monolayer [4], electrochemical pre- treatment [5], polymer film [6-17] and covalent modified [18]. It is well known that DA exists as a cation at physiological pH 7.0, while AA exists as an anion [19]. It is a possible way to overcome this problem by coating the electrodes with a thin film of Nafion [20-22]. The SO3 – of Nafion film can repel AA anion to eliminate interference of AA. The kind of electrode usually suffers from a slow response due to low diffusion coefficients [23,24] of analytes in the films. The other method is to cover the electrode surface with double-layer film [25-27]. This kind of modified electrode is coated first with an electroactive material having catalytic effect on the oxidation of DA and then with a Nafion layer. An- other approach is to cover the electrode surface with electropolymerized films. In this paper, we apply m-ABSA as a modifier to fab- ricate polymer modified electrodes by electropolymeri- zation method. In m-ABSA, there are electron-rich N atom and high electron density of sulfonic group. Hence, the poly (m-ABSA) film is negatively charged. The mo- dified electrodes show an electrocatalytic activity for the oxidation of DA and AA. The obvious separation of po- tential to DA and AA can be obtained at modified elec- trode. It means that AA has no interference for detection DA. On the contrary, poly(m-ABSA) film modified glassy carbon electrode was utilized for electrocatalytic effect on the electrooxidation of DA in the presence of AA at physiological pH. The modified electrode showed good stability and reproducibility. 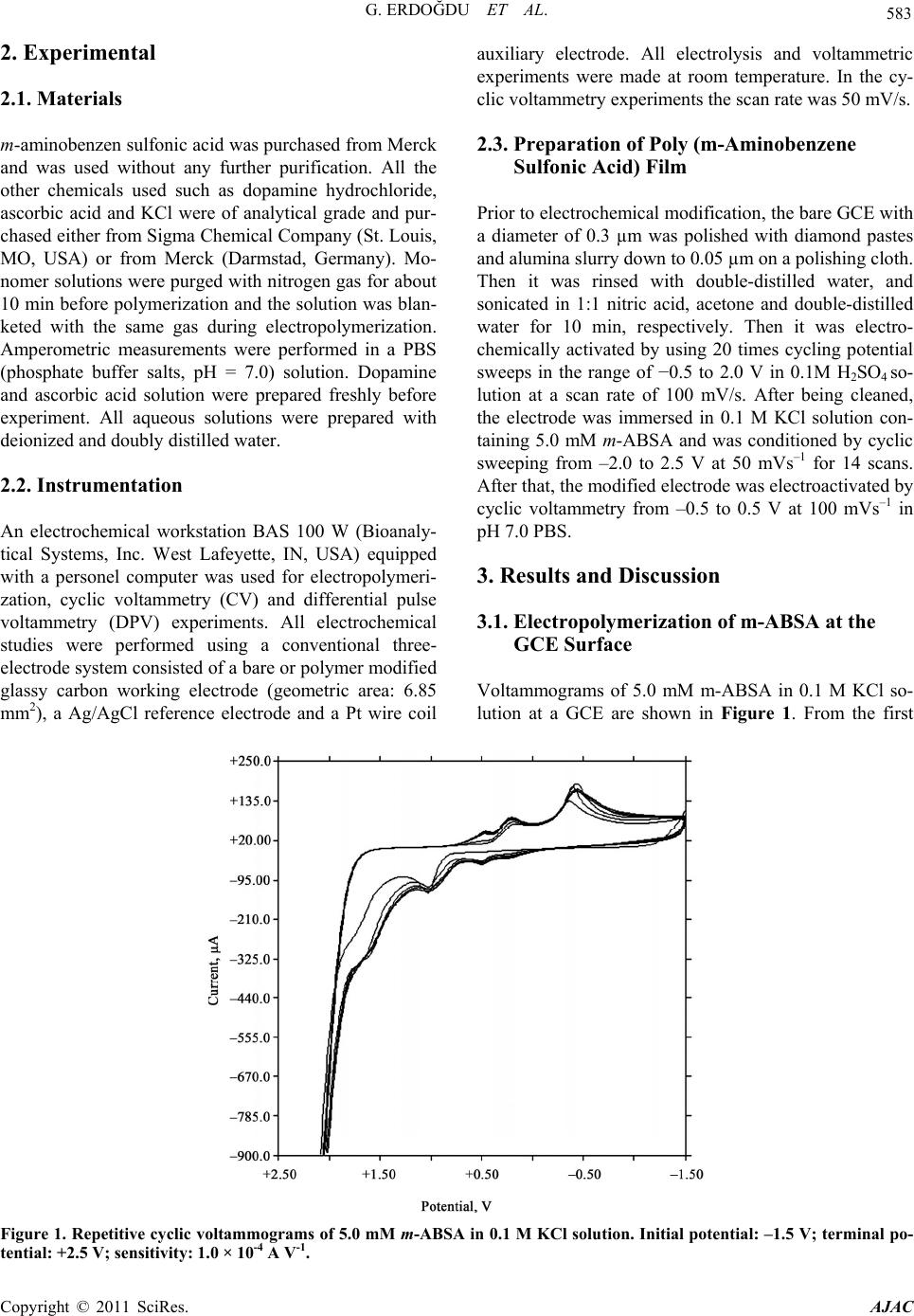 G. ERDOĞDU ET AL. 583 2. Experimental 2.1. Materials m-aminobenzen sulfonic acid was purchased from Merck and was used without any further purification. All the other chemicals used such as dopamine hydrochloride, ascorbic acid and KCl were of analytical grade and pur- chased either from Sigma Chemical Company (St. Louis, MO, USA) or from Merck (Darmstad, Germany). Mo- nomer solutions were purged with nitrogen gas for about 10 min before polymerization and the solution was blan- keted with the same gas during electropolymerization. Amperometric measurements were performed in a PBS (phosphate buffer salts, pH = 7.0) solution. Dopamine and ascorbic acid solution were prepared freshly before experiment. All aqueous solutions were prepared with deionized and doubly distilled water. 2.2. Instrumentation An electrochemical workstation BAS 100 W (Bioanaly- tical Systems, Inc. West Lafeyette, IN, USA) equipped with a personel computer was used for electropolymeri- zation, cyclic voltammetry (CV) and differential pulse voltammetry (DPV) experiments. All electrochemical studies were performed using a conventional three- electrode system consisted of a bare or polymer modified glassy carbon working electrode (geometric area: 6.85 mm2), a Ag/AgCl reference electrode and a Pt wire coil auxiliary electrode. All electrolysis and voltammetric experiments were made at room temperature. In the cy- clic voltammetry experiments the scan rate was 50 mV/s. 2.3. Preparation of Poly (m-Aminobenzene Sulfonic Acid) Film Prior to electrochemical modification, the bare GCE with a diameter of 0.3 µm was polished with diamond pastes and alumina slurry down to 0.05 µm on a polishing cloth. Then it was rinsed with double-distilled water, and sonicated in 1:1 nitric acid, acetone and double-distilled water for 10 min, respectively. Then it was electro- chemically activated by using 20 times cycling potential sweeps in the range of −0.5 to 2.0 V in 0.1M H2SO4 so- lution at a scan rate of 100 mV/s. After being cleaned, the electrode was immersed in 0.1 M KCl solution con- taining 5.0 mM m-ABSA and was conditioned by cyclic sweeping from –2.0 to 2.5 V at 50 mVs–1 for 14 scans. After that, the modified electrode was electroactivated by cyclic voltammetry from –0.5 to 0.5 V at 100 mVs–1 in pH 7.0 PBS. 3. Results and Discussion 3.1. Electropolymerization of m-ABSA at the GCE Surface Voltammograms of 5.0 mM m-ABSA in 0.1 M KCl so- lution at a GCE are shown in Figure 1. From the first Figure 1. Repetitive cyclic voltammograms of 5.0 mM m-ABSA in 0.1 M KCl solution. Initial potential: –1.5 V; terminal po- tential: +2.5 V; sensitivity: 1.0 × 10-4 A V-1. Copyright © 2011 SciRes. AJAC 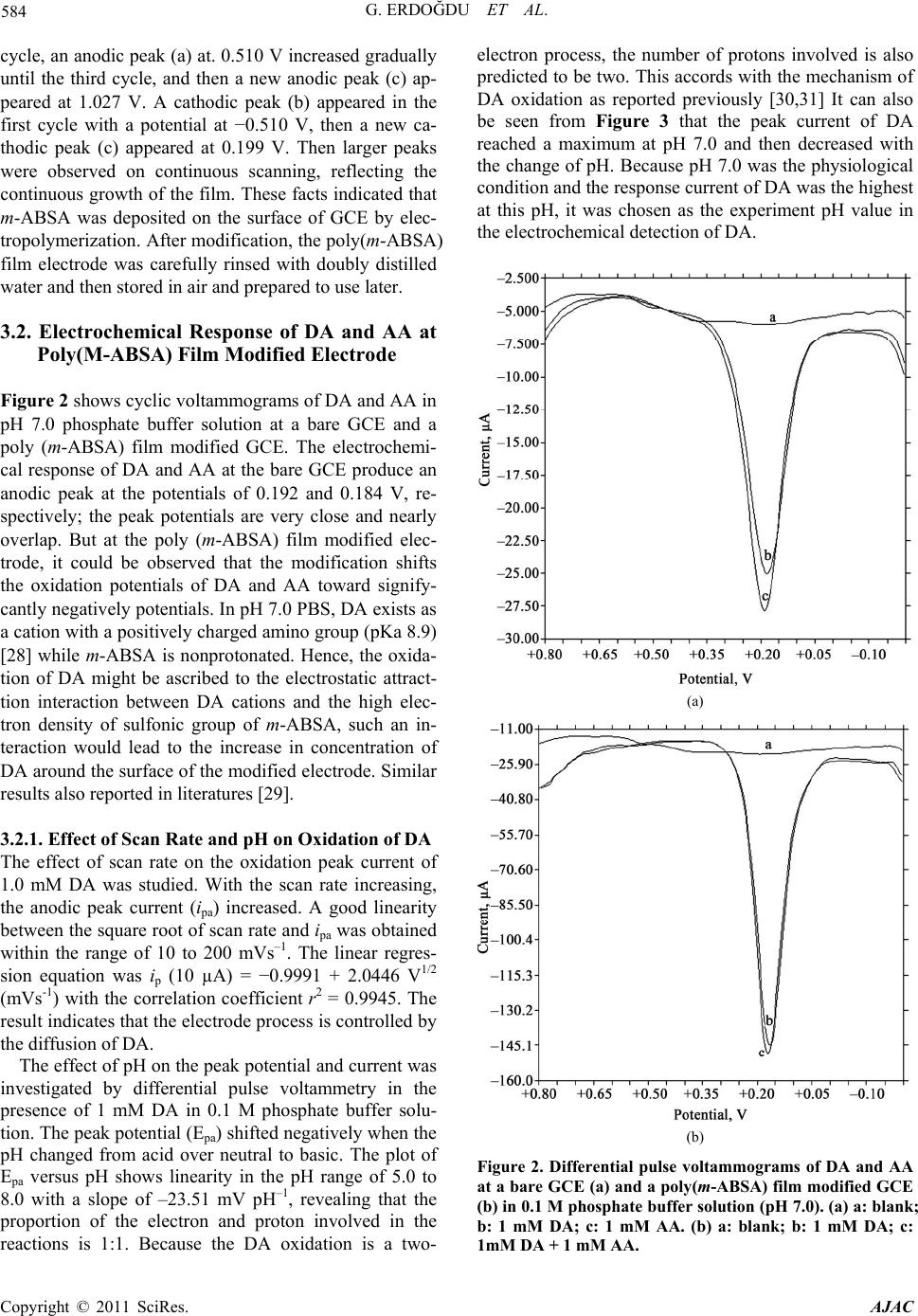 G. ERDOĞDU ET AL. Copyright © 2011 SciRes. AJAC 584 cycle, an anodic peak (a) at. 0.510 V increased gradually until the third cycle, and then a new anodic peak (c) ap- peared at 1.027 V. A cathodic peak (b) appeared in the first cycle with a potential at −0.510 V, then a new ca- thodic peak (c) appeared at 0.199 V. Then larger peaks were observed on continuous scanning, reflecting the continuous growth of the film. These facts indicated that m-ABSA was deposited on the surface of GCE by elec- tropolymerization. After modification, the poly(m-ABSA) film electrode was carefully rinsed with doubly distilled water and then stored in air and prepared to use later. 3.2. Electrochemical Response of DA and AA at Poly(M-ABSA) Film Modified Electrode Figure 2 shows cyclic voltammograms of DA and AA in pH 7.0 phosphate buffer solution at a bare GCE and a poly (m-ABSA) film modified GCE. The electrochemi- cal response of DA and AA at the bare GCE produce an anodic peak at the potentials of 0.192 and 0.184 V, re- spectively; the peak potentials are very close and nearly overlap. But at the poly (m-ABSA) film modified elec- trode, it could be observed that the modification shifts the oxidation potentials of DA and AA toward signify- cantly negatively potentials. In pH 7.0 PBS, DA exists as a cation with a positively charged amino group (pKa 8.9) [28] while m-ABSA is nonprotonated. Hence, the oxida- tion of DA might be ascribed to the electrostatic attract- tion interaction between DA cations and the high elec- tron density of sulfonic group of m-ABSA, such an in- teraction would lead to the increase in concentration of DA around the surface of the modified electrode. Similar results also reported in literatures [29]. 3.2.1. Effect of Scan Rate and pH on Oxidation of DA The effect of scan rate on the oxidation peak current of 1.0 mM DA was studied. With the scan rate increasing, the anodic peak current (ipa) increased. A good linearity between the square root of scan rate and ipa was obtained within the range of 10 to 200 mVs–1. The linear regres- sion equation was ip (10 µA) = −0.9991 + 2.0446 V1/2 (mVs-1) with the correlation coefficient r2 = 0.9945. The result indicates that the electrode process is controlled by the diffusion of DA. The effect of pH on the peak potential and current was investigated by differential pulse voltammetry in the presence of 1 mM DA in 0.1 M phosphate buffer solu- tion. The peak potential (Epa) shifted negatively when the pH changed from acid over neutral to basic. The plot of Epa versus pH shows linearity in the pH range of 5.0 to 8.0 with a slope of –23.51 mV pH–1, revealing that the proportion of the electron and proton involved in the reactions is 1:1. Because the DA oxidation is a two- electron process, the number of protons involved is also predicted to be two. This accords with the mechanism of DA oxidation as reported previously [30,31] It can also be seen from Figure 3 that the peak current of DA reached a maximum at pH 7.0 and then decreased with the change of pH. Because pH 7.0 was the physiological condition and the response current of DA was the highest at this pH, it was chosen as the experiment pH value in the electrochemical detection of DA. (a) (b) Figure 2. Differential pulse voltammograms of DA and AA at a bare GCE (a) and a poly(m-ABSA) film modified GCE (b) in 0.1 M phosphate buffer solution (pH 7.0). (a) a: blank; b: 1 mM DA; c: 1 mM AA. (b) a: blank; b: 1 mM DA; c: 1mM DA + 1 mM AA. 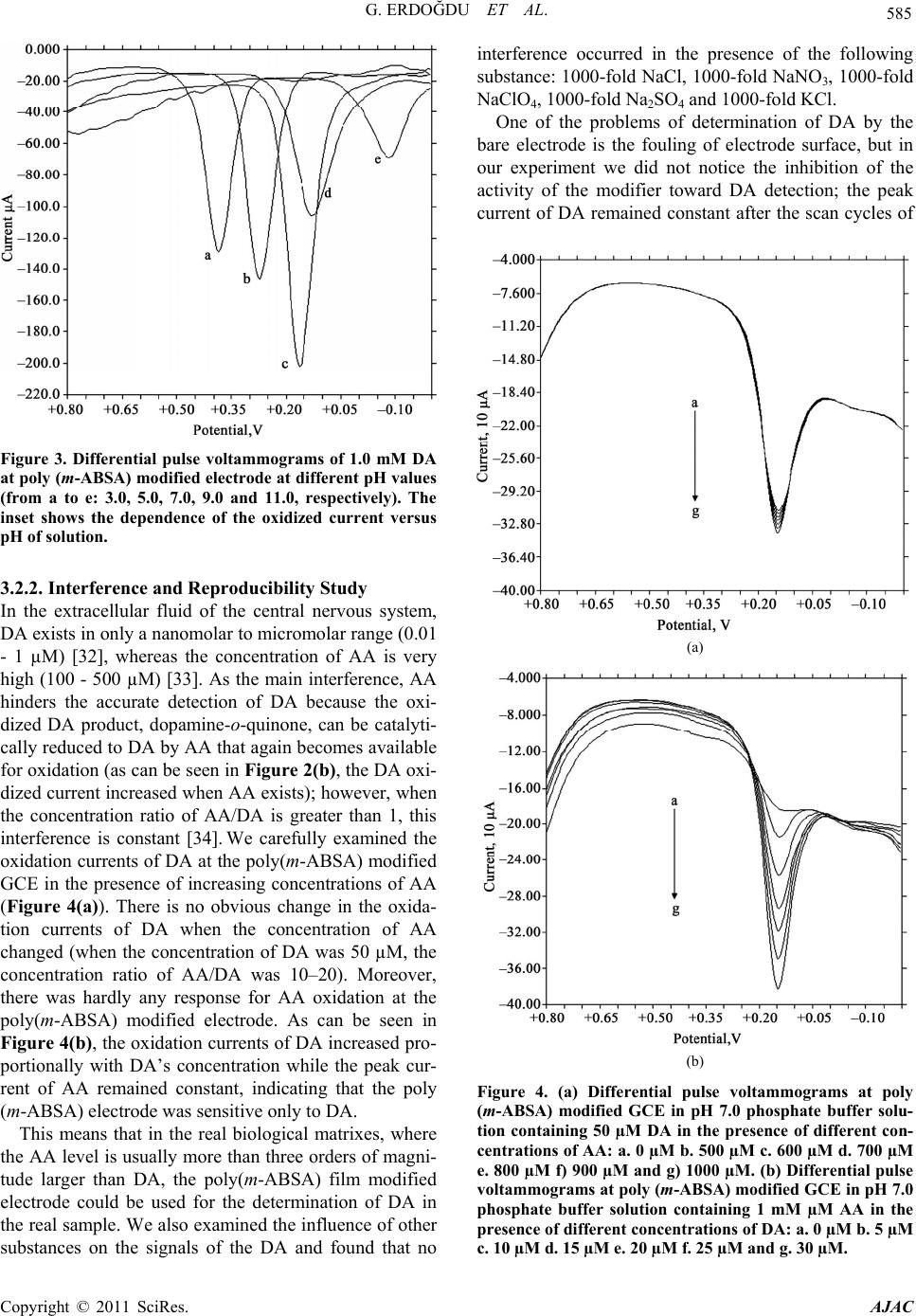 G. ERDOĞDU ET AL. 585 Figure 3. Differential pulse voltammograms of 1.0 mM DA at poly (m-ABSA) modified electrode at different pH values (from a to e: 3.0, 5.0, 7.0, 9.0 and 11.0, respectively). The inset shows the dependence of the oxidized current versus pH of solution. 3.2.2. Interference and Reproducibility Study In the extracellular fluid of the central nervous system, DA exists in only a nanomolar to micromolar range (0.01 - 1 µM) [32], whereas the concentration of AA is very high (100 - 500 µM) [33]. As the main interference, AA hinders the accurate detection of DA because the oxi- dized DA product, dopamine-o-quinone, can be catalyti- cally reduced to DA by AA that again becomes available for oxidation (as can be seen in Figure 2(b), the DA oxi- dized current increased when AA exists); however, when the concentration ratio of AA/DA is greater than 1, this interference is constant [34]. We carefully examined the oxidation currents of DA at the poly(m-ABSA) modified GCE in the presence of increasing concentrations of AA (Figure 4(a)). There is no obvious change in the oxida- tion currents of DA when the concentration of AA changed (when the concentration of DA was 50 µM, the concentration ratio of AA/DA was 10–20). Moreover, there was hardly any response for AA oxidation at the poly(m-ABSA) modified electrode. As can be seen in Figure 4(b), the oxidation currents of DA increased pro- portionally with DA’s concentration while the peak cur- rent of AA remained constant, indicating that the poly (m-ABSA) electrode was sensitive only to DA. This means that in the real biological matrixes, where the AA level is usually more than three orders of magni- tude larger than DA, the poly(m-ABSA) film modified electrode could be used for the determination of DA in the real sample. We also examined the influence of other substances on the signals of the DA and found that no interference occurred in the presence of the following substance: 1000-fold NaCl, 1000-fold NaNO3, 1000-fold NaClO4, 1000-fold Na2SO 4 and 1000-fold KCl. One of the problems of determination of DA by the bare electrode is the fouling of electrode surface, but in our experiment we did not notice the inhibition of the activity of the modifier toward DA detection; the peak current of DA remained constant after the scan cycles of (a) (b) Figure 4. (a) Differential pulse voltammograms at poly (m-ABSA) modified GCE in pH 7.0 phosphate buffer solu- tion containing 50 µM DA in the presence of different con- centrations of AA: a. 0 µM b. 500 µM c. 600 µM d. 700 µM e. 800 µM f) 900 µM and g) 1000 µM. (b) Differential pulse voltammograms at poly (m-ABSA) modified GCE in pH 7.0 phosphate buffer solution containing 1 mM µM AA in the presence of different concentrations of DA: a. 0 µM b. 5 µM c. 10 µM d. 15 µM e. 20 µM f. 25 µM and g. 30 µM. Copyright © 2011 SciRes. AJAC 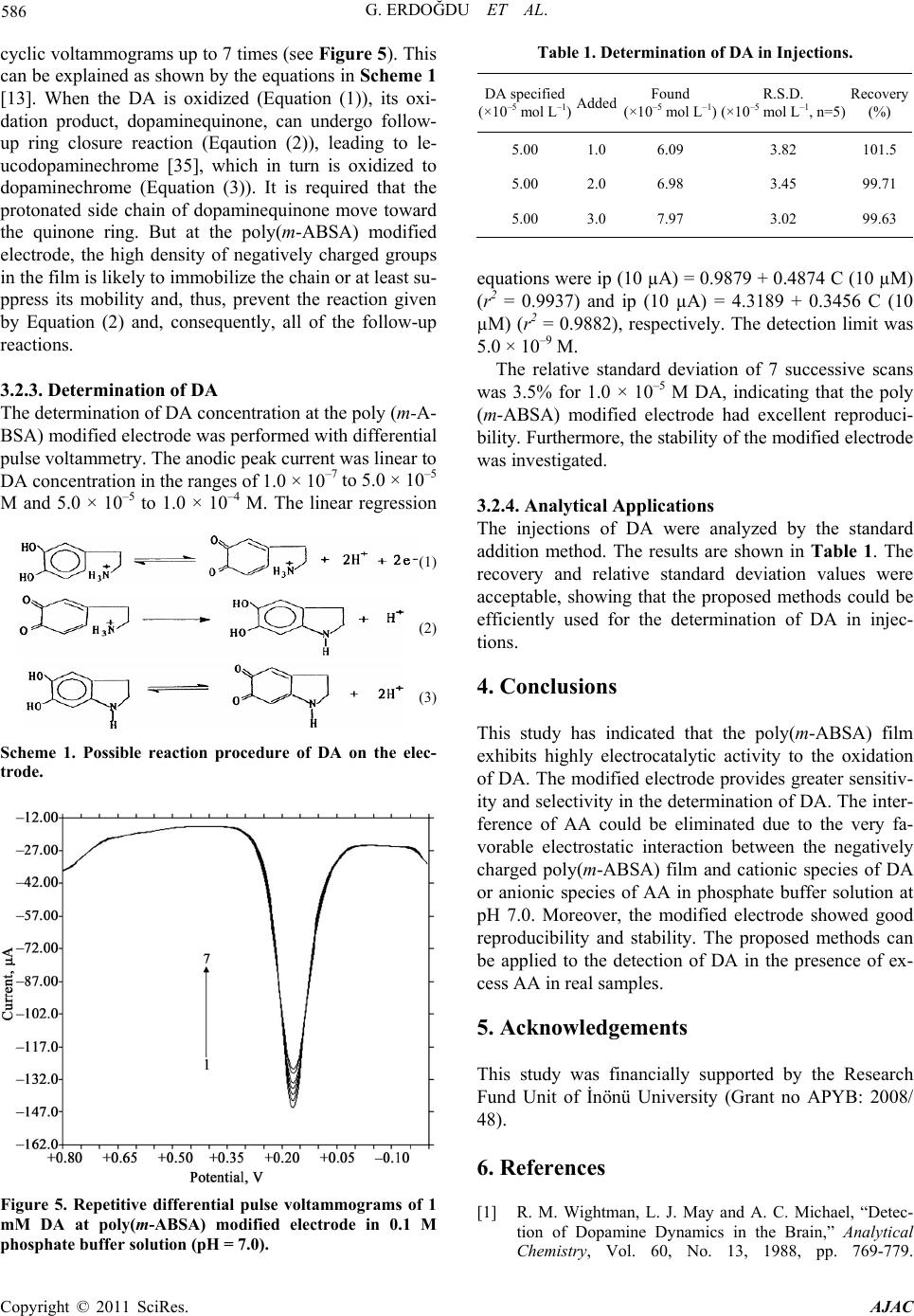 586 G. ERDOĞDU ET AL. cyclic voltammograms up to 7 times (see Figure 5). This can be explained as shown by the equations in Scheme 1 [13]. When the DA is oxidized (Equation (1)), its oxi- dation product, dopaminequinone, can undergo follow- up ring closure reaction (Eqaution (2)), leading to le- ucodopaminechrome [35], which in turn is oxidized to dopaminechrome (Equation (3)). It is required that the protonated side chain of dopaminequinone move toward the quinone ring. But at the poly(m-ABSA) modified electrode, the high density of negatively charged groups in the film is likely to immobilize the chain or at least su- ppress its mobility and, thus, prevent the reaction given by Equation (2) and, consequently, all of the follow-up reactions. 3.2.3. Determination of DA The determination of DA concentration at the poly (m-A- BSA) modified electrode was performed with differential pulse voltammetry. The anodic peak current was linear to DA concentration in the ranges of 1.0 × 10–7 to 5.0 × 10–5 M and 5.0 × 10–5 to 1.0 × 10–4 M. The linear regression (1) (2) (3) Scheme 1. Possible reaction procedure of DA on the elec- trode. Figure 5. Repetitive differential pulse voltammograms of 1 mM DA at poly(m-ABSA) modified electrode in 0.1 M phosphate buffer solution (pH = 7.0). Table 1. Determination of DA in Injections. DA specified (×10–5 mol L–1)Added Found (×10–5 mol L–1) R.S.D. (×10–5 mol L–1, n=5) Recovery (%) 5.00 1.06.09 3.82 101.5 5.00 2.06.98 3.45 99.71 5.00 3.07.97 3.02 99.63 equations were ip (10 µA) = 0.9879 + 0.4874 C (10 µM) (r2 = 0.9937) and ip (10 µA) = 4.3189 + 0.3456 C (10 µM) (r2 = 0.9882), respectively. The detection limit was 5.0 × 10–9 M. The relative standard deviation of 7 successive scans was 3.5% for 1.0 × 10–5 M DA, indicating that the poly (m-ABSA) modified electrode had excellent reproduci- bility. Furthermore, the stability of the modified electrode was investigated. 3.2.4. Analytical Applications The injections of DA were analyzed by the standard addition method. The results are shown in Table 1. The recovery and relative standard deviation values were acceptable, showing that the proposed methods could be efficiently used for the determination of DA in injec- tions. 4. Conclusions This study has indicated that the poly(m-ABSA) film exhibits highly electrocatalytic activity to the oxidation of DA. The modified electrode provides greater sensitiv- ity and selectivity in the determination of DA. The inter- ference of AA could be eliminated due to the very fa- vorable electrostatic interaction between the negatively charged poly(m-ABSA) film and cationic species of DA or anionic species of AA in phosphate buffer solution at pH 7.0. Moreover, the modified electrode showed good reproducibility and stability. The proposed methods can be applied to the detection of DA in the presence of ex- cess AA in real samples. 5. Acknowledgements This study was financially supported by the Research Fund Unit of İnönü University (Grant no APYB: 2008/ 48). 6. References [1] R. M. Wightman, L. J. May and A. C. Michael, “Detec- tion of Dopamine Dynamics in the Brain,” Analytical Chemistry, Vol. 60, No. 13, 1988, pp. 769-779. Copyright © 2011 SciRes. AJAC  G. ERDOĞDU ET AL. 587 doi:org/10.1021/ac00164a001 [2] X. Cao, L. Luo, Y. Ding, X. Zou and R. Bian, “Electroch- emical Methods for Simultaneous Determi- nation of Dopamine and Ascorbic Acid using Cetylpy-Ridine Bromide/chitosan Composite Film-Modified Glassy Car- bon Electrode,” Sensors and Actuators B, Vol. 129, No. 2, 2008, pp. 941-946. doi:org/10.1016/j.snb.2007.10.008 [3] T. Zetterstrom, T.Sharp, C. A. Marsden and U. Ung- erstedt, “In vivo Measurement of Dopamine and Its Me- tabolites by Intracerebral Dialysis-Changes after D-Am- pheta-Mine,” Journal of Neurochemistry, Vol. 41, No. 6, 1983, pp. 1769-1773. doi:org/10.1111/j.1471-4159.1983.tb00893.x [4] F. Malem and D. Mandler, “Self-Assembled Monolayers in Electroanalytical Chemistry-Application of Omega- Mercapto Carboxylic-Acid Monolayers for the Electro- chemical Detection of Dopamine in the Presence of A High-Concentration of Ascorbic-Acid,” Analytical Che- mistry, Vol. 65, No. 1, 1993, pp. 37-41. [5] F. Gonon, M. Buda, R. Cespuglio, J. Jouvet and J. F. Pujol, “Differential Pulse Voltammetry in Brain-Tissue 1. Detection of 5-Hydroxyindoles in the Rat Striatum,” Brain Research, Vol. 223, No. 2, 1981, pp. 69-80. doi:org/10.1016/0006-8993(81)90807-6 [6] R. A. Saraceno, J. G. Pack and A. G. Ewing, “Catalysis of Slow Charge-Transfer Reactions at Polypyrrole-Coated Glassy-Carbon Electrodes,” Journal of Electroanalytical Chemistry, Vol. 97, No. 1-2, 1986, pp. 265-278. doi:org/10.1016/0022-0728(86)80154-1 [7] G. Erdogdu, H. B. Mark and A. E. Karagozler, “Resolu- tion of Ascorbic Acid and Dopamine at Conducting Polymer Electrodes,” Analytical Letters, Vol. 29, No. 2, 1996, pp. 221-231. [8] G. Erdogdu and A. E. Karagozler, “Investigation and Comparison of the Electrochemical Behavior of Some Organic and Biological Molecules at Various Conducting Polymer Electrodes,” Talanta, Vol. 44, No. 11, 1997, pp. 2011-2018. [9] G. Erdogdu, E. Ekinci and A. E. Karagozler, “Preparation and Electrochemical Behavior of Dopamine-Selective Polymeric Membrane,” Polymer Bulletin , Vol. 44, No. 2, pp. 195-201. [10] E. Ekinci, G. Erdogdu and A. E. Karagözler, “Preparation, Optimization, and Voltammetric Characteristics of Poly (o-phenylenediamine) Film as a Dopamine-Selective Polymeric Membrane,” Journal of Applied Polymer Sci- ence, Vol. 79, No. 2, 2001, pp.327-332. doi:org/10.1002/1097-4628(20010110)79:2<327::AID-A PP150>3.0.CO;2-Z [11] E. Ekinci, G. Erdogdu and A. E. Karagozler, “Investiga- tion of Polymerization Parameters Affecting Dopamine Selectivity of a Polymeric Membrane,” Polymer Bulletin, Vol. 44, No. 5-6, 2000, pp. 547-553. doi:org/10.1007/s002890070077 [12] G. Erdogdu and A. E. Karagozler, “Investigation of the Voltammetric Characteristics of Poly (1,4-Diaminoben- zene) Film as a Dopamine-Selective Polymer Electrode,” Turkish Journal of Chemistry, Vol. 31, No. 2, 2007, pp. 171-178. doi:org/10.1021/ac9808223 [13] A. Ciszewski and G. Milczarek, “Polyeugenol-Modified Platinum Electrode for Selective Detection of Dopamine in the Presence of Ascorbic Acid,” Analytical Chemistry, Vol. 71, No. 5, 1999, pp. 1055-1061. [14] H. Zhao, Y. Z. Zhang and Z. B. Yuan, “Determination of Dopamine in the Presence of Ascorbic Acid using Poly(Hippuric Acid) Modified Glassy Carbon Electrode,” Electroanalysis, Vol. 14, No. 14, 2002, pp.1031-1034. [15] H. Zhao, Y. Z. Zhang and Z. B. Yuan, “Electrochemical Determination of Dopamine Using a Poly (2-picolinic acid) Modified Glassy Carbon Electrode,” Analyst, Vol. 126, No. 3, 2001, pp. 358-360. [16] H. Zhao, Y. Z. Zhang and Z. B. Yuan, “Study on the Electrochemical Behavior of Dopamine with Poly(Sulf- osali-Cylic Acid) Modified Glassy Carbon Electrode,” Analytica Chimica Acta, Vol. 441, No. 1, 2001, pp. 117-122. doi:org/10.1016/S0003-2670(01)01086-8 [17] U. Chandra, B. E. K. Swamy, O. Gilbert and B. S. Sheri- gara, “Voltammetric Resolution of Dopamine in the Presence of Ascorbic Acid and Uric Acid at Poly (Cal- magite) Film Coated Carbon Paste Electrode,” Electro- chimica Acta, Vol. 55, No. 24, 2010, pp. 7166-7174. doi:org/10.1016/j.electacta.2010.06.091 [18] A. J. Downard, A. D. Roddick and A. M. Bond, “Cova- lent Modification of Carbon Electrodes for Voltammetric Differentiation of Dopamine and Ascorbic Acid,” Analy- tica Chimica Acta, Vol. 317, No. 1-3, 1995, pp. 303-310. [19] M. J. Giz, B. Duong and N. J. Tao, “In Situ STM Study of Self-Assembled Mercaptopropionic Acid Monolayers for Electrochemical Detection of Dopamine,” Journal of Electroanalytical Chemistry, Vol. 465, No. 1, 1999, pp. 72-79. doi:org/10.1016/S0022-0728(99)00056-X [20] K. T. Kawagoe and R. M. Wightman, “Characterization of Amperometry for in-vivo Measurement of Dopamine Dynamics in the Rat-Brain,” Talanta, Vol. 41, No. 2, 1994, pp. 865-874. [21] G. A. Gerhardt, A. F. Oke, G. Nagy, B. Moghaddam and R. N. Adams, “Nafion-Coated Electrodes with High Se- lectivity for CNS Electrochemistry,” Brain Research, Vol. 290, No. 2, 1984, pp. 390-395. doi:org/10.1016/0006-8993(84)90963-6 [22] J. A. Ni, H. X. Ju, H. Y. Chen and D. Leech, “Electro- catalytic Oxidation and Trace Determination of Cate- cholamine Neurotransmitters at Osmium Complex Poly- mer and Nafion Dual-layer Membrane Carbon-Base elec- trode,” Chemical Journal of Chinese Universi- ties-Chinese, Vol. 20, No. 2, 1999, pp. 224-226. [23] J. Wang and T. Z. Peng, “Selectivity and Sensitivity Im- provements at Perfluorinated Ionomer Cellulose-Acetate Bilayer Electrodes,” Analytical Chemistry, Vol. 58, No. 14, 1986, pp. 3257-3261. doi:org/10.1021/ac00127a076 [24] A. R. Guadalupe and H. D. Abruna, “Electroanalysis with Chemically Modified Electrodes,” Analytical Chemistry, Vol. 57, No. 1, 1985, pp. 142-149. [25] Z. Salamon and G. Tollin, “Reduction of Cytochrome-C Copyright © 2011 SciRes. AJAC  G. ERDOĞDU ET AL. Copyright © 2011 SciRes. AJAC 588 at a Lipid Bilayer Modified Electrode,” Bioelectrochem- istry and Bioenergetics, Vol. 25, No. 3, 1991, pp. 447- 454. doi:org/10.1016/0302-4598(91)80009-R [26] J. A. Ni, H. X. Ju, H. Y. Chen and D. Leech, “Am- perometric Determination of Epinephrine with an Os- mium Complex and Nafion Double-Layer Membrane Modified Electrode,” Analytica Chimica Acta, Vol. 378, No. 1-3, 1999, pp. 151-157. doi:org/10.1016/S0003-2670(98)00569-8 [27] Q. D. Huang, Z. Q. Lu and J. F. Rusling, “Composite Films of Surfactants, Nafion, and Proteins with Electro- chemical and Enzyme Activity,” Langmuir, Vol. 12, No. 22, 1996, pp. 5472-5480. [28] Y. Z. Zhang, G. Y. Jin, Y. L. Wang and Z. S. Yang, “De- termination of Dopamine in the Presence of Ascorbic Acid using Poly (Acridine Red) Modified Glassy Carbon Electrode,” Sensor, Vol. 3, 10, 2003, pp. 443-450. [29] P. R. Roy, T. Okajima and T. Ohsaka, “Simultaneous Electroanalysis of Dopamine and Ascorbic Acid using Poly (N,N-Dimethylaniline)-Modified Electrodes,” Bio- electrochemistry, Vol. 59, No. 1-2, 2003, pp. 11-19. [30] H. Zhao, Y. Z. Zhang and Z. B. Yuan, “Study on the Electrochemical Behavior of Dopamine with Poly(Sulf- osali-Cylic Acid) Modified Glassy Carbon Electrode, Analytica Chimica Acta, Vol. 441, No. 1, 2001, pp. 117-122. doi:org/10.1016/S0003-2670(01)01086-8 [31] H. Zhao, Y. Z. Zhang and Z. B. Yuan, “Electrochemical Determination of Dopamine using a Poly(2-picolinic acid) Modified Glassy Carbon Electrode,” Analyst, Vol. 126, No. 3, 2001, pp. 358-360. [32] P. Capella, B. Ghasemzadeh, K. Mitchell and R. N. Ad- ams, “Nafion-Coated Carbon-Fiber Electrodes for Neu- rochemical Studies in Brain-Tissue,” Electroanalysis, Vol. 2, No. 3, 1990, pp. 175-182. [33] R. D. O’Neill, “Microvoltammetric Techniques and Sensors for Monitoring Neurochemical Dynamics in vivo —a Review,” Analyst, Vol. 119, No. 1, 1994, pp. 767-779. [34] Y. H. Xiao, C. X. Guo, C. M. Li, Y. B. Li, J. Zhang, R. H. Xue and S. Zhang, “Highly Sensitive and Selective Method to Detect Dopamine in the Presence of Ascorbic Acid by a New Polymeric Composite Film,” Analytical Biochemistry, Vol. 371, No. 2, 2007, pp. 229-237. doi:org/10.1016/j.ab.2007.07.025 [35] E. S. Forzani, G. A. Rivas G. A and V. M. Solis, “Am- perometric Determination of Dopamine on Vegetal-Tis- sue Enzymatic Electrodes. Analysis of Interferents and Enzymatic Selectivity,” Journal of Electroanalytical Chemistry, Vol. 435, No. 1-2, 1997, pp. 77-84. doi:10.1016/S0022-0728(97)00049-1 |

