Journal of Materials Science and Chemical Engineering
Vol.04 No.12(2016), Article ID:72408,7 pages
10.4236/msce.2016.412003
Composites on Base of the Ultradispersed Polytetrafluoroethylene and Chitosan Salts
Vitaly I. Saldin, Alexander K. Tsvetnikov
Far-Eastern Branch of Russian Academy of Science, Institute of Chemistry, Vladivostok, Russia Federation




Received: October 10, 2016; Accepted: November 25, 2016; Published: December 1, 2016
ABSTRACT
Composites based on ultradispersed polytetrafluoroethylene and chitosan salts (nitrate, perchlorate and dodecahydro-closo-dodecaborate) and methods of their fabrication have been developed. For this purpose, the ultrasonic effect on the mixture of the aqueous gels of chitosan salts with dry UPTHE or UPTFE ethanol dispersion with propeller agitators. Introduction of ultradispersed polytetrafluoroethylene improving their functioning properties, namely high combustion efficiency and resistance to air moisture.
Keywords:
Composites, Ultradispersed Polytetrafluoroethylene, Chitosan, Chitosan Nitrate, Perchlorate, Dodecahydro-Closo-Dodecaborate, Graphite Oxid, Ultrasonic Treatment, Composites, XRD, IR

1. Introduction
Polytetrafluoroethylene (PTFE) is one of the most thoroughly studied polymers that has found rather extensive practical applications [1] [2]. In particular, PTFE-M (M-Al, Mg, Ti, Zr etc.) composites were suggested as mixed energy-rich powders [3]. They are produced using the method of combined grinding in ball mills. The phenomenon of transition of heavy polymer molecules into the gas phase in the process of PTFE thermal destruction with subsequent homophase nucleation and condensation in the form of nanofilms of a thickness of 2 - 10 nm was discovered for the first time in the Institute of Chemistry FEBRAS in 1982. Depending on the condensation conditions, nanofilms can grow up to sizes of 100 × 100 µm and roll up into microtubes of a length of up to 150 µm or a size of about 2 × 2 µm with subsequent formation of a pack of nanofilms as a microsphere of a diameter of about 1 µm [4]. This material comprising a mixture of PTFE oligomers with the polymer chain length containing from ≈101 to 103 CF2-groups was named ultradispersed polytetrafluoroethylene (UPTFE) [5]. A significant difference between UPTFE and PTFE (in the form of fluoroplast-4) is related to their powders particle sizes: 0.1 - 3.0 µm and 160 - 200 µm, respectively. The latter fact opened new possible fields of practical application of UPTFE or significantly improved the available ones [6]. For example, fabrication of composites based on UPTFE and intercalated GO compounds with dodecahydro-closo-dodecaborate acid (Н2В12Н12) [7], which are rather promising as energy-rich materials, can be performed through ultrasonic treatment of components aqueous solutions without the pre-pounding of fluoropolymer [8]. This is simpler and safer than application of mechanochemical methods [3]. Fabrication of this kind of composites can be further streamlined using instead of dry UPTHE, its dispersion in ethanol [9]. In these composites, Н2В12Н12 serves as a combustible, while combined UPTFE and GO―as an oxidizer. The advantage of such composites is the completeness of combustion of the borohydride fragment, since the combustion products are gaseous fluoride of boron compounds. In pure oxygen systems, combustion products are oxygen compounds that form a protective film on the surface of a burning particle. This leads to the incompleteness of combustion and the inability to realize the potentially high energy borohydride compounds.
The relentless scientific and practical interest is chitosan, a natural biopolymer [10] [11]. In particular, the composite chitosan perchlorate-chitosan dodecahydro-closo- dodecaborate is developed, which is a promising energy-intensive vigorously burning material [12].
The purpose of this study is to develop composites UPTFE with salts of chitosan, methods for their preparation and the study of their properties.
2. Experimental
Studies of the process of thermal destruction of PTFE (fluoroplast-4) yielded the development of the thermal gas-dynamic (TGD) method and the technology of UPTFE production capable to create UPTFE manufacture on an industrial scale [13]. To obtain the composites was used as the powder itself UPTFE and its ethanol dispersion.
The chitosan produced by OOO Biopolimery (Russia) according to TU 283−174− 200472012−03 with a degree of deacetylation of 75.0% and the following elemental composition, wt%: C, 45.5; H, 6.8, N, 8.1, and O, 39.6 was used as a source compound for preparation of its salts. This corresponds to the brutto formula С6.5О4.25Н9.5NН2 and its relative molecular mass of 171.7 a .m.u.
Chitosan nitrate С6.5О4.25Н9.5NН3NO3 and perchlorate С6.5О4.25Н9.5NН3ClO4 were prepared by dissolving of the chitosan in the respective acids, taken with a small drawback according to the method given in [14].
The synthesis of (С6.5О4.25Н9.5NН3)2В12Н12 through interaction between chitosan and aqueous solutions of Н2В12Н12 or exchange reactions in aqueous solutions of chitosan salt (chitosan acetate, fluoride, nitrate and cloride) with acid or its salts (Na2В12Н12 and K2В12Н12) in molar ratio of 2: 1 was described in detail in [15].
Dodecahydro-closo-dodecaboric acid and its salts obtained from pyrolysis products of the mixture NaВН4−КВF4 [16].
The content of the В12Н122−-anion in solutions of acid and salts was determined using the gravimetric method as Ag2В12Н12 [17].
Preparation of composites UPTFE-chitosan salts was carried out 2 methods.
The first procedure included ultrasonic treatment of the mixture of dry UPTFE with the aqueous gels of chitosan salts using the apparatus “Sona Prep. 150 (GB)”.
The second method was carried out mixing ethanol UPTHE dispersion with aqueous gels of chitosan salts using a propeller stirrer.
The X-ray diffraction analysis was carried out on a DRON?3 and a D8 ADVANCE diffractometers (Bragg-Brentano method, λCuKa).
The IR adsorption spectra of the samples were recorded in the range 350 - 4000 cm−1 on an FSEQUINOX-55S IR spectrometer at room temperature. Samples were prepared in the form of films, as mineral oil mulls and KBr pellets.
3. Results and Discussion
First of all, it should be noted that the gels of chitosan nitrate and perchlorate have sufficient viscous properties for the production of a stable homogeneous liquid composites with UPTFE. As a result of their drying can be obtained thin coatings with high adhesion to a solid surface such as glass and even Teflon. With increasing of gel composite layer deposited on a solid surface, after drying can be obtained a quite strong film. Unlike chitosan nitrate and perchlorate, fine dispersed chitosan dodecahydro-closo-do- decaborate does not form gels that can give with UPTFE homogeneous composites. Therefore, its combination with UPTFE can be carried out on the basis of the gels of chitosan nitrate and perchlorate. They are film-forming the basis of such three-com- ponent composites. In some cases, a film-forming basis may be a mixture of chitosan nitrate and perchlorate with obtaining a four-component composites.
Secondly, for obtaining of the above composites are suitable both methods. But there are features.
Ultrasonic method of UPTFE introduction in gels of chitosan nitrate and perchlorate is a more safe compared to its introduction in the gels of graphite oxide (GО) [8]. In the case of the gels of the GO in excess of the power of ultrasonic impact (USI) can be its transform into the so-called reduced graphene oxide, (rGO) [18]. This is evident in the change in viscosity of the gel. At low power of USI is occurred a gradual increase of the viscosity of the gel. This is due to the splitting of multilayer packages of GO into packages with fewer layers, ideally to a monolayer rGO. This increases the number of functional oxygen-containing groups present on the surface of rGO oligomers around which is formed a hydrated shell. This reduces the concentration of free water in the mixture and increase the viscosity of the gel. The uncontrolled increase in the power of USI is carried out of the oxygen-containing groups reduction, the collapse of the hydration shell with a sharp decrease in the viscosity of the gel.
In the case of the chitosan nitrate and perchlorate gels USI also causes a viscosity increase. But because of the higher chemical stability of the chitosan component is not is its decomposition. Reducing the viscosity of the chitosan nitrate and perchlorate gels was not observed in the modes of USI, in which the viscosity of the GO gels reduced sharply. But, of course, to avoid overheating the mixture.
Upon obtaining of the composites in the second method, found that by mixing ethanol dispersion UPTFE with chitosan nitrate and perchlorate gels not observed their coagulation or formation of immiscible phases. Under the action of propeller agitators for such mixtures are formed homogeneous opaque light gels. The viscosity of the chitosan nitrate or perchlorate gels should be sufficient to prevent the sedimentation of UPTFE particles in the process of drying. For the chitosan used in this study it was found experimentally that the concentration of chitosan nitrate and perchlorate should be not less than 2.5%.
An important moment in the development of such composites is the choice of the ratio of their components.
It is known that when exposed to chitosan open flames it burns pretty slow. More actively burns chitosan dodecahydro-closo-dodecaborate [15] (Figure 1), thanks to thin, on a molecular level, the distribution of fuel (B12H122--anions) and oxidizer (oxygen-containing groups of chitosan), the free access of oxygen and air to the centers of burning, swelling when heated with a sharp increase in the volume of burning material. But even in this case, the formation of a protective layer of boric acids and oxygen compounds of boron on the surface of the burning particles prevents their complete combustion [19] [20]. Much faster and more complete burn composites of chitosan dodecahydro-closo-dodecaborate with chitosan perchlorate [12] (Figure 2) because they improved oxygen balance. Combustion of such composites in an open channel occurs at velocities of 0.6 m/sec and higher, depending on the ratio of 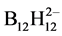 -и
-и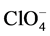 - anions fixed on polycationic matrix of chitosan (Figure 2). But in this case, the problem of the protective layer is not eliminated. Introduction to the composition of such composites powder UPTFE can solve it.
- anions fixed on polycationic matrix of chitosan (Figure 2). But in this case, the problem of the protective layer is not eliminated. Introduction to the composition of such composites powder UPTFE can solve it.
In this work, as actively burning base is used composites of composition
(С6О4Н9NН3)2В12Н12 × 3С6О4Н9NН3NO3 and (С6О4Н9NН3)2В12Н12 × 3С6О4Н9NН3ClO4,
Figure 1. The fragment of the structure model of chitosan dodecahydro-closo-dodecaborate.
Figure 2. The fragment of the structure model of composite (С6О4Н9NН3)2В12Н12 × 2С6О4Н9NН3ClO4.
in which was introduced UPTHE. Thus, its content in the composite must provide combustion of boron to boron oxyfluoride (BOF)3 under the schemes:
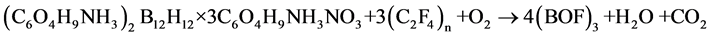 (1)
(1)
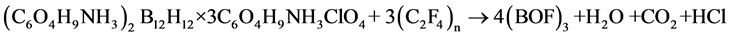 (2)
(2)
It is easily to calculate that the content UPTFE in these composites should be 20.1% and 18.7%, respectively.
The qualitative composition of these composites is confirmed by XRD and IR. The presence of UPTHE in the diffraction patterns of the composites evidenced by the number of reflections (the strongest at 4.88 A ) [1] [6] on the background of x-ray amorphous phases of the chitosan salts [10] [11]. The presence of the characteristic absorption bands of UPTFE (1158, 1220 cm−1) [6], 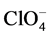 (1090 cm−1) [21],
(1090 cm−1) [21], 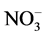 (1383 cm−1) [21] and
(1383 cm−1) [21] and 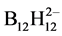 (2480 - 2490 cm−1) [7] in the IR spectra of the composites indicates the absence of redox interactions between components in their production process or drying. Thanks UPTHE included in the composites they have a higher resistance to moisture of the air.
(2480 - 2490 cm−1) [7] in the IR spectra of the composites indicates the absence of redox interactions between components in their production process or drying. Thanks UPTHE included in the composites they have a higher resistance to moisture of the air.
In the course of thermodynamic studies of these composites by combustion in a bomb calorimeter (constant volume) in an oxygen atmosphere is found that the combustion occurs completely and without solid residue [21] with the formation of gaseous products and water [22].
To sum up, in this study the developed composites based on UPTFE and chitosan salts and methods for their preparation. The composites UPTFE with chitosan nitrate or perchlorate of represent an oxidative polymer base, in which can be introduced combustible components, as shown in the example of chitosan dodecahydro-closo- dodecaborate. As such compounds there may be other salts of dodecahydro-closo-do- decaborane acid mainly with organic cations. This requires additional testing, the results of which will be announced later.
Acknowledgements
The work financially supported by the Program of Basic Research “”Far East” (project no. 265-2015-0011).
Cite this paper
Saldin, V.I. and Tsvetnikov, A.K. (2016) Composites on Base of the Ultradispersed Polytetrafluoroethylene and Chitosan Salts. Journal of Materials Science and Chemical Engineering, 4, 22-28. http://dx.doi.org/10.4236/msce.2016.412003
References
- 1. Wall, L.A. (1972) Fluoropolymers. Wiley-Interscience, New York.
- 2. Pashnin, Yu.A., Malkevich, S.G. and Dunaevskaya, Ts.S. (1975) Fluoropolymers. Khimia, L.
- 3. Dolgoborodov, A.Yu., Makhov, M.N., Kolbanev, I.V. and Fortov, V.E. (2006) The Pyrotechnic Composi-tion Based ON Mixtures of Mechanically Activated Metal-Oxidizer. Proceedings of III All-Russian Symposium “Energetic Condensed Sys-tems”, Chernogolovka, 31 September-2 November 2006, 32-33.
- 4. Tsvetnikov, A.K. and Uminskiy, A.A. (1990) A Method of Polyfluo-rocarbon Producing. RF Patent No 1808194.
- 5. Pavlov, A.D., Sukhoverkhov, S.V. and Tsvetnikov, A.K. (2007) The Use of Pyrolytic Gas Chromatography/Mass Spectrometry to Determine the Composition of the Forum and Its Fractions. Bulletin of the Feb RAS, 51-55.
- 6. Buznik, V.M. and Tsvetnikov, A.K. (1993) Ultradispersed Polytetrafluoroethylene as a Basis for New Advanced Materials. Bulletin of the Feb RAS, 39-47.
- 7. Saldin, V.I., Tsvetnikov, A.K., Ignatieva, L.N. and Buznik, V.M. (2004) Intercalation of Graphite Oxide with Dodecahydro-Closo-Dodecaboric Acid. Russian Journal of Inorganic Chemistry, 49, 1775-1779.
- 8. Saldin, V.I. and Sukhovey, V.V. (2013) A Method of Producing of Boron-Fluorine- Containing Energy-Intensive Compositions. RF Patent No 2479560.
- 9. Saldin, V.I. and Tsvetnikov, A.K. (2015) Composites on Base of the Ultradispersed Polytetrafluoroethylene and Graphite Oxide Intercalated Compounds. Journal of Materials Science and Chemical Engineering, 3, 12-16.
- 10. Plisko, E.A., Nudga, L.A. and Danilov, S.N. (1977) Chitin and Its Chemical Transformation. Uspekhi Khimii, 46, 1470-1487. https://doi.org/10.1070/rc1977v046n08abeh002171
- 11. Rinaudo, M. (2006) Chitin and Chitosan: Properties and Applications. Progress in Polymer Science, 31, 603-632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
- 12. Saldin, V.I., Ignatieva, L.N. and Buznik, V.M. (2006) Chitosan Perchlorate, Method of Its Producing and Energy-Intensive Composition Containing It. RF Patent No 2315774.
- 13. Tsvetnikov, A.K. (1995) Installation for Processing of Polytetrafluoroethylene. RF Patent No 2035308.
- 14. Saldin, V.I., Ignatieva, L.N., Savchenko, N.N. and Sukhovey, V.V. (2011) Study of Interaction of Chitosan with Some Oxidizers . Bulletin of the Feb RAS, 45-51.
- 15. Saldin, V.I., Ignatieva, L.N. and Nikolenko, Yu.M. (2006)Reactions of Dodecahydro-Closo- Dodecaboric Acid with Chitosan. Journal of Structural Chemistry, 47, 41-46. https://doi.org/10.1007/s10947-006-0262-3
- 16. Saldin, V.I., Sukhovey, V.V., Buznik, V.M., Mikhailov, Yu.M., Merkin, A.A., Rybin, V.E. and Komarov, A.A. (2016) A Method of Producing of Potassium Dodecahydro-Closo- Dodecaborate. Pat. RF 2573679.
- 17. Kuznetsov, N.T., Kulikova, L.N. and Kanaeva, O.A. (1976) The Gravymetric Determination of the Closo-Dodecaborates. Journal of Analytical Chemistry, 31, 1382-1383.
- 18. Zhu, Y., Murali, S., Cai, W., et al. (2010) Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Advance Materials, 22, 3906-3924. https://doi.org/10.1002/adma.201001068
- 19. Saldin, V.I., Ignateva, L.N., Nikolenko, Y.M., Buznik, V.M. and Mikhailov, Y.M. (2010) Thermal Conversion of Chitosanium Dodecahydro-Closo-Dodecaborate. Russian Journal of Inorganic Chemistry, 55, 1221-1227.
- 20. Saldin, V.I., Buznik, V.M., Mikhailov, Y.M. and Ganina, L.V. (2014) Thermodynamic Properties of Chitosan Dodecahy-dro-Closo-Dodecaborate. Russian Journal of Physical Chemistry A, 88, 377-380. https://doi.org/10.1134/S0036024414020216
- 21. Nakamoto, K. (1986) Infrared and Raman Spectra of Inorganic and Coordination Com-pounds, Interscience, New York.
- 22. Saldin, V.I. et al. Not published data.



