Open Access Library Journal
Vol.03 No.11(2016), Article ID:72375,12 pages
10.4236/oalib.1103212
Immunoglobulin A Antibody Responses in Patients with Primary or Secondary Dengue Infections
Didye Ruiz*, Susana Vázquez, Hernán C. Ríos, Naifi Calzada, María G. Guzmán
“Pedro Kouri” Tropical Medicine Institute (IPK), PAHO/WHO Collaborating Center for the Study of Dengue and Its Vector, Havana, Cuba

Copyright © 2016 by authors and Open Access Library Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 7, 2016; Accepted: November 26, 2016; Published: November 29, 2016
ABSTRACT
IgM serological diagnostic plays an important role in the dengue epidemiological surveillance. However, IgA has been identified as a possible diagnostic marker for early stage and recent infections. The aim of this study was to know the IgA response in dengue patients in relation to type of infection, serotype specificity and role as early and recent infection marker. IgA behavior in dengue patients, using a method defined as Indirect Sandwich ELISA-IgA (ELISA-IgA) and Capture IgM ELISA (MAC- ELISA) as reference assay was studied. The sensibility and specificity of the system were 91.06% and 83.73% respectively. In acute phase of illness, IgA was only detected in secondary cases with 32.3%. IgM appears later than IgA in secondary cases. No significant differences in IgA or IgM responses between primary and secondary cases were found in samples collected after day 5 of onset of symptoms. In days 41 - 54, the percentage of IgA was lower respect to IgM. IgA was detected until day 47 in primary and until day 50 in secondary cases. An elevated cross-reactivity in IgA was observed between dengue serotypes contrary to IgM. The usefulness of IgA, as alternative early diagnostic tool, could be influenced by the absence or very low percentages of positives cases in the first four days of onset of symptoms. However, for definition of secondary infection could be important. IgA could be an alternative tool, better than IgM, as recent infection marker for dengue fever, but this requires more studies in future investigations.
Subject Areas:
Immunology, Infectious Diseases
Keywords:
Cross-Reactivity, Dengue Infections, Early Detection, IgA, Recent Infection

1. Introduction
Dengue viruses (DENVs) are transmitted to humans by infected mosquitoes, mainly Aedes aegypti and Aedes albopictus. Dengue is the most important mosquito-borne viral disease of humans and an enormous public health burden in affected countries. An estimated of 50 - 100 million dengue cases occur annually, including 250,000 - 500,000 cases of severe illness and around 25,000 deaths. Approximately 2.5 billion people live in dengue endemic countries and the illness is reported in Southeast Asia, Western Pacific, the Americas, Africa and Mediterranean regions [1] [2] [3] .
DENVs belong to the Flaviviridae family, genus flavivirus. There are four dengue virus serotypes (1, 2, 3, and 4), which are responsible for this disease. Infection by each of the four dengue serotypes can lead to a broad spectrum of outcomes, from asymptomatic and dengue with or without alarm signs and dengue severe form [4] [5] .
There are several reasons why early and accurate diagnosis of dengue is important. An early and accurate diagnosis can assist in patient management by directing clinical attention to the appearance of major warning signs of severe or even life threatening complications, a prompt diagnosis of index cases may facilitate vector control activities in the community and also, an accurate dengue diagnosis prevents unnecessary and possibly expensive antibiotic usage [6] . To count with quick, sure, reliable methods in an early diagnosis, it is of supreme importance for an appropriate surveillance that allows the control of viral transmission and vector [7] [8] .
The IgM antibodies detection against dengue virus is one of the serological markers broadly used in the diagnosis of this illness. They are detectable only starting from 5 to 6 days of onset of fever, which represent a delay in the diagnosis. Another problem is that they may persist in some individuals up to 3 months or more, being complicated the definition of recent infection, mainly in those countries where the illness is endemic [7] . Therefore, some authors have conferred certain value to IgA antibody in regard to early detection, its use in secondary dengue infections and its short duration [9] [10] [11] [12] . However, the studies of this immunoglobulin have been few and its role in the dengue diagnostic is not even well elucidated.
One method used for the detection of IgA antibodies is the capture IgA enzyme- linked immunosorbent assay, known as AAC-ELISA [13] [14] [15] . A group of researchers (Yap et al., 2011) developed an ELISA that was defined as ACA-ELISA for the detection of IgA in serum and saliva samples, achieving a high sensitiveness in the detection of these antibodies. With the aim of know the IgA response in dengue patients in relation to primary and secondary infections, serotype specificity and its role as early and recent infection marker, a similar method defined as indirect sandwich ELISA-IgA (ELISA-IgA) was applied in this work.
2. Material and Methods
2.1. Serum Specimens
The DENV-positive and negative samples used in this work were from the dengue sera bank of Arbovirus Laboratory at the “Pedro Kouri” Tropical Medicine Institute of Havana, Cuba. Three panels of sera were selected for the study.
First panel was constituted by 345 samples, of them 179 corresponding to IgM positive samples, collected between days 5 and 7 of onset of fever, from patients of DENV-3 confirmed outbreak by RT-PCR and virus isolation, 67 samples from healthy blood donors and 99 from non-dengue confirmed febrile cases.
Second panel was constituted by 765 samples, which were sectioned in three groups: Group 1: 260 samples collected within the first 4 days since onset of fever, from confirmed DENV 2,3 and 4 patients by RT-PCR and/or virus isolation, classified in 130 primary and 130 secondary cases; Group 2: 230 samples collected between days 5 and 7 of onset of fever, from patients of DENV-3 confirmed outbreak by RT-PCR and virus isolation, with IgM and/or IgG positive serology and classified as 85 primary and 145 secondary cases; Group 3: 230 samples collected between days 14 and 54 of onset of fever, from dengue patients confirmed by seroconvertion or increment of four fold IgG titers in paired serums, constituted by 85 primary and 145 secondary cases and corresponding to pair serum of samples of group 2.
Third panel was established by 34 monosera from group 2, constituted by 16 primary and 18 secondary patients to determinate cross-reactivity of IgA between dengue serotypes.
All sera were collected from adult dengue patients, which were confirmed by the routine methods, such as virus isolation in C6/36 cell line, RT-PCR detection and serological diagnosis for the detection of IgM (MAC-ELISA) and IgG (ELISA inhibition method, EIM) [15] [16] [17] . The dengue patients were also classified according to the type of infection (primary or secondary). Ethical approval was obtained from the Ethics Review Committee of IPK Institution.
2.2. Virus and Antigens
Dengue antigens used in the serological studies were obtained from infected suckling mice brain extracted by the sucrose-acetone method [18] . DENV-1 (Hawaii strain), DENV-2 (New Guinea C strain), DENV-3 (H-87 strain) and DENV-4 (H-241 strain) were used.
2.3. Capture IgM ELISA Assay (MAC-ELISA)
MAC-ELISA for the detection of IgM dengue antibody was performed as previously described by Vazquez et al., 2005 [11] . A serum sample was considered positive when the optical density ratio (OD ratio) was ≥2. This value was calculated as P/N where P represents the OD of each serum sample and N represents the mean OD of the negative control wells.
2.4. ELISA Inhibition Method (EIM)
EIM for the detection of IgG dengue antibody was performed as previously described by Vazquez et al., 2009 [17] . The inhibition percentage was calculated as:
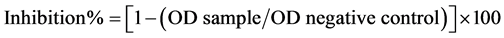
The antibody titer of each serum was considered as the highest dilution with a percentage of inhibition ≥50. A serum with a percentage of inhibition <50 was considered negative for IgG dengue antibodies (<20).
2.5. Indirect Sandwich ELISA-IgA Assay (ELISA-IgA)
Briefly, each microtiter plate (Greiner Bio-one) was coated with 2.5 µg/mL (100 µL/well) of monoclonal antibody H3/6 anti-dengue complex in sodium carbonate-bicarbonate buffer (pH 9.5) produced in the IPK [19] . The plates were incubated overnight at 4˚C. After washing the plates were blocked with 1% of bovine serum albumin (BSA, Sigma) and incubated during 1 h at 37˚C. One hundred microliter of mixed virus antigens (16 hemaglutinate units) from mice brain infected with dengue 1, 2, 3 and 4 were incubated for 1 h at 37˚C. After washing, 100 µL of sera and control (1:100) dilutions in PBS-T20 with 5% of milk were added and incubated (1 h at 37˚C). A dilution of 1:20,000 peroxidase-conjugated goat anti-IgA (α chain specific, Sigma) in PBS-T20 with 5% of milk was added (100 µL/well). After incubation (1 h at 37˚C) and washing, orthophenilendiamine (OPD, Merck) and hydrogen peroxide (Merck) in buffer phosphate citrate pH 5 was used as substrate. The optical density (OD) was measured at 492 nm. A serum sample was considered positive when OD ratio was ≥2. This value was calculated as P/N where P represents the OD of each serum sample and N represents the mean OD of the negative control wells.
2.6. Statistical Analysis
For the analysis of the results were designated the statistical Epidat 3.1 (2006, OPS/ OMS) and GraphPad Prism version 6.0 (2007) programs. Also, for the pictures was used EXCEL program, Office 2010.
3. Results
Comparison between ELISA-IgA and MAC-ELISA using serum samples collected on days 5 to 7 after fever onset (panel 1).
Three hundred forty five sera corresponding to panel 1 were assayed for the evaluation of ELISA-IgA, using the MAC-ELISA as gold standard. Table 1 show 163 positive and 139 negative concordant samples by both assays, 16 were positive by MAC-ELISA and negative by ELISA-IgA, and 27 were positive to IgA detection but negative by the reference assay. The sensitivity and specificity of IgA method were 91.06% (IC 95%: 86.60 - 95.52) and 83.73% (IC 95%: 77.82 - 89.65) respectively. The concordance between assays was 87.54% (IC 95%: 83.91 - 91.17).
IgA responses in serum samples collected in different days after onset of symptoms (panel 2).
The Figure 1 shows the positive percentages of IgA in serum samples collected in different days since onset of symptoms in response to primary and secondary dengue infections. A first group, corresponding to 260 serum samples collected between day 0 and 4, showed percentages of 0% (0/130) for primary and 32.3% (42/130) for secondary
Table 1. Comparison of ELISA-IgA and MAC-ELISA assays in samples collected between days 5 and 7 since onset of symptoms.
Sensitivity: 91.06% (IC 95%: 86.60-95.52); Specificity: 83.73% (IC 95%: 77.82- 89.65); Concordance: 87.54% (IC 95%: 83.91-91.17).

Figure 1.Positive percentages of IgA in samples collected in different days after onset of symptoms. N = Total samples from each group of collection days.
dengue cases. The second group, with 230 sera collected between day 5 and 7 revealed percentages of 88.2% (75/85) for primary and 86.9% (126/145) for secondary cases. Third group, constituted by 230 samples collected between days 14 and 54 after onset, showed percentages of 81.2% (69/85) and 71.0% (103/145) respectively. No significant differences in the percentages between primary and secondary cases in samples collected after day 5 of onset of symptoms were found (5 - 7 d: p = 0.93; 14 - 54 d: p = 0.12). However, a significant decrease of IgA in secondary cases was observed (5 - 7 d/ 14 - 50 d: p = 0.002), contrary to primary cases (5 - 7 d/14 - 54 d: p = 0.29). IgA was detected in serum until day 47 in primary and day 50 in secondary cases.
Percentages of IgA and IgM from sera collected in acute (0 - 4 d) and convalescent (5 - 54 d) phases (panel 2: group 2 and 3)
Two hundred sixty sera collected in the four first days since onset of fever from pri- mary and secondary cases were used in the analysis of the IgA/IgM response in the acute phase of illness. In the Figure 2(a) is observed absent percentages of IgA in pri- mary cases, while secondary cases are detectable from day 0 with value of 67.0% (2/3). In primary, IgM is detectable at day 2 in only two samples, but higher percentages were observed at day 4 (67.0%), while in secondary is detectable at day 1 (3%) increasing the percentage until day 4 (53%). Figure 2(b) shows positive percentages in primary and secondary cases from 430 serum samples collected in days after day 4 of onset of symp- toms. No significant difference in IgA or IgM percentages between primary and secondary cases was observed. The global analysis showed higher percentages for IgM than


Figure 2. IgA and IgM positive percentages from sera of primary and secondary dengue cases in relation to collection days. (a) Serum samples collected between days 0 - 4; (b) Serum samples collected between days 5 - 54. N = number serum sample by day.
IgA in sera collected after day 4, independently of infection type (primary: 95.3% vs. 84.6%, p = 0.002; secondary: 95.4% vs. 78.9%, p = 0.000). It is necessary to point out that between days 41 and 54 a higher decrease in the percentage of positives was observed, mainly in IgA antibodies.
IgA and IgM cross-reactive responses (panel 3).
Figure 3 shows the IgA/IgM cross-reactive responses in 16 primary and 18 secondary serum samples from dengue 3 patients, against the four dengue antigens. In primary cases (Figure 3(a)), the comparison between the infecting serotype (Den-3) and the others serotypes showed significant differences with Den-1 (p = 0.03), but not with Den-2 (p = 0.25) and Den-4 (p = 0.28). Meanwhile, a low IgM cross-reactivity with significant differences (p < 0.001) was found.
In secondary samples (Figure 3(b)) it was also observed cross-reactivity of IgA, but with higher increase in relation to primary cases. The p values for Den-1, Den-2 and Den-4 were 0.017, 0.21 and 0.83 respectively. The comparison of IgM response of Den- 3 with Den-1, 2, 4 serotypes showed significant differences (p = 0.01; p < 0.00).
4. Discussion
IgM detection in sera is still the preferred marker for the serological diagnosis of dengue infection. However, it could be detected early within 3 - 4 days after fever onset, but the majority of infected individuals become positive by day 5 - 6 [20] . Also, IgM have been described as persisting until about 3 to 8 months post onset. This implicates a delayed diagnostic and the difficulty in the definition of recent infection, mainly in endemic countries. So, other markers as IgA antibodies and NS1 protein have been se-

Figure 3. Specific IgA and IgM antibody reactivity to the four dengue virus serotypes ( D1,
D1,  D2,
D2,  D3, and
D3, and  D4) in serum samples from primary (a) and secondary (b) dengue cases. The OD ratio values ± standard deviations were included.
D4) in serum samples from primary (a) and secondary (b) dengue cases. The OD ratio values ± standard deviations were included.
lected as markers for early diagnosis in the latest years.
Few studies have been developed to evaluate the behaviour of IgA serum antibodies in patients with dengue infections. Some researchers have enunciated its value as diagnostic marker in the early phase of illness, the usefulness for definition of secondary dengue infections, prognostic marker of severity and its limited persistence on time [9] [10] [11] [12] .
In the present work, the ELISA-IgA assay showed a good sensibility (91.06%) in samples from confirmed dengue patients, although the specificity (83.73%) was influenced by IgA detection in febrile no-dengue patients. In two different moments, Balmaseda et al. 2003/2008 [21] working an IgA Capture ELISA reported similar values of sensibility and specificity (94.40% and 74.70% // 93.00% and 86.00%), observing the influence of febrile no-dengue cases in the specificity. Besides, recently De Deker et al., 2015 [22] using a Dengue IgA Capture assay reported a specificity of 88% associated to the response in 6 sera of non-dengue group and expounded the possible cross-reaction induced by another etiological agent.
IgA percentages in different stages of dengue illness have been commented by different authors. Balmaseda et al. (2003) reported positive percentages of IgA of 96.0% in serum samples collected in the first 7 days of symptom onset and 90% between days 8 and 78. Vazquez et al. (2005) found positive percentages of 23.8% and 85.4% in samples collected between days 5 and 7 from primary and secondary cases. Also, Vazquez et al. (2007) found IgA percentages around 20% at day 4 with an increase until 100% to day 7 from primary cases and around 40% at day 4 getting a 100% also at day 7 in secondary cases. Yap et al. 2011 using ACA-ELISA method observed percentages of 15% for primary and 94 for secondary cases in samples collected in the first 3 days of symptom onset, showing an increase in later days. In this study, it was not observed significant differences in IgA percentages between primary and secondary dengue samples collected after day 4, however, in the first four days for primary cases the IgA detection was absent (0%) contrary to 32.3% showed by secondary cases.
As it has been previously commented, in a primary infection IgA antibodies should be detectable after the release of IgM around day 5 of fever onset, while in secondary infection IgA presents an analogous behavior like IgG, appearing early in acute phase due to the common epitopes recognition by memory B cell clones circulating on blood. [20] Balmaseda et al. (2003) found that IgA antibodies were detected in more acute- phase sera than were IgM antibodies, pointing out that one explanation for these results could be that IgA levels are higher in secondary infections, which constituted the vast majority of their samples. However, Yap et al. (2011) found higher percentage of IgA that IgM so much in primary as in secondary cases, in the acute phase of the illness. Ahmed et al. 2010 [23] , using a new commercial IgA rapid test, reported similar percentages of IgM and IgA as much in primary as secondary cases, but De la Cruz et al. 2012 [24] using the same commercial system found higher percentages of IgM in relation to IgA in secondary and inverse in primary cases. The kinetics of IgA detection with respect to the onset of fever seems to vary according to the immune status and seems to appear late in primary cases but very early in secondary cases.
In secondary infection, memory B cells play an important role in rapid and strong production of humoral response that may give higher detectable levels of IgA. Skin immunization utilizes potent bone marrow-derived DCs that are resident in the outer epidermal layers of skin, such as Langerhans cells. These DCs provide immune-sur- veillance functions, and when they are activated migrate out of the skin to the draining lymph nodes (DLNs) and induce strong antigen specific responses by B and T lymphocytes. This immunization strategy induces robust secretory IgA responses [25] . But, it is common that antigenic stimulation occurs in the secondary lymphoid organ (spleen), where the T and B cell zones are located. However, an early generation of IgA may takes place in local and peripheral lymph nodes due to the directly enter of memory lymphocytes from the blood [26] [27] . On the other hand, a low IgA levels in primary infection reported in previous studies [12] [15] [24] [28] may be explained by the presence of one type of B cell (named B-1 cells) that produce antibodies with poor specificity and do not appear to require help from T cells in their responses to antigens, but may be influenced by the cytokines produced by T cells [27] .
In addition, in this work a significant decrease of IgA percentages in serum samples collected between days 41 and 54 from primary and secondary cases was observed, which could suggest a short life of IgA with respect to IgM. Previous studies have commented this characteristic. Talarmin et al. (1998) reported that IgA antibodies were not detectable after day 40 of onset of fever. Nawa et al. 2005 [29] found a positive detection of IgA until day 23 of fever in serum samples from primary dengue patients. Balmaseda et al. (2003) showed positivity of DEN-specific IgA in sera collected until day 78 of onset of symptoms. Also, Yap et al. (2011) detected IgA in samples collected until day 34 after onset of symptoms from primary and secondary dengue cases. In the present work positive percentage of IgA, above 50%, in the last group of collection (41 - 54 d), exactly until day 47 in primary and day 50 in secondary cases were found. Different results have been found in relation to the life period of IgA, for that reason future studies must be developed to elucidate the role of IgA as a possible marker of recent infection, working with samples collected in days higher than 3 months of onset of sym- ptoms.
The low cross-reactivity of IgM antibodies and its application for the identification of the dengue virus infecting serotypes has been reported by some authors [30] [31] [32] while IgA cross-reactivity had been poor studied [33] [34] . We found high IgA cross- reactivity as much in primary as in secondary cases, contrary to IgM, that showed high specificity against the infecting serotype (dengue 3) in agreement with previous studies. De Decker et al. [22] showed similar IgA detection for Den-2, 3 and 4, using a commercial Capture IgA ELISA. This result could be described by a high recognition of common epitopes for primary cases and memory B cells activation, producing IgA antibodies against previous serotypes in secondary cases.
5. Conclusion
Finally, we can say that the utility of IgA as alternative early diagnostic tool could be affected by the absence or very low percentages of positives cases in the first four days of onset of symptoms. However, it’s utility in the definition of secondary infection could be important considering the incidence of these cases in (hyper)-endemic areas where more cases are frequently associated with severe outcomes. In sera collected at day 5 or more of fever onset, the IgM may be still considered the favorite marker for serological diagnostic, although IgA could be an alternative tool for primary and secondary cases, keeping in mind its possible use as recent infection marker, which requires more studies in future investigations. The cross-reactivity present in the recognition against the dengue serotypes does not permit the identification of circulating serotype of outbreak. It is necessary to continue the studies of the behaviour of this immunoglobulin to insure a best diagnostic of this illness.
Acknowledgements
We thank Drs. M. Pupo and J.S. González for their suggestions and reviewing this manuscript. Also, special thank to Dr. Anavaj Sakuntabhai and Cecile Roux for their help in the publishing process.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Funding Information
This work was funded by the European Union 7th Framework Health Program (grant agreement 282378-DENFREE). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Cite this paper
Ruiz, D., Vázquez, S., Ríos, H.C., Calzada, N. and Guzmán, M.G. (2016) Immunoglobulin A Antibody Responses in Patients with Primary or Sec- ondary Dengue Infections. Open Access Li- brary Journal, 3: e3212. http://dx.doi.org/10.4236/oalib.1103212
References
- 1. Halstead, S.B. (2007) Dengue. Lancet, 370, 1644-1652.
https://doi.org/10.1016/S0140-6736(07)61687-0 - 2. Guzman, M.G., Halstead, S.B., Artsob, H., Buchy, P., Farrar, J., Gubler, D.J., Hunsperger, E., Kroeger, A., Margolis, H.S., Martinez, E., Nathan, M.B., Pelegrino, J.L., Simmons, C., Yoksan, S. and Peeling, R.W. (2010) Dengue: A Continuing Global Threat. Nature Reviews Microbiology, 8, S7-S16.
https://doi.org/10.1038/nrmicro2460 - 3. Bhatt, S., Gething, P.W., Brady, O.J., Messina, J.P., Farlow, A.W., Moyes, C.L., Drake, J.M., Brownstein, J.S., Hoen, A.G., Sankoh, O., Myers, M.F., George, D.B., Jaenisch, T., Wint, G.R., Simmons, C.P., Scott, T.W., Farrar, J.J. and Hay, S.I.(2013) The Global Distribution and Burden of Dengue. Nature, 496, 504-507.
https://doi.org/10.1038/nature12060 - 4. PAHO (1994) Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control. Scientific Publication, Pan American Health Organization, 548.
- 5. WHO/TDR (2009) Dengue Case Classification. Dengue, Guidelines for Diagnosis Treatment, Prevention and Control. Geneva.
- 6. Tricou, V., Vu, H.T., Quynh, N.V., Nguyen, C.V., Tran, H.T., Farrar, J., Wills, B. and Simmons, C.P. (2010) Comparison of Two Dengue NS1 Rapid Tests for Sensitivity, Specificity and Relationship to Viraemia and Antibody Responses. BMC Infectious Diseases, 10, 142.
https://doi.org/10.1186/1471-2334-10-142 - 7. Guzman, M.G., Rosario, D. and Kouri, G. (2006) Diagnosis of Dengue Virus Infection. In: Kalitzy, M. and Borowski, P., Eds., Molecular Biology of the Flavivirus, Horizon Bioscience, Norfolk, 191-223.
- 8. Senanayake, S. (2006) Dengue Fever and Dengue Haemorrhagic Fever—A Diagnostic Challenge. Australian Family Physician, 35, 609-612.
- 9. Talarmin, A., Labeau, B., Lelarge, J. and Sarthou, J.L. (1998) Immunoglobulin A-Specific Capture Enzyme-Linked Immunosorbent Assay for Diagnosis of Dengue Fever. Journal of Clinical Microbiology, 36, 1189-1192.
- 10. Balmaseda, A., Guzman, M.G., Hammond, S., Robleto, G., Flores, C., Tellez, Y., Videa, E., Saborio, S., Perez, L., Sandoval, E., Rodriguez, Y. and Harris, E. (2003) Diagnosis of Dengue Virus Infection by Detection of Specific Immunoglobulin M (IgM) and IgA Antibodies in Serum and Saliva. Clinical and Diagnostic Laboratory Immunology, 10, 317-322.
https://doi.org/10.1128/cdli.10.2.317-322.2003 - 11. Vazquez, S., Perez, A.B., Ruiz, D, Rodriguez, R., Pupo, M., Calzada, N., González, L., González, D., Castro, O., Serrano, T. and Guzmán, M.G. (2005) Serological Markers during Dengue 3 Primary and Secondary Infections. Journal of Clinical Virology, 33, 132-137.
https://doi.org/10.1016/j.jcv.2004.10.013 - 12. Yap, G., Sil, B.K. and Ng, L.C. (2011) Use of Saliva for Early Dengue Diagnosis. PLOS Neglected Tropical Diseases, 5, e1046.
https://doi.org/10.1371/journal.pntd.0001046 - 13. Cuzzubbo, A.J., Vaughn, D.W., Nisalak, A., Suntayakorn, S., Aaskov, J. and Devine, P.L. (1998) Detection of Specific Antibodies in Saliva during Dengue Infection. Journal of Clinical Microbiology, 36, 3737-3739.
- 14. Koraka, P., Suharti, C., Setiati, T.E., Mairuhu, A.T., Van Gorp, E., Hack, C.E., Juffrie, M., Sutaryo, J., Van Der Meer, G.M., Groen, J. and Osterhaus, A.D. (2001) Kinetics of Dengue Virus-Specific Serum Immunoglobulin Classes and Sub-classes Correlate with Clinical Outcome of Infection. Journal of Clinical Microbiology, 39, 4332-4338.
https://doi.org/10.1128/JCM.39.12.4332-4338.2001 - 15. Vazquez, S., Cabezas, S., Perez, A.B., Pupo, M., Ruiz, D., Calzada, N., Bernardo, L., Castro, O., Gonzalez, D., Serrano, T., Sanchez, A. and Guzman, M.G. (2007) Kinetics of Antibodies in Sera, Saliva, and Urine Samples from Adult Patients with Primary or Secondary Dengue 3 Virus Infections. International Journal of Infectious Diseases, 11, 256-262.
https://doi.org/10.1016/j.ijid.2006.05.005 - 16. Lanciotti, R.S., Calisher, C.H., Gubler, D.J., Chang, G.J. and Vorndam, A.V. (1992) Rapid Detection and Typing of Dengue Viruses from Clinical Samples by Using Reverse Transcriptase-Polymerase Chain Reaction. Journal of Clinical Microbiology, 30, 545-551.
- 17. Vazquez, S., Acosta, N., Ruiz, D., Calzada, N., Alvarez, A.M. and Guzman, M.G. (2009) Immunoglobulin G antibody Response in Children and Adults with Acute Dengue 3 Infection. Journal of Virological Methods, 159, 6-9.
https://doi.org/10.1016/j.jviromet.2009.02.017 - 18. Clark, D.H. and Casals, J. (1958) Techniques for Hemagglutination Inhibition with Arthropodborne Viruses. American Journal of Tropical Medicine and Hygiene, 7, 561-573.
- 19. Hermida Diaz, C., Pupo, M., Guzman Tirado, M.G., Gonzalez Garriga, M. and Marcet Sanchez, R. (1992) [Use of a Dengue Anti-Complex Monoclonal Antibody in Viral Purification]. Revista Cubana de Medicina Tropical, 44, 171-176.
- 20. Blacksell, S.D., Jarman, R.G., Gibbons, R.V., Tanganuchitcharnchai, A., Mammen Jr., M.P., Nisalak, A., Kalayanarooj, S., Bailey, M.S., Premaratna, R., de Silva, H.J., Day, N.P. and Lalloo, D.G. (2012) Comparison of Seven Commercial Antigen and Antibody Enzyme-Linked Immunosorbent Assays for Detection of Acute Dengue Infection. Clinical and Vaccine Immunology, 19, 804-810.
https://doi.org/10.1128/CVI.05717-11 - 21. Balmaseda, A., Saborio, S., Tellez, Y., Mercado, J.C., Perez, L., Hammond, S.N., Rocha, C., Kuan, G. and Harris, E. (2008) Evaluation of Immunological Markers in Serum, Filter-Paper Blood Spots, and Saliva for Dengue Diagnosis and Epidemiological Studies. Journal of Clinical Virology, 43, 287-291.
https://doi.org/10.1016/j.jcv.2008.07.016 - 22. De Decker, S., Vray, M., Sistek, V., Labeau, B., Enfissi, A., Rousset, D. and Matheus, S. (2015) Evaluation of the Diagnostic Accuracy of a New Dengue IgA Capture Assay (Platelia Dengue IgA Capture, Bio-Rad) for Dengue Infection Detection. PLoS Neglected Tropical Diseases, 9, e0003596.
https://doi.org/10.1371/journal.pntd.0003596 - 23. Ahmed, F., Mursalin, H., Alam, M.T., Amin, R., Sekaran, S.D., Wang, S.M., Tan, Y.Y., Sil, B.K. and Hossain, M.A. (2010) Evaluation of ASSURE(R) Dengue IgA Rapid Test Using Dengue-Positive and Dengue-Negative Samples. Diagnostic Microbiology and Infectious Disease, 68, 339-344.
https://doi.org/10.1016/j.diagmicrobio.2010.07.007 - 24. De la Cruz Hernandez, S.I., Gonzalez Mateos, S., Flores Aguilar, H., Lopez Martinez, I., Alpuche Aranda, C., Ludert, J.E. and Del Angel, R.M. (2012) Evaluation of A Novel Commercial Rapid Test for Dengue Diagnosis Based on Specific IgA Detection. Diagnostic Microbiology and Infectious Disease, 72, 150-155.
https://doi.org/10.1016/j.diagmicrobio.2011.11.002 - 25. Belyakov, I.M., Hammond, S.A., Ahlers, J.D., Glenn, G.M. and Berzofsky, J. (2004) Transcutaneous Immunization Induces Mucosal CTLs and Protective Immunity by Migration of Primed Skin Dendritic Cells. The Journal of Clinical Investigation, 113, 998-1007.
https://doi.org/10.1172/JCI20261 - 26. Jeurissen, S.H.M., Claassen, E., Van Rooijen, N. and Kraal, G. (1985) Intra-Intestinal Priming Leads to Antigen-Specific IgA Memory Cells in Peripheral Lymphoid Organs. Immunology, 56, 417-423.
- 27. Eales, L.-J. (2003) Cells and Tissues of the Immune System. In: Eales, L.-J., Ed., Immunology for Life Scientists, John Wiley & Sons, Hoboken, 18-24.
https://doi.org/10.1002/047086821x.ch1 - 28. Andries, A.C., Duong, V., Ly, S., Cappelle, J., Kim, K.S., Lorn Try, P., Ros, S., Ong, S., Huy, R., Horwood, P., Flamand, M., Sakuntabhai, A., Tarantola, A. and Buchy, P. (2015) Value of Routine Dengue Diagnostic Tests in Urine and Saliva Specimens. PLoS Neglected Tropical Diseases, 9, e0004100.
https://doi.org/10.1371/journal.pntd.0004100 - 29. Nawa, M., Takasaki, T., Ito, M., Inoue, S., Morita, K. and Kurane, I. (2005) Immunoglobulin a Antibody Responses in Dengue Patients: A Useful Marker for Serodiagnosis of Dengue Virus Infection. Clinical and Diagnostic Laboratory Immunology, 12, 1235-1237.
- 30. Chungue, E., Boutin, J.P. and Roux, J. (1989) [Significance of IgM Titration by an Immu-noenzyme Technic for the Serodiagnosis and Epidemiological Surveillance of Dengue in French Polynesia]. Research in Virology, 140, 229-240.
https://doi.org/10.1016/S0923-2516(89)80100-1 - 31. Nawa, M., Yamad,a K.I., Takasaki, T., Akatsuka, T. and Kurane, I. (2000) Serotype-Cross-Reactive Immunoglobulin M Responses in Dengue Virus Infections Determined by Enzyme-Linked Immunosorbent Assay. Clinical and Diagnostic Laboratory Immunology, 7, 774-777.
https://doi.org/10.1128/cdli.7.5.774-777.2000 - 32. Delgado, I., Vazquez, S., Bravo, J.R. and Guzmán, M.G. (2002) Predicción del serotipo del virus del dengue mediante la respuesta de anticuerpos IgM. Revista Cubana de Medicina Tropical, 54, 113-117.
- 33. Inouye, S., Matsuno, S., Kono, R., Sangkawibha, N. and Thongcharoen, P. (1980) Hemagglutination-Inhibiting Immunoglobulin a Antibody in the Serum of Patients with Dengue Hemorrhagic Fever. Japanese Journal of Medical Science and Biology, 33, 181-184.
https://doi.org/10.7883/yoken1952.33.181 - 34. Summers, P.L., Eckels, K.H., Dalrymple, J.M., Scott, R.M. and Boyd, V.A. (1984) Antibody Response to Dengue-2 Vaccine Measured by Two Different Radioimmunoassay Methods. Journal of Clinical Microbiology, 19, 651-659.


