Facile Synthesis and Thermal Stability of Nanocrystalline Molybdenum Carbide1315
0100 200 300 400 500 600 700 800 900
-100
-90
-80
-70
-60
-50
-40
-30
-20
-10
0
Weight/%
Temperature/ oC
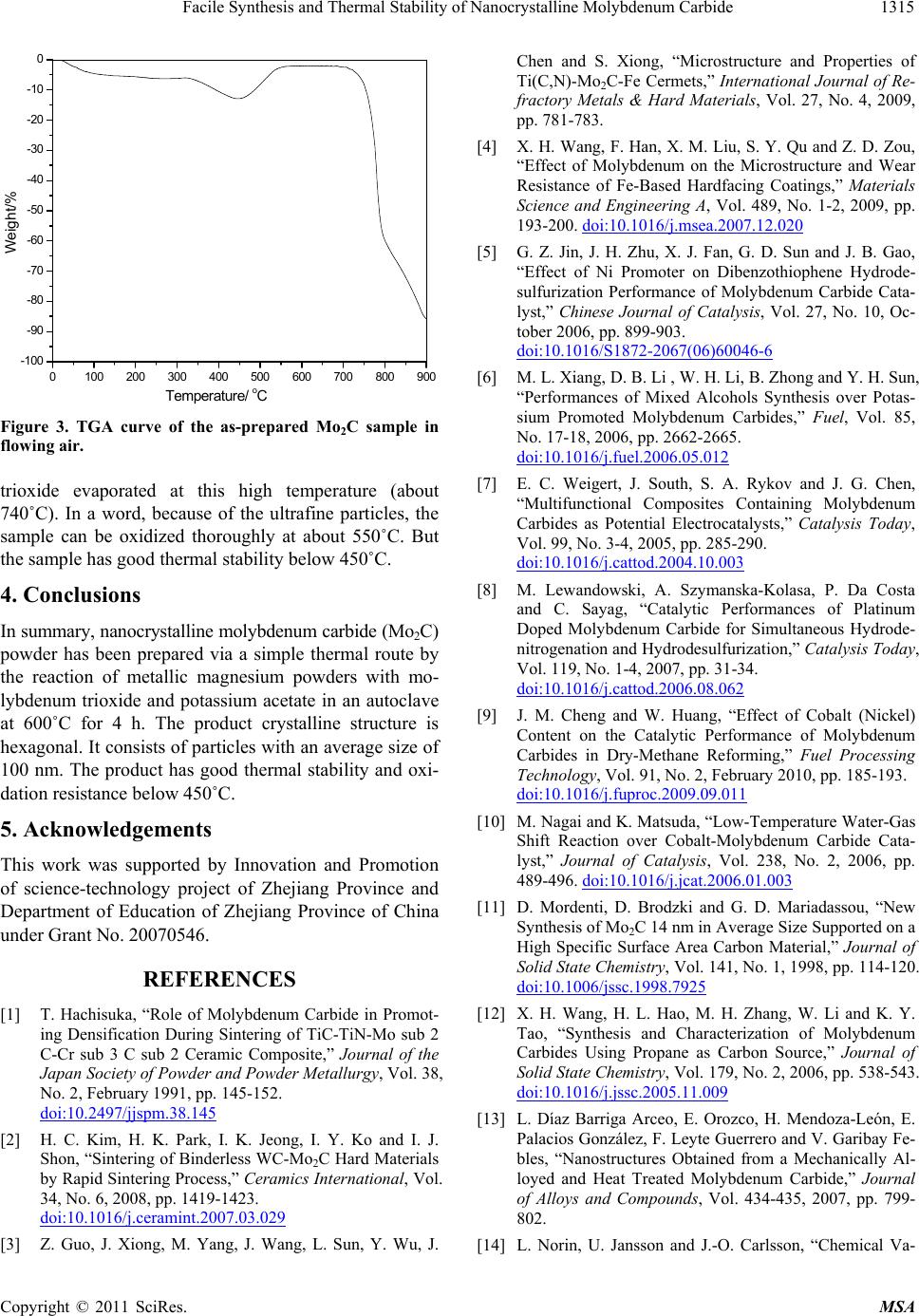
Figure 3. TGA curve of the as-prepared Mo2C sample in
flow ing air.
trioxide evaporated at this high temperature (about
740˚C). In a word, because of the ultrafine particles, the
sample can be oxidized thoroughly at about 550˚C. But
the sample has good thermal stability below 450˚C.
4. Conclusions
In summary, nanocrystalline molybdenum carbide (Mo2C)
powder has been prepared via a simple thermal route by
the reaction of metallic magnesium powders with mo-
lybdenum trioxide and potassium acetate in an autoclave
at 600˚C for 4 h. The product crystalline structure is
hexagonal. It consists of particles with an average size of
100 nm. The product has good thermal stability and oxi-
dation resistance below 450˚C.
5. Acknowledgements
This work was supported by Innovation and Promotion
of science-technology project of Zhejiang Province and
Department of Education of Zhejiang Province of China
under Grant No. 20070546.
REFERENCES
[1] T. Hachisuka, “Role of Molybdenum Carbide in Promot-
ing Densification During Sintering of TiC-TiN-Mo sub 2
C-Cr sub 3 C sub 2 Ceramic Composite,” Journal of the
Japan Society of Powder and Powder Metallurgy, Vol. 38,
No. 2, February 1991, pp. 145-152.
doi:10.2497/jjspm.38.145
[2] H. C. Kim, H. K. Park, I. K. Jeong, I. Y. Ko and I. J.
Shon, “Sintering of Binderless WC-Mo2C Hard Materials
by Rapid Sintering Process,” Ceramics International, Vol.
34, No. 6, 2008, pp. 1419-1423.
doi:10.1016/j.ceramint.2007.03.029
[3] Z. Guo, J. Xiong, M. Yang, J. Wang, L. Sun, Y. Wu, J.
Chen and S. Xiong, “Microstructure and Properties of
Ti(C,N)-Mo2C-Fe Cermets,” International Journal of Re-
fractory Metals & Hard Materials, Vol. 27, No. 4, 2009,
pp. 781-783.
[4] X. H. Wang, F. Han, X. M. Liu, S. Y. Qu and Z. D. Zou,
“Effect of Molybdenum on the Microstructure and Wear
Resistance of Fe-Based Hardfacing Coatings,” Materials
Science and Engineering A, Vol. 489, No. 1-2, 2009, pp.
193-200. doi:10.1016/j.msea.2007.12.020
[5] G. Z. Jin, J. H. Zhu, X. J. Fan, G. D. Sun and J. B. Gao,
“Effect of Ni Promoter on Dibenzothiophene Hydrode-
sulfurization Performance of Molybdenum Carbide Cata-
lyst,” Chinese Journal of Catalysis, Vol. 27, No. 10, Oc-
tober 2006, pp. 899-903.
doi:10.1016/S1872-2067(06)60046-6
[6] M. L. Xiang, D. B. Li , W. H. Li, B. Zhong and Y. H. Sun,
“Performances of Mixed Alcohols Synthesis over Potas-
sium Promoted Molybdenum Carbides,” Fuel, Vol. 85,
No. 17-18, 2006, pp. 2662-2665.
doi:10.1016/j.fuel.2006.05.012
[7] E. C. Weigert, J. South, S. A. Rykov and J. G. Chen,
“Multifunctional Composites Containing Molybdenum
Carbides as Potential Electrocatalysts,” Catalysis Today,
Vol. 99, No. 3-4, 2005, pp. 285-290.
doi:10.1016/j.cattod.2004.10.003
[8] M. Lewandowski, A. Szymanska-Kolasa, P. Da Costa
and C. Sayag, “Catalytic Performances of Platinum
Doped Molybdenum Carbide for Simultaneous Hydrode-
nitrogenation and Hydrodesulfurization,” Catalysis Today,
Vol. 119, No. 1-4, 2007, pp. 31-34.
doi:10.1016/j.cattod.2006.08.062
[9] J. M. Cheng and W. Huang, “Effect of Cobalt (Nickel)
Content on the Catalytic Performance of Molybdenum
Carbides in Dry-Methane Reforming,” Fuel Processing
Technology, Vol. 91, No. 2, February 2010, pp. 185-193.
doi:10.1016/j.fuproc.2009.09.011
[10] M. Nagai and K. Matsuda, “Low-Temperature Water-Gas
Shift Reaction over Cobalt-Molybdenum Carbide Cata-
lyst,” Journal of Catalysis, Vol. 238, No. 2, 2006, pp.
489-496. doi:10.1016/j.jcat.2006.01.003
[11] D. Mordenti, D. Brodzki and G. D. Mariadassou, “New
Synthesis of Mo2C 14 nm in Average Size Supported on a
High Specific Surface Area Carbon Material,” Journal of
Solid State Chemistry, Vol. 141, No. 1, 1998, pp. 114-120.
doi:10.1006/jssc.1998.7925
[12] X. H. Wang, H. L. Hao, M. H. Zhang, W. Li and K. Y.
Tao, “Synthesis and Characterization of Molybdenum
Carbides Using Propane as Carbon Source,” Journal of
Solid State Chemistry, Vol. 179, No. 2, 2006, pp. 538-543.
doi:10.1016/j.jssc.2005.11.009
[13] L. Díaz Barriga Arceo, E. Orozco, H. Mendoza-León, E.
Palacios González, F. Leyte Guerrero and V. Garibay Fe-
bles, “Nanostructures Obtained from a Mechanically Al-
loyed and Heat Treated Molybdenum Carbide,” Journal
of Alloys and Compounds, Vol. 434-435, 2007, pp. 799-
802.
[14] L. Norin, U. Jansson and J.-O. Carlsson, “Chemical Va-
Copyright © 2011 SciRes. MSA