A Top-Down Approach of Making Sn-3.5Ag Nanosolder Alloy by Swirl Method
1300
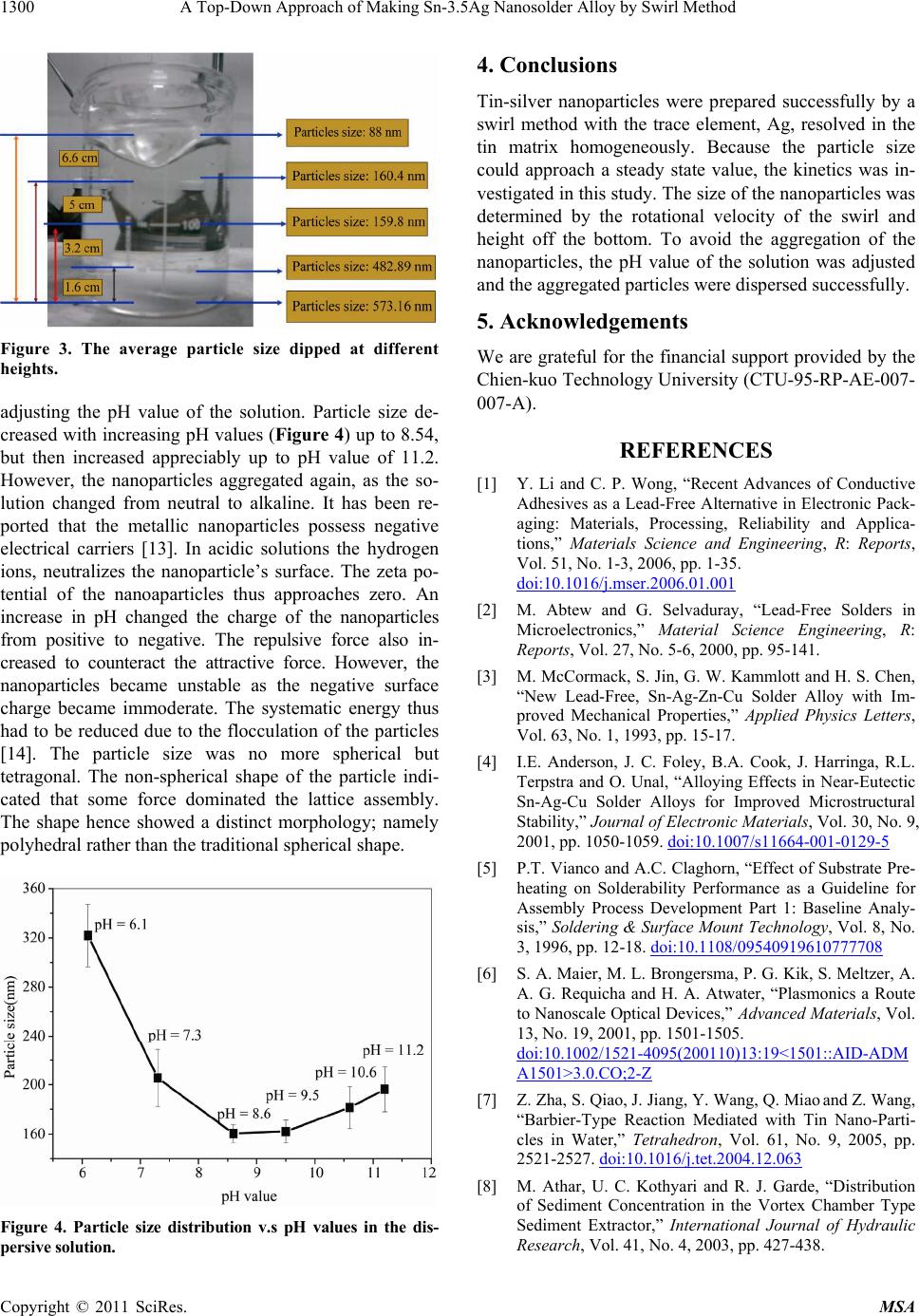
Figure 3. The average particle size dipped at different
heights.
adjusting the pH value of the solution. Particle size de-
creased with increasing pH values (Figure 4) up to 8.54,
but then increased appreciably up to pH value of 11.2.
However, the nanoparticles aggregated again, as the so-
lution changed from neutral to alkaline. It has been re-
ported that the metallic nanoparticles possess negative
electrical carriers [13]. In acidic solutions the hydrogen
ions, neutralizes the nanoparticle’s surface. The zeta po-
tential of the nanoaparticles thus approaches zero. An
increase in pH changed the charge of the nanoparticles
from positive to negative. The repulsive force also in-
creased to counteract the attractive force. However, the
nanoparticles became unstable as the negative surface
charge became immoderate. The systematic energy thus
had to be reduced due to the flocculation of the particles
[14]. The particle size was no more spherical but
tetragonal. The non-spherical shape of the particle indi-
cated that some force dominated the lattice assembly.
The shape hence showed a distinct morphology; namely
polyhedral rather than the traditiona l spherical shape.
Figure 4. Particle size distribution v.s pH values in the dis-
persive solution.
4. Conclusions
Tin-silver nanoparticles were prepared successfully by a
swirl method with the trace element, Ag, resolved in the
tin matrix homogeneously. Because the particle size
could approach a steady state value, the kinetics was in-
vestigated in this study. Th e size of the nanoparticles was
determined by the rotational velocity of the swirl and
height off the bottom. To avoid the aggregation of the
nanoparticles, the pH value of the solution was adjusted
and the aggregated particles were dispersed successfully.
5. Acknowledgements
We are grateful for the financial support provided by the
Chien-kuo Techno logy University (C TU-95-RP-AE-007-
007-A).
REFERENCES
[1] Y. Li and C. P. Wong, “Recent Advances of Conductive
Adhesives as a Lead-Free Alternative in Electronic Pack-
aging: Materials, Processing, Reliability and Applica-
tions,” Materials Science and Engineering, R: Reports,
Vol. 51, No. 1-3, 2006, pp. 1-35.
doi:10.1016/j.mser.2006.01.001
[2] M. Abtew and G. Selvaduray, “Lead-Free Solders in
Microelectronics,” Material Science Engineering, R:
Reports, Vol. 27, No. 5-6, 2000, pp. 95-141.
[3] M. McCormack, S. Jin, G. W. Kammlott and H. S. Chen,
“New Lead-Free, Sn-Ag-Zn-Cu Solder Alloy with Im-
proved Mechanical Properties,” Applied Physics Letters,
Vol. 63, No. 1, 1993, pp. 15-17.
[4] I.E. Anderson, J. C. Foley, B.A. Cook, J. Harringa, R.L.
Terpstra and O. Unal, “Alloying Effects in Near-Eutectic
Sn-Ag-Cu Solder Alloys for Improved Microstructural
Stability,” Journal of Electronic Materials, Vol. 30, No. 9,
2001, pp. 1050-1059. doi:10.1007/s11664-001-0129-5
[5] P.T. Vianco and A.C. Claghorn, “Effect of Substrate Pre-
heating on Solderability Performance as a Guideline for
Assembly Process Development Part 1: Baseline Analy-
sis,” Soldering & Surface Mount Technology, Vol. 8, No.
3, 1996, pp. 12-18. doi:10.1108/09540919610777708
[6] S. A. Maier, M. L. Brongersma, P. G. Kik, S. Meltzer, A.
A. G. Requicha and H. A. Atwater, “Plasmonics a Route
to Nanoscale Optical Devices,” Advanced Materials, Vol.
13, No. 19, 2001, pp. 1501-1505.
doi:10.1002/1521-4095(200110)13:19<1501::AID-ADM
A1501>3.0.CO;2-Z
[7] Z. Zha, S. Qiao, J. Jiang, Y. Wang, Q. Miao and Z. Wang,
“Barbier-Type Reaction Mediated with Tin Nano-Parti-
cles in Water,” Tetrahedron, Vol. 61, No. 9, 2005, pp.
2521-2527. doi:10.1016/j.tet.2004.12.063
[8] M. Athar, U. C. Kothyari and R. J. Garde, “Distribution
of Sediment Concentration in the Vortex Chamber Type
Sediment Extractor,” International Journal of Hydraulic
Research, Vol. 41, No. 4, 2003, pp. 427-438.
Copyright © 2011 SciRes. MSA