 Materials Sciences and Applicatio ns, 2011, 2, 1285-1292 doi:10.4236/msa.2011.29173 Published Online September 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1285 Effect of Nano Size TiO2 Particles on Mechanical Properties of AWS E 11018M Type Electrode Tapan Kumar Pal, Utpal Kumar Maity Welding Technology Centre, Metallurgical and Material Engineering Department, Jadavpur University, Kolkata, India. Email: tkpal.ju@gmail.com Received February 21st, 2011; revised May 16th, 2011; accepted July 1st, 2011. ABSTRACT Addition of nano size particles of TiO2 in the coating of shielded metal arc welding electrode (E 11018M) pa rtia lly sub- stituting the con ventional micro size TiO2 was studied for possible enhanced electrode characteristics. The results show that the nano size particle of TiO2 improved recovery of elements such as Mn, Ni, Mo, Ti etc. as well as increased all-weld-metal tensile and ch arp y impa ct properties a t –51˚C. Furthermore, the charpy impact properties were found to be very sensitive to variations in Ti content of the weld deposit. Keywords: High Strength Steel, Coated Electrode, Nano Size Tio2, Strength and Toughne ss 1. Introduction High strength steels are increasingly employed in many applications due to the advantage they offer such as size and weight reduction along with greater load bearing capacities [1]. Increased use of high strength structural steels poses a need for adequate welding consumable for such materials. This leads to a significant advance in electrode formulation to obtain weld deposits with high values for strength and good toughness [2]. Structural safety in welded joints are obtained by imposing re- quirement on toughness by setting charpy V-notch levels at the lowest design temperature [3]. The achievement of adequate toughness value increasingly difficult as the weld metal tensile strength increases. It has been well established that maintaining good toughness become more problematic as strength increases above in the re- gion of 690 Mpa [1,4]. One way to obtain improved weld metal toughness is through micro structural control, which requires taking into account the weld metal chem- istry. Steel manufacturers have addressed the challenges of increasing toughness in steel through grain refinement, precipitating hardening, solid solution hardening, thermo mechanical treatment and the promotion of low tem- perature transformation products such as martensite and bainite. Since weld metals are not usually given any thermo mechanical or special heat treatment, many re- search works have carried out by varying elemental compositions or welding parameters with the aim of op- timizing weld metal properties [5-7]. For the weld metal of 780 MPa or lower strength steel, the effect of micro- structure and oxygen content has been clearly demon- strated. Acicular ferrite is an ideal microstructure for these weld metals, and the increase in the amount of acicular ferrite leads to improvement both in strength and toughness [8,9]. The prior austenite grain size, inclusion and specific alloying addition that hinder grain boundary ferrite nucleation all are important consideration in achieving optimum weld metal acicular ferrite and prop- erties [10]. Weld consumables generally contain a small amount of Ti to achieve stabilized transfer of molten droplets during arc welding and it had been well known that Ti bearing weld metal is preferably refined. Watanabe et al. [11] experimentally changed oxygen contents in Ti bear- ing gas metal arc weld metals and found that Ti oxides effectively nucleate fine intragranular acicular ferrite. Mori et al. [12] also proposed that oxide particles having surface coating of TiO or those particles which are wholly TiO would be the most potent site for nucleation of acicular ferrite since disregestry between TiO and fer- rite is only three percent. In the present investigation, a novel approach has been attempted to develop AWS E11018M electrode. The study consisted of an addition of nano size particles of TiO2 to the coating of shielded metal arc welding elec- trode (E11018M) partially substituting the original (mi- cro size) TiO2 and the effect of such addition on micro- 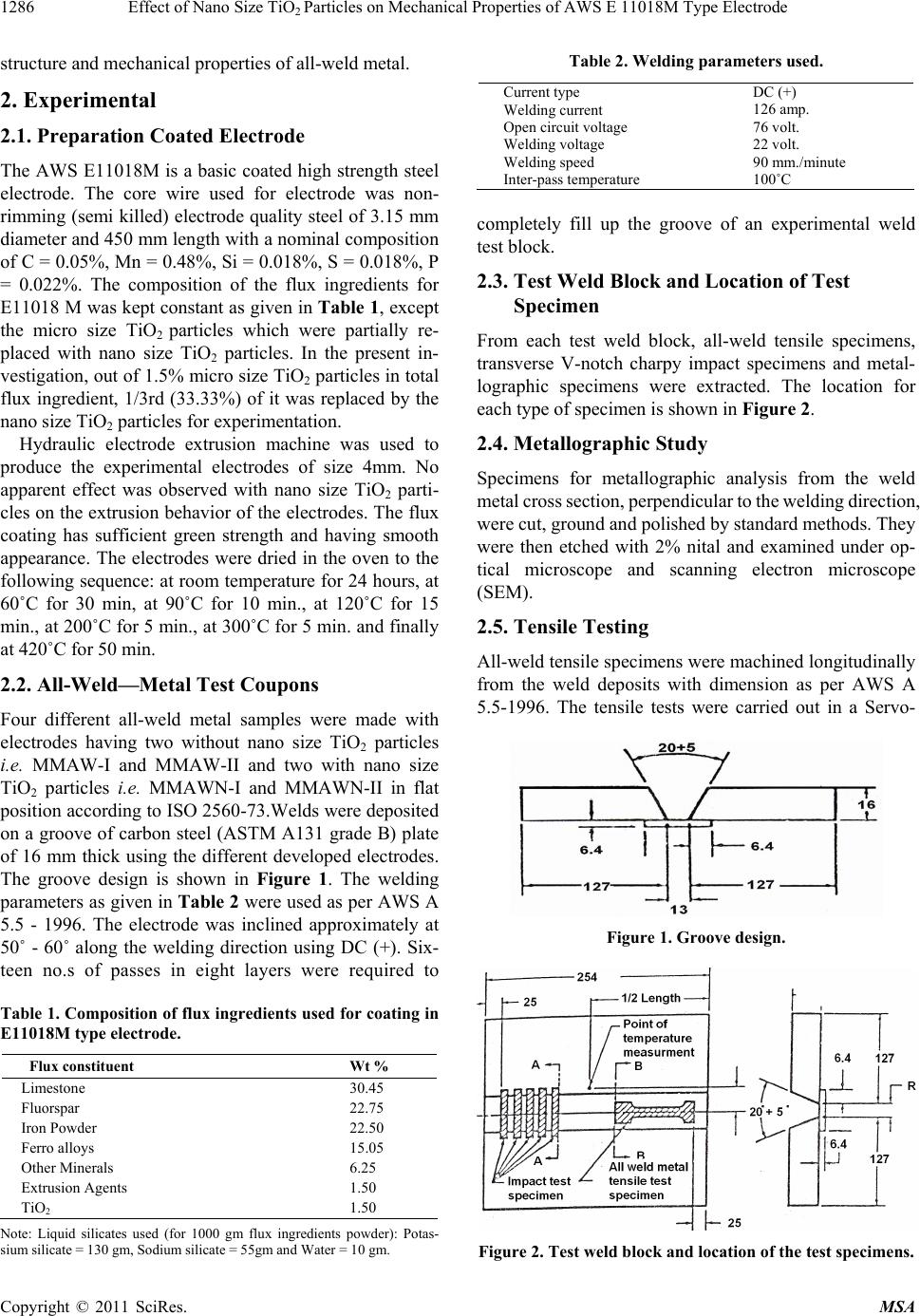 Effect of Nano Size TiOParticles on Mechanical Properties of AWS E 11018M Type Electrode 1286 2 structure and mechanical properties of all-weld metal. 2. Experimental 2.1. Preparation Coated Electrode The AWS E11018M is a basic coated high strength steel electrode. The core wire used for electrode was non- rimming (semi killed) electrode quality steel of 3.15 mm diameter and 450 mm length with a nominal composition of C = 0.05%, Mn = 0.48%, Si = 0.018%, S = 0.018%, P = 0.022%. The composition of the flux ingredients for E11018 M was kept constant as given in Table 1, except the micro size TiO2 particles which were partially re- placed with nano size TiO2 particles. In the present in- vestigation, out of 1.5% micro size TiO2 particles in total flux ingredient, 1/3rd (33.33%) of it was replaced by the nano size TiO2 particles for experimentation. Hydraulic electrode extrusion machine was used to produce the experimental electrodes of size 4mm. No apparent effect was observed with nano size TiO2 parti- cles on the extrusion behavior of the electrodes. The flux coating has sufficient green strength and having smooth appearance. The electrodes were dried in the oven to the following sequence: at room temperature for 24 hours, at 60˚C for 30 min, at 90˚C for 10 min., at 120˚C for 15 min., at 200˚C for 5 min., at 300˚C for 5 min. and finally at 420˚C for 50 min. 2.2. All-Weld—Metal Test Coupons Four different all-weld metal samples were made with electrodes having two without nano size TiO2 particles i.e. MMAW-I and MMAW-II and two with nano size TiO2 particles i.e. MMAWN-I and MMAWN-II in flat position according to ISO 2560-73.Welds were deposited on a groove of carbon steel (ASTM A131 grade B) plate of 16 mm thick using the different developed electrodes. The groove design is shown in Figure 1. The welding parameters as given in Table 2 were used as per AWS A 5.5 - 1996. The electrode was inclined approximately at 50˚ - 60˚ along the welding direction using DC (+). Six- teen no.s of passes in eight layers were required to Table 1. Composition of flux ingredients used for coating in E11018M type electrode. Note: Liquid silicates used (for 1000 gm flux ingredients powder): Potas- sium silicate = 130 gm, Sodium silicate = 55gm and Water = 10 gm. Table 2. Welding parameters used. Current type Welding current Open circuit voltage Welding voltage Welding speed Inter-pass temperature DC (+) 126 amp. 76 volt. 22 volt. 90 mm./minute 100˚C completely fill up the groove of an experimental weld test block. 2.3. Test Weld Block and Location of Test Specimen From each test weld block, all-weld tensile specimens, transverse V-notch charpy impact specimens and metal- lographic specimens were extracted. The location for each type of specimen is shown in Figure 2. 2.4. Metallographic Study Specimens for metallographic analysis from the weld metal cross section, perpendicular to the welding direction, were cut, ground and polished by standard methods. They were then etched with 2% nital and examined under op- tical microscope and scanning electron microscope (SEM). 2.5. Tensile Testing All-weld tensile specimens were machined longitudinally from the weld deposits with dimension as per AWS A 5.5-1996. The tensile tests were carried out in a Servo- Figure 1. Groove design. Figure 2. Test weld block and location of the test specimens. Flux constituent Wt % Limestone 30.45 Fluorspar 22.75 Iron Powder 22.50 Ferro alloys 15.05 Other Minerals 6.25 Extrusion Agents 1.50 TiO2 1.50 Copyright © 2011 SciRes. MSA  Effect of Nano Size TiO2 Particles on Mechanical Properties of AWS E 11018M Type Electrode Copyright © 2011 SciRes. MSA 1287 variations in the behaviour of the flux system, which in turn leads to large variations in the metallurgical proc- esses occurring in the weld pool. Electric (Instron 8862) type 100 kN capacity universal testing machine at a cross head speed of 0.5 mm/min. Tensile test data such as YS, UTS and % elongation was recorded for each all-weld sample. For each electrode three samples were tested and average of three samples was reported. Although chemical equilibrium is not achieved in a weld pool, a trend toward equilibrium is generally ob- served that can be estimated using fundamental thermo- dynamic principles. Oxidation reactions in the weld pool can be described by the following generic reaction: 2.6. Vickers Hardness Testing Hardness test was performed on metallography samples using Vicker’s hardness testing m/c with a square based diamond pyramid indenter having an included angle of 136˚ under a load of 30 kg. xy XMyOM O (1) According to the law of Mass Action, the equilibrium constant, k, for Equation (1) can be written as follows: x MOy y x MO a k aa (2) 2.7. Charpy Impact Testing and Fracture Surface Study For charpy impact testing, standard 55 × 10 × 10 × mm size transverse specimens were machined and notch was kept in weld metal perpendicular to the weld direction. Charpy impact specimens were tested at –51˚C which was achieved by adding acetone in liquid nitrogen. The temperature was measured with electronic thermometer. For each electrode five samples were tested and average of five samples was reported. The broken charpy impact specimens were examined under Scanning Electron Mi- croscope to understand fracture micro-mechanism. where, M a and O a are the activities of the weld metal alloying element, M and oxygen, respectively, and x MOy a is the activity of the metal-oxide inclusion in the weld metal. For equilibrium considerations, the activity of the metal-oxide can be taken as unity, which leads to the following relation: y x MO 1 k aa (3) 3. Results and Discussion With knowledge of the oxidation reactions that will occur in the solidified weld pool, it is possible to predict the direction toward which these reactions will go as changes are made in the welding flux. 3.1. Chemical Composition of Weld Deposits The chemical composition of different weld deposits is given in Table 3. The final weld metal chemical compo- sition is determined by the slag-metal reactions that oc- cur in the weld pool [13-15]. Research into slag-metal reactions has generally been done within the limited scope of a single flux system. However, the complexity of the welding environment as well as the nonideal be- haviour of the reactions that occur in the arc, lead to too many variables, which make accurate prediction of the effect of specific changes in system parameters difficult. Even small changes in the flux coating can result in large Competing chemical reactions within the weld pool will also affect the final weld metal chemical composi- tions. As local concentrations of alloy elements vary with solidification [16,17], the local activity of the alloying elements will change, altering the amount of alloying additions that will be oxidized. As a result, these com- petitive reactions will alter the predicted weld metal chemical composition. Results of all-weld metal chemical composition as presented in Table 3 demonstrate that the recovery of the Table 3. All-weld metal chemical composition (wt %). Weld Element MMAW-I MMAWN-I MMAW-II MMAWN-II E11018M (AWS A5.5 - 1996) C 0.053 0.046 0.058 0.051 0.10 max Mn 1.49 1.76 1.62 1.73 1.3 - 1.8 Si 0.58 0.46 0.60 0.40 0.60 max S 0.02 0.02 0.019 0.018 0.03 max P 0.025 0.028 0.024 0.028 0.03 max Cr 0.158 0.152 0.160 0.156 0.40 max Ni 2.31 2.45 2.23 2.47 1.25 - 2.50 Mo 0.412 0.451 0.391 0.432 0.25 - 0.50 Cu 0.021 0.027 0.025 0.023 - Al 0.003 0.005 0.005 0.005 - V 0.012 0.019 0.019 0.020 0.05 max Ti 0.0023 0.0040 0.0088 0.0200 -  Effect of Nano Size TiOParticles on Mechanical Properties of AWS E 11018M Type Electrode 1288 2 elements such as Mn, Ni, Mo, Ti etc. have improved in weld metal with the addition of nano size TiO2 particles (sample MMAWN-I and MMAWN-II). When alloying elements are transferred from the coating (slag) to weld metal, the equilibrium is [18] do slox MM MM (4) where Md is the amount of alloy transferred to deposit, Mo is the initial amount of the alloy in the electrode (Core and flux coating), Ms is the remaining amount of the alloy in the slag and Mox is the amount of the oxidised alloy. The unique physical properties of nano particles, resulting from quantum size and surface effects presumably en- hance the recovery of the alloying elements by facilitating the slag-metal reactions. Arguments based on high reac- tivity of nano particles suggest that nano size TiO2 would dissociate resulting in more instant generation of O2 which is likely to provide more stirring effect of the weld pool. This in turn will improve slag-metal reaction. A similar behaviour was reported for nano scale marble added in a hardfacing electrode [19]. However, increasing oxygen concentration with dissociation of TiO2 should increase the loss of alloying elements by oxidation. The chemical composition of weld deposit as presented in Table 3 rather shows opposite trend except Cr which shows some loss. This observation suggests that the two potentially competing effects would determine the final amount of alloy components transferred to the weld pool. However, considering affinity of Ti towards oxygen compared to Cr, it appears that gain of Ti can be attributed due to 1) neg- ligible difference between the partial pressure of oxygen in the arc atmosphere and dissociation pressure of TiO2 at high temperature and 2) reduction of TiO2 at the boundary of metal/slag in the reaction zone of welding as per fol- lowing equation: 2 TiO2(Si)2(SiO) (Ti) (5) This is not unexpected considering some loss of Si in the weld deposit with addition nano size TiO2. 3.2. Microstructure and Mechanical Properties of Weld Deposit Figure 3 shows the optical and Scanning Electron mi- crographs of weld deposits with and without nano size TiO2. The weld metal microstructure is affected by melt- ing, gas dissolution, solidification and solid-state trans- formations. Since the weld pool region is heated to tem- perature as high as 2500 k, the liquid steel dissolves oxy- gen. The extent of oxygen dissolution depends upon the thermodynamic properties of liquid metal, gas and slag phases [20]. As the liquid weld metal cools from this temperature in the temperature range 2000˚C - 1700˚C, (a) (b) (c) (d) Figure 3. Typical microstructures of all-weld metals: (a) optical micrograph (× 500) & (b) SEM micrographs without nano ize TiO2, (c) optical micrograph (× 500) and (d) SEM micrographs with nano size TiO2. s Copyright © 2011 SciRes. MSA 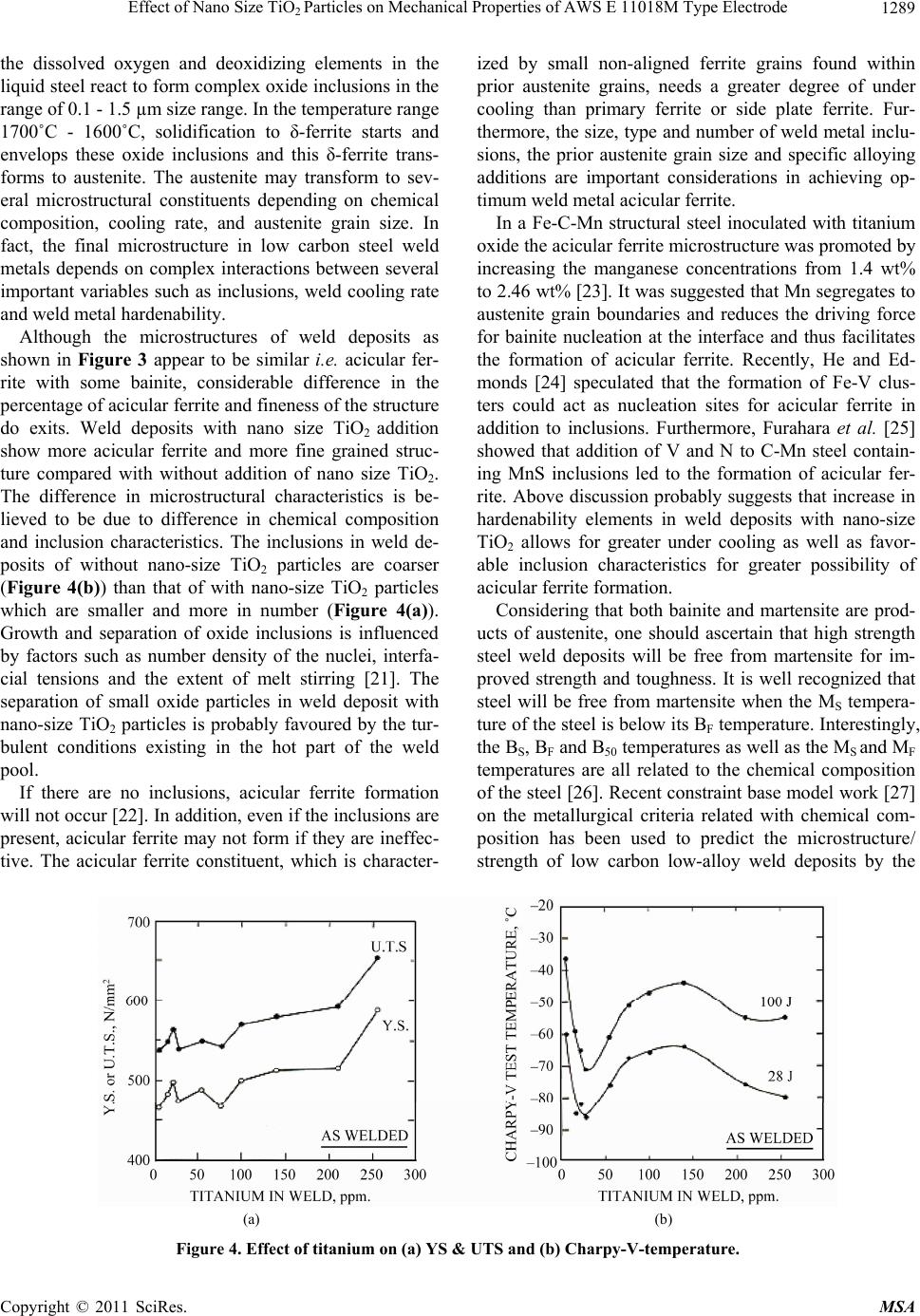 Effect of Nano Size TiOParticles on Mechanical Properties of AWS E 11018M Type Electrode 1289 2 the dissolved oxygen and deoxidizing elements in the liquid steel react to form complex oxide inclusions in the range of 0.1 - 1.5 µm size range. In the temperature range 1700˚C - 1600˚C, solidification to δ-ferrite starts and envelops these oxide inclusions and this δ-ferrite trans- forms to austenite. The austenite may transform to sev- eral microstructural constituents depending on chemical composition, cooling rate, and austenite grain size. In fact, the final microstructure in low carbon steel weld metals depends on complex interactions between several important variables such as inclusions, weld cooling rate and weld metal hardenability. Although the microstructures of weld deposits as shown in Figure 3 appear to be similar i.e. acicular fer- rite with some bainite, considerable difference in the percentage of acicular ferrite and fineness of the structure do exits. Weld deposits with nano size TiO2 addition show more acicular ferrite and more fine grained struc- ture compared with without addition of nano size TiO2. The difference in microstructural characteristics is be- lieved to be due to difference in chemical composition and inclusion characteristics. The inclusions in weld de- posits of without nano-size TiO2 particles are coarser (Figure 4(b)) than that of with nano-size TiO2 particles which are smaller and more in number (Figure 4(a)). Growth and separation of oxide inclusions is influenced by factors such as number density of the nuclei, interfa- cial tensions and the extent of melt stirring [21]. The separation of small oxide particles in weld deposit with nano-size TiO2 particles is probably favoured by the tur- bulent conditions existing in the hot part of the weld pool. If there are no inclusions, acicular ferrite formation will not occur [22]. In addition, even if the inclusions are present, acicular ferrite may not form if they are ineffec- tive. The acicular ferrite constituent, which is character- ized by small non-aligned ferrite grains found within prior austenite grains, needs a greater degree of under cooling than primary ferrite or side plate ferrite. Fur- thermore, the size, type and number of weld metal inclu- sions, the prior austenite grain size and specific alloying additions are important considerations in achieving op- timum weld metal acicular ferrite. In a Fe-C-Mn structural steel inoculated with titanium oxide the acicular ferrite microstructure was promoted by increasing the manganese concentrations from 1.4 wt% to 2.46 wt% [23]. It was suggested that Mn segregates to austenite grain boundaries and reduces the driving force for bainite nucleation at the interface and thus facilitates the formation of acicular ferrite. Recently, He and Ed- monds [24] speculated that the formation of Fe-V clus- ters could act as nucleation sites for acicular ferrite in addition to inclusions. Furthermore, Furahara et al. [25] showed that addition of V and N to C-Mn steel contain- ing MnS inclusions led to the formation of acicular fer- rite. Above discussion probably suggests that increase in hardenability elements in weld deposits with nano-size TiO2 allows for greater under cooling as well as favor- able inclusion characteristics for greater possibility of acicular ferrite formation. Considering that both bainite and martensite are prod- ucts of austenite, one should ascertain that high strength steel weld deposits will be free from martensite for im- proved strength and toughness. It is well recognized that steel will be free from martensite when the MS tempera- ture of the steel is below its BF temperature. Interestingly, the BS, BF and B50 temperatures as well as the MS and MF temperatures are all related to the chemical composition of the steel [26]. Recent constraint base model work [27] on the metallurgical criteria related with chemical com- position has been used to predict the microstructure/ strength of low carbon low-alloy weld deposits by the (a) (b) Figure 4. Effect of titanium on (a) YS & UTS and (b) Charpy-V-temperature. Copyright © 2011 SciRes. MSA  Effect of Nano Size TiOParticles on Mechanical Properties of AWS E 11018M Type Electrode 1290 2 following equations: 50 Cn r B(o)770(270C)(90M ) 37(Ni)(70C )83(Mo) (6) C Ms(o )561(474C)(33Mn) (17Ni)(21 Mo) (7) The calculated metallurgical characteristics of differ- ent weld deposits are presented in Table 4. The calculated data of B50 temperature as given in Ta- ble 4 indicates the presence of bainite in all the weld de- posits as the higher strength bainitic steels exhibits a B50 temperature in the range of 420˚C to 550˚C. Furthermore, weld deposits having lower B50 temperature attributes higher strength [28,29], Since bainite is a transformation product of austenite, lowering the transformation tem- peratures allows one to refine the grain size of the trans- formation product, leading to simultaneous increase in both tensile strength and ductility. The average measured tensile and impact properties are summarized in Table 5 for four weld deposits with and without nano size TiO2. The addition of nano size TiO2 in the coating of E11018M electrode significantly elevates both YS and UTS as well as low temperature toughness. The fracture surfaces of with nano size TiO2 shows quasi-cleavage mode of fracture(Figure 5(a)) compared to fracture surface of without nano size TiO2 which exhibits almost smooth typical cleavage frac- ture(Figure 5(b)). Beside microstructural control to achieve mechanical property goal, it is necessary to con- trol oxygen and nitrogen in the weld metal for low tem- perature toughness. Prior investigation using experimen- tal flux-cored wire electrodes has shown the beneficial effect of titanium addition in controlling weld metal ni- trogen content [30]. Titanium addition also served to refine the weld metal grains. Using C-Mn steel weld de- posits, Evans [31] determined that there are two optimum Table 4. Calculated metallurgical characteristics of differ- ent weld deposits. Weld sample B50 Temperature (˚C) MS Temperature (˚C) MMAW-I 491 439 MMAWN-I 460 430 MMAW-II 479 434 MMAWN-II 463 428 Table 5. Mechanical properties of different weld deposits. Weld deposits YS (MPa) UTS (MPa) % Elongation Cha rp y Impac t toughness at –51˚C (J) MNAW-I MMAWN-I MMAW-II MMAWN-II 690 710 701 734 765 791 770 823 23 24 22 25 28 33 29 35 titanium concentration regarding impact toughness e.g. 30 and 200 ppm as shown schematically in Figure 5(b). Also both YS and UTS increased with increase in Ti content in the weld metal (Figure 5(a)). Evans [32] also reported a complex interaction between Mn and Ti. The optimum impact toughness occurred at 35 ppm Ti and 1.4 pct. Mn. The effect of Ti was more pronounced at higher level of Mn. Titanium therefore acts as deoxidizer, grain refiner and nitrogen getter and at the same time it is very sensitive to the performance of weld deposits. The beneficial effect of Ti on mechanical properties of weld deposits, toughness in particular, at some critical concen- tration is supported in the present study. Thus, to enhance the performance of weld deposits particularly the tough- ness, it is very important to control the amount of Ti in weld deposits. 4. Conclusions The following conclusions may be drawn from the study: 1) The introduction of nano size TiO2 in the coating of coated electrode (E11018M) improved recovery of ele- ments such as Mn, Ni, Mo, Ti etc. (a) (b) Figure 5. SEM fractographs (a) weld metal with nano size TiO2 and (b) weld metal without nano size TiO2. Copyright © 2011 SciRes. MSA  Effect of Nano Size TiOParticles on Mechanical Properties of AWS E 11018M Type Electrode 1291 2 (a) (b) Figure 6. Optical micrographs showing inclusions: (a) weld metal with nano size TiO2 and (b) weld metal without nano size TiO2. 2) A significant influence on microstructure and me- chanical properties of weld deposits has been observed with addition of nano size TiO2. Both strength and toughness at –51˚C of weld deposits with nano size TiO2 increased compared to without nano size TiO2. 3) In order to enhance the performance of weld depos- its particularly the toughness, it is very important to con- trol the amount of Ti in weld deposits. REFERENCES [1] L. E. Svensson, “Consumable for Welding High Strength Steels,” Svetsaren, Vol. 54, No. 1-2, 1999, pp. 29-33. [2] M. George, J. Still and P. Terry, “Gas Metal Arc Welds for High Toughness Applications-Microstructure and Other Factors,” Metal Construction, Vol. 13, No. 12, 1981, pp. 730-737. [3] T. Keeler, “Innershield Welding Part 1-Development and Applications,” Metal Construction, Vol. 13, No. 11, 1981, pp. 667-673. [4] D. J. Widgery, L. Karlsson, M. Murugananth and E. Keehan, “Approaches to the Development of High- Strength Weld Metals,” Proceedings of 2nd International Symposium on High Strength Steel, Stikklestad, Verdal, Norway: European Coal and Steel Community (ECSC), B-1049 Brussels, Belgium, 2002. [5] W. C. Leslie, “The Physical Metallurgy of Steels,” McGraw-Hill, London, 1981. [6] L. E. Svensson, “Control of Microstructure and Properties in Steel Arc Welds,” CRC Press, Inc., 1994. [7] G. M. Evans and N. Bailey, “Metallurgy of Basic Weld Metal,” Abington Publishing, 1997. doi:10.1533/9781845698850 [8] M. Nakanishi, Journal of the Japan Welding Society, Vol. 50, 1981, p. 5. [9] J. A. Gianetto, et al., Welding Journal, Vol. 71, 1992, p. 407-S. [10] S. Liu and D. L. Olson, “The Role of Non-metallic Inclu- sions in Controlling HSLA Weld Microstructures,” Weld- ing Journal, Vol. 65, 1986, p.134-s-149-s. [11] I. Watanabe and T. Kojima, Journal of the Japan Welding Society, Vol. 49, 1980, p. 772 N. Mori: IIW Doc. IX – 1158-1180. [12] N. Mori, H. Homma, S. Ohkita and M. Wakabayashi, “Mechanisms of Notch Toughness Improvement in Ti-B Bearing Weld Metal,” IIW Doc. IX-1196-81. [13] N. Christensen and J. Chipman, “Slag-Metal Interaction in Arc Welding,” Welding Research Council, Bulletin, Vol. 15, January 1953, pp. 1-14. [14] U. Mitra and T. W. Eagar, “Slag-Metal Reaction during Submerged Arc Welding of Alloy Steels,” Metallurgical and Materials Transactions A, Vol. 15A, 1984, pp. 217- 227. doi:10.1007/BF02644404 [15] G. R. Bolton, T. J. Moore and E. S. Tankins, “Slag-Metal Reactions in Submerged Arc Welding,” Welding Journal, Vol. 42, No. 7, 1963, pp. 289-s-297-s. [16] M. C. Flemings, “Solidification Processing,” McGraw- Hill, New York, 1974. [17] R. H. Forst, D. L. Olson and S. Liu, “Influence of Solidi- fication on Inclusion Formation in Welds,” Proceedings of the 3rd International Conference on Trends in Welding Research, ASM International, Gatlinburg, Tennessee, 1992. [18] W. Y. Zhang, “Welding Metallurgy,” Mechanical Engi- neering Press, Beijing, 2003. [19] B. Chen, F. Han, Y. Huang, K. Lu, Y. Liu and L. Li, “In- fluence of Nanoscale Marble (Calcium Carbonate CaCO3) on Properties of D600 R Surfacing Electrode,” Welding Journal, Vol. 88, March 2009, pp. 99-s-103-s. [20] S. A. David and T. Deb Ray, “Current Issues and Prob- lems in Welding Science,” Science, Vol. 257, 1992, pp. 497-502. doi:10.1126/science.257.5069.497 [21] U. Lindborg and K. Torsell, Transactions on TMS-AIME, Vol. 242, 1968, pp. 94-102. [22] J.S. Byun, J.H. Shim and Y. W. Cho, “Influence of Mn on Microstructure Evolution in Ti-Killed C-Mn Steel,” Scripta Materialia, Vol. 48, No. 4, 2003, pp. 449-454. doi:10.1016/S1359-6462(02)00437-2 Copyright © 2011 SciRes. MSA  Effect of Nano Size TiO2 Particles on Mechanical Properties of AWS E 11018M Type Electrode Copyright © 2011 SciRes. MSA 1292 [23] S. K. Liu and J. Zhang, “The Influence of the Si and Mn Concentrations on the Kinetics of the Bainite Transfor- mation in Fe-C-Si-Mn Alloys,” Metallurgical and Mate- rials Transactions A, Vol. 21, No. 6, 1990, pp. 1517- 1525. [24] K. He and D. V. Edmonds, “Formation of Acicular Fer- rite and Influence of Vanadium Alloying,” Materials Science and Technology, Vol. 18, 2002, pp. 289-296. doi:10.1179/026708301225000743 [25] T. Furuhara, J. Yamaguchi, N. Sugita, N. Miyamoto and T. Maki, “Nucleation of Procutectoid Ferrite on Complex Precipitates in Austenite,” ISIJ International, Vol. 43, No. 10, 2003, pp. 1630-1639. doi:10.2355/isijinternational.43.1630 [26] W. Steven and A. G. Haynes, “The Temperature of For- mation of Martensite and Bainite in Low-Alloy Steels,” Journal of the Iron and Steel Institute, Vol. 183, No. 8, 1956, pp. 349-359 [27] K. Sampath, “Constraints-Based Modeling Enables Suc- cessful Development of a Welding Electrode Specifica- tion for Critical Navy Applications,” Welding Journal, August 2005, pp. 131-s-138-s. [28] K. J. Irvine and F. B. Pickering, “Low-Carbon Bainitic Steels,” Journal of the Iron and Steel Institute, Vol. 184, No. 12, 1957, pp. 292-309. [29] W. C. Leslie, “The Physical Metallurgy of Steels,” McGraw-Hill International Book Company, London, 1981, pp. 201-205. [30] R. J. Wrong and M. D. Hayes, “The Metallurgy, Welding and Qualification of Microalloyed (HSLA) Steel Weld- ments,” AWS, Miami, 1990, pp. 450-489. [31] G. M. Evans, “Factors Affecting the Microstructure and Properties of C-Mn All-Weld Metal Deposits,” Welding Research Abroad, Vol. 28, No. 1, 1983, pp. 1-69. [32] G. M. Evans, “The Effect of Titanium on the Microstruc- ture and Properties of C-Mn All-Weld Metal Deposits,” OERLIKON-Schweibmitt, Vol. 49, No. 125, 1991, pp. 22- 33.
|