Discontinuous Precipitation in Al-8% Mass.Mg Alloy under the Effect of Temperature1283
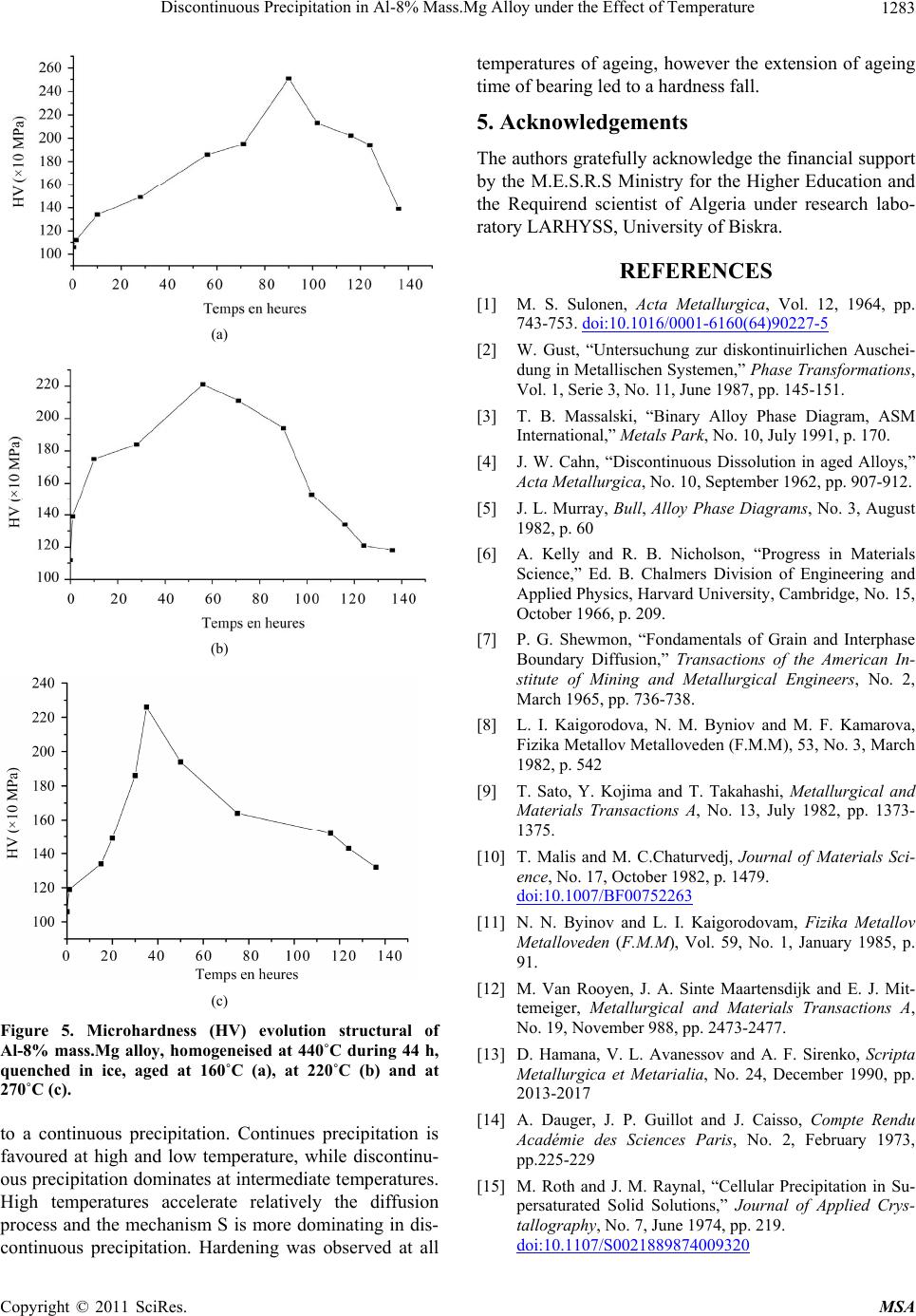
(a)
(b)
(c)
Figure 5. Microhardness (HV) evolution structural of
Al-8% mass.Mg alloy, homogeneised at 440˚C during 44 h,
quenched in ice, aged at 160˚C (a), at 220˚C (b) and at
270˚C (c).
to a continuous precipitation. Continues precipitation is
favoured at high and low temperature, while discontinu-
ous precipitation dominates at intermediate temperatures.
High temperatures accelerate relatively the diffusion
process and the mechanism S is more dominating in dis-
continuous precipitation. Hardening was observed at all
temperatures of ageing, however the extension of ageing
time of bearing led to a hardness fall.
5. Acknowledgements
The authors gratefully acknowledge the financial support
by the M.E.S.R.S Ministry for the Higher Education and
the Requirend scientist of Algeria under research labo-
ratory LARHYSS, University of Biskra.
REFERENCES
[1] M. S. Sulonen, Acta Metallurgica, Vol. 12, 1964, pp.
743-753. doi:10.1016/0001-6160(64)90227-5
[2] W. Gust, “Untersuchung zur diskontinuirlichen Auschei-
dung in Metallischen Systemen,” Phase Transformations,
Vol. 1, Serie 3, No. 11, June 1987, pp. 145-151.
[3] T. B. Massalski, “Binary Alloy Phase Diagram, ASM
International,” Metals Park, No. 10, July 1991, p. 170.
[4] J. W. Cahn, “Discontinuous Dissolution in aged Alloys,”
Acta Metallurgica, No. 10, September 1962, pp. 907-912.
[5] J. L. Murray, Bull, Alloy Phase Diagrams, No. 3, August
1982, p. 60
[6] A. Kelly and R. B. Nicholson, “Progress in Materials
Science,” Ed. B. Chalmers Division of Engineering and
Applied Physics, Harvard University, Cambridge, No. 15,
October 1966, p. 209.
[7] P. G. Shewmon, “Fondamentals of Grain and Interphase
Boundary Diffusion,” Transactions of the American In-
stitute of Mining and Metallurgical Engineers, No. 2,
March 1965, pp. 736-738.
[8] L. I. Kaigorodova, N. M. Byniov and M. F. Kamarova,
Fizika Metallov Metalloveden (F.M.M), 53, No. 3, March
1982, p. 542
[9] T. Sato, Y. Kojima and T. Takahashi, Metallurgical and
Materials Transactions A, No. 13, July 1982, pp. 1373-
1375.
[10] T. Malis and M. C.Chaturvedj, Journal of Materials Sci-
ence, No. 17, October 1982, p. 1479.
doi:10.1007/BF00752263
[11] N. N. Byinov and L. I. Kaigorodovam, Fizika Metallov
Metalloveden (F.M.M), Vol. 59, No. 1, January 1985, p.
91.
[12] M. Van Rooyen, J. A. Sinte Maartensdijk and E. J. Mit-
temeiger, Metallurgical and Materials Transactions A,
No. 19, November 988, pp. 2473-2477.
[13] D. Hamana, V. L. Avanessov and A. F. Sirenko, Scripta
Metallurgica et Metarialia, No. 24, December 1990, pp.
2013-2017
[14] A. Dauger, J. P. Guillot and J. Caisso, Compte Rendu
Académie des Sciences Paris, No. 2, February 1973,
pp.225-229
[15] M. Roth and J. M. Raynal, “Cellular Precipitation in Su-
persaturated Solid Solutions,” Journal of Applied Crys-
tallography, No. 7, June 1974, pp. 219.
doi:10.1107/S0021889874009320
Copyright © 2011 SciRes. MSA