 Materials Sciences and Applicatio ns, 2011, 2, 1260-1267 doi:10.4236/msa.2011.29170 Published Online September 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 70 - 30 in NaCl 3% Solution Tayeb Chieb1,2, Kamel Belmokre2, Mohammed Benmessaoud1,3, Sidi El Hassane Drissi3, Najat Hajjaji1, Abdellah Srhiri1 1Laboratory of Electrochemistry, Materials and Environment (LEME), Faculty of Science, Ibn Tofail University, Kenitra, Morocco; 2Laboratory Corrosion and Surface Treatment (LCST), University of Skikda, Faculty of Sciences, Skikda, Algeria; 3University of Mohammed V Agdal, Energy Systems Laboratory, Materials and Environment, High School of Technology, Salé Medina, Morocco. Email: chiebtayeb2003@yahoo.fr Received May 3rd, 2011; revised May 26th, 2011; accepted June 11th, 2011. ABSTRACT The effect of 3-methyl 4-amino 1,2,4 triazole (MATA) on the corrosion behaviour of copper-nickel 70 - 30 (Monel) in NaCl 3% solution is investigated at 298 K by weight loss measurements, potentiodynamic polarisation and impedance spectroscopy (EIS) methods. Preliminary screening of the inhibition efficiency (ECR) was carried out with using weight- loss measurements. Polarization measurements showed that the organic compound investigated is mixed type inhibitor (it acts on the cathod ic and the cathodic reac tions), inhibiting the corrosion of Monel by blocking the active sites of the metal surface. Changes in the impedan ce parameters charge transfer resistance (Rct) and double layer capacitance (Cdl) are related to adsorption of organic inhibitor of the metal surface, leading to the formation of protective film, which grows with increasing exposure time. Inhibition efficiencies obtained from cathodic Tafel plots, gravimetric and EIS methods are in good agreement. Resu lts obtained shows that the (MA T A) is good inhibitor for copper – nickel, and its efficiency reaches more than 95% at 60 ppm after 30 mn of immersion. Keywords: Copper-Nickel, Triazole, Inhibitor, Efficiency, Polarisation, Impedance 1. Introduction Copper and its alloys are widely used in industry because of their excellent electrical and thermal conductivity and their corrosion resistance; copper alloys are often used in heating and cooling system [1-3]. Copper-nickel 70 - 30 (Monel) has been widely used as tubing material con- densers and heat exchangers in various water cooling systems [4-9]. However, the presence of certain pollut- ants such as sulphurs and ammonia compromise their corrosion resistance especially in sea water. Sure enough, Macdonald et al. [10,11] studied the impact of sulphur on the corrosion behaviour of copper nickel in airless sea water, these authors showed that sulphurs increases cor- rosion rates. On the other hand Syrett et al. [12] noticed that the sulphurs are not dangerous in the absence of oxygen. Other authors [13,14] investigated the effect of ammonia, they have observed that the presence of am- monia favour the selective corrosion of copper nickel alloys by the formation of complexes compounds with copper. Sure enough, copper complexes which formed with ammonia molecules destabilize easily corrosion products layer which generally protect copper alloys [15]. During more then thirty years ago, many techniques have been used to minimise corrosion of copper-nickel. One of the techniques used for minimising corrosion is the use of inhibitors. The effectiveness of the inhibitors va- ries with its concentration, the corrosive medium and the surface properties of the alloy. Many inhibitors have been tested to minimise the corrosion of Monel in dif- ferent media [16]. Particularly, heterocyclic organic com- pounds containing nitrogen, sulphur and/or oxygen are often used to protect metals from corrosion. Among them, azoles have been intensively investigated as effective copper corrosion inhibitors [17-20]. Benzotriazole (BTA), for example, has been studied and found to have excel-  The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 1261 70 - 30 in NaCl 3% Solution lent inhibition properties in several corrosive environ- ments [21-25]. This molecule contains nitrogen atoms and has been found useful in preventing copper staining and tarnishing. The effectiveness of BTA has been re- lated to the formation of a [Cu+-BTA−]n film, the film formed is considered to be insoluble and polymeric [26]. Bag et al. [27] investigated the protective action of azo- les on the corrosion of 70 - 30 brass in ammonia solution and concluded that the inhibitors could control corrosion effectively. Shukla and Pitre [28] studied the electro- chemical behaviour of brass and the inhibitive effect of imidazole in acid solution. Walker [29] showed that the addition of small amounts of the 1,1,3 benzotriazole and 1,2,4 triazole inhibited the corrosion of brass in various acidic, neutral and alkaline solutions. Fenelon and Bre- zlin [30] studied the formation of BTA films on copper, Cu-Zn alloys and Zn in chloride solution. Aramaki et al. [31] proposed mechanisms of benzotriazole derivatives on copper in sulphate solution at several values of pH by an impedance technique and enhanced Raman scattering spectroscopy. Subramanian and Lakshimina- rayanan [32] studied the adsorption properties and the effect of some azoles such as benzotriazole, mercaptobenzotriazole, benzimidazole, imidazole and tetrazole on the growth of oxide film on copper in 0.1 M NaOH. All these com- pounds contain nitrogen and/or sulphur atoms which can co-ordinate with copper through the lone pair of elec- trons to form complexes. These complexes are generally believed to be polymeric in nature and form a protective film on the copper surface, which acts as a barrier of ox- ide film formation. The inhibitor action of these azoles may also be due to physisorption or chimisorption onto the copper surface. Trachli et al. [33] studied the protect- tive effect of electropolymerized aminotriazole towards corrosion of copper in 0.54 M NaCl solution. Nagiub and Mansfeld [34] investigated the corrosion behaviour of 26,000 brasses in artificial sea water using EIS and ENA techniques. BTA, gluconic acid sodium salt and poly- phosphoric acid sodium salt were evaluated as corrosion inhibitors. Qafsaoui et al. [35] studied the quantitative characterization of protective film developed on copper by anodic polarization in a borate buffered solution con- taining benzotriazole and aminotriazole. Al-kharafi and Ateya [36] investigated the effect of sulphides on the electrochemical impedance of copper in benzotriazole- inhibited media. Tommesani [37] studied protective ac- tion of 1,2,3-benzotriazole derivative films against cop- per corrosion. Although there is an extensive literature on the corrosion properties of triazole such as imidazole and benzotriazole on steel and copper, there remains little information on the effect of various functional groups in the BTA derivatives on the corrosion of copper nickel alloys. Based on this, the inhibitors have been considered and synthesized according to the previous report. In the present investigation, it is proposed to study the electro- chemical behaviour of copper nickel 70 - 30 in artificial seawater with 3-Methyl 4-Amino 1,2,4 triazole (MATA). Weight-loss method and electrochemical studies such as potentiodynamic polarization, impedance spectroscopy. Solution analysis was carried out to find out the concen- tration of Cu and Ni leached out from the copper nickel alloy using atomic absorption spectroscopy. 2. Experimental Conditions Monel strips having chemical compositions (wt%): 69.3% Cu, 29.6% Ni, 0.7% Mn, 0.4% Fe. The NaCl 3% solution was prepared by dissolving 30 g of pure NaCl in 1 l of dis- tilled water. The inhibitor: 3-Methyl 4-Amino 1,2,4 triazole (MATA) was synthesized according to the reported proce- dures [38] and its structure is shown in Figure 1. Weight-loss method measurements were performed with rectangular Monel coupons (5 cm × 3 cm× 0.3 cm). The coupons were immersed in 300 ml of NaCl 3% solu- tion with and without inhibitors and allowed to for 5 days at room temperature (25˚C). Afterwards, the coupons were rinsed with distilled water and adherent corrosion products were removed by immersing the coupons in 6% H2SO4 for 20 s. Then the coupons were rinsed with dis- tilled water, cleaned with acetone, dried and weighed. Duplicate tests were conducted with for each experiment. The corrosion rate CR and the percentage of inhibition efficiency ECR (%) over the exposure period were calcu- lated using the following equations [39]: 87.6 W CR DAT (1) % inh CR CR CR ECR 100 (2) Figure 1. 3-Methyl 4-Amino 1,2,4 triazole (MATA). Copyright © 2011 SciRes. MSA 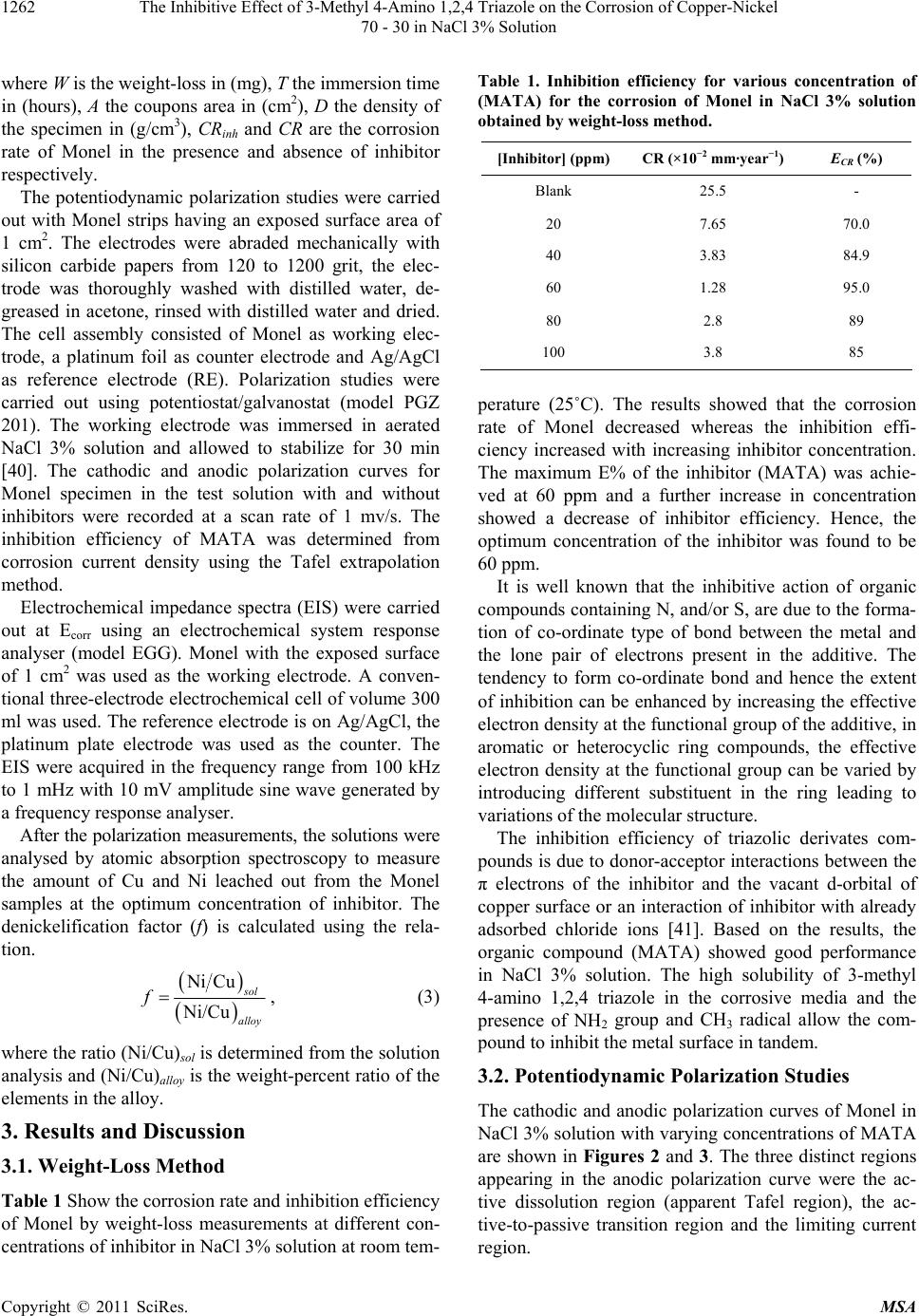 The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 1262 70 - 30 in NaCl 3% Solution where W is the weight-loss in (mg), T the immersion time in (hours), A the coupons area in (cm2), D the density of the specimen in (g/cm3), CRinh and CR are the corrosion rate of Monel in the presence and absence of inhibitor respectively. The potentiodynamic polarization studies were carried out with Monel strips having an exposed surface area of 1 cm2. The electrodes were abraded mechanically with silicon carbide papers from 120 to 1200 grit, the elec- trode was thoroughly washed with distilled water, de- greased in acetone, rinsed with distilled water and dried. The cell assembly consisted of Monel as working elec- trode, a platinum foil as counter electrode and Ag/AgCl as reference electrode (RE). Polarization studies were carried out using potentiostat/galvanostat (model PGZ 201). The working electrode was immersed in aerated NaCl 3% solution and allowed to stabilize for 30 min [40]. The cathodic and anodic polarization curves for Monel specimen in the test solution with and without inhibitors were recorded at a scan rate of 1 mv/s. The inhibition efficiency of MATA was determined from corrosion current density using the Tafel extrapolation method. Electrochemical impedance spectra (EIS) were carried out at Ecorr using an electrochemical system response analyser (model EGG). Monel with the exposed surface of 1 cm2 was used as the working electrode. A conven- tional three-electrode electrochemical cell of volume 300 ml was used. The reference electrode is on Ag/AgCl, the platinum plate electrode was used as the counter. The EIS were acquired in the frequency range from 100 kHz to 1 mHz with 10 mV amplitude sine wave generated by a frequency response analyser. After the polarization measurements, the solutions were analysed by atomic absorption spectroscopy to measure the amount of Cu and Ni leached out from the Monel samples at the optimum concentration of inhibitor. The denickelification factor (f) is calculated using the rela- tion. Ni Cu Ni/Cu ol alloy f, (3) where the ratio (Ni/Cu)sol is determined from the solution analysis and (Ni/Cu)alloy is the weight-percent ratio of the elements in the alloy. 3. Results and Discussion 3.1. Weight-Loss Method Table 1 Show the corrosion rate and inhibition efficiency of Monel by weight-loss measurements at different con- centrations of inhibitor in NaCl 3% solution at room tem- Table 1. Inhibition efficiency for various concentration of (MATA) for the corrosion of Monel in NaCl 3% solution obtained by weight-loss method. [Inhibitor] (ppm) CR (×102 mm·year1) ECR (%) Blank 25.5 - 20 7.65 70.0 40 3.83 84.9 60 1.28 95.0 80 2.8 89 100 3.8 85 perature (25˚C). The results showed that the corrosion rate of Monel decreased whereas the inhibition effi- ciency increased with increasing inhibitor concentration. The maximum E% of the inhibitor (MATA) was achie- ved at 60 ppm and a further increase in concentration showed a decrease of inhibitor efficiency. Hence, the optimum concentration of the inhibitor was found to be 60 ppm. It is well known that the inhibitive action of organic compounds containing N, and/or S, are due to the forma- tion of co-ordinate type of bond between the metal and the lone pair of electrons present in the additive. The tendency to form co-ordinate bond and hence the extent of inhibition can be enhanced by increasing the effective electron density at the functional group of the additive, in aromatic or heterocyclic ring compounds, the effective electron density at the functional group can be varied by introducing different substituent in the ring leading to variations of the molecular structure. The inhibition efficiency of triazolic derivates com- pounds is due to donor-acceptor interactions between the π electrons of the inhibitor and the vacant d-orbital of copper surface or an interaction of inhibitor with already adsorbed chloride ions [41]. Based on the results, the organic compound (MATA) showed good performance in NaCl 3% solution. The high solubility of 3-methyl 4-amino 1,2,4 triazole in the corrosive media and the presence of NH2 group and CH3 radical allow the com- pound to inhibit the metal surface in tandem. 3.2. Potentiodynamic Polarization Studies The cathodic and anodic polarization curves of Monel in NaCl 3% solution with varying concentrations of MATA are shown in Figures 2 and 3. The three distinct regions appearing in the anodic polarization curve were the ac- tive dissolution region (apparent Tafel region), the ac- tive-to-passive transition region and the limiting current region. Copyright © 2011 SciRes. MSA  The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 1263 70 - 30 in NaCl 3% Solution Figure 2. Cathodic polarization curves of Monel in aerated 3% NaCl without and with various concentrations of MATA at 25˚C: = 1000 rpm; |dE/dt| = 1 m·Vs1. Figure 3. Anodic polarization curves of Monel in aerated 3% NaCl without and with various concentrations of MATA at 25˚C: = 1000 rpm; |dE/dt| = 1 m·Vs1. It is evident that in the presence of inhibitor, the ca- thodic and anodic curves were shifted towards positive region and the shift was found to be dependent on in- hibitor concentration. The Table 2 illustrates the corre- sponding electrochemical parameters. The Ecorr were marginally shifted in the presence of MATA. This ob- servation clearly indicated that the inhibitor control the anodic and cathodic reaction and thus act as mixed type inhibitor. The current density also decreased with in- creasing concentration of inhibitor. The corrosion rates [42] and the inhibition efficiency [43] were calculated from polarization curves using the following equations: 3 3.27 10 corr Ew I CR D (4) Table 2. Polarisation parameters and corresponding inh ibition efficiency for the corrosion of cupper-nickel 70 - 30 in NaCl 3% without and with addition of various c oncentration of inhibitor. Solution Blank 20 ppm 40 ppm60 ppm bc (mV/dec) −288 −238 −218 −196 Ecorr (mV/Ag/AgCl) −222 −214 −195 −167 icorr (A·cm2) 24.5 6.92 3.28 0.8 CR (× 10-2 mm·year1)26.00 7.36 3.49 0.85 E (%) - 71.7 86.6 96.7 % corrcorr inh corr II EI100 (5) where CR is the corrosion rate (mm·year−1), D the density (g·cm−3), EW the equivalent weight of the specimen and corr inh and corr I are the corrosion current density (A·cm−2) values with and without inhibitor respectively. The values of cathodic Tafel slope (bc) it found to change with inhibitor concentrations, indicates that in- hibitor controlled both the reactions. The inhibition effi- ciency of MATA attained a maximum value of 96.7% at 60 ppm. The values of inhibition efficiency increase with increasing concentration of inhibitor, indicating that a higher surface coverage was obtained in a solution with the optimum concentration of inhibitor. The corrosion rate in blank solution was found to be 26 × 10−2 mm·year−1 and it was minimized by adding the inhibitor to a lower value of 0.85 × mm·year−1 for the Monel due to the presence of MATA on the metal surface. 3.3. Impedance Studies The impedance diagrams are represented in Nyquist plot, obtained in solution with and without the MATA inhibit- tor, for different concentrations, are presented in Figure 4. The electrode was prepolarized at the free corrosion potential until a steady-state was attained. The impedance spectra in the absence of MATA pre- sent one capacitive loop. In the context of a detailed study published elsewhere [44], this loop is attributed to a charge transfer process, the value of associated resis- tance is about 1.5 kOhm·cm2, the capacity is in the order of 168 F·cm−2. In the presence of inhibitor, we note that the impedance display of the electrode in MATA con- taining the solution changes in shape and size, on the other hand it can be noticed that the impedance modulus increased dramatically in presence of inhibitor from 20 ppm of MATA. The presence of two capacitive loops seems to indicate a diffusion contribution to the begin- ning of the experiment. Copyright © 2011 SciRes. MSA  The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 70 - 30 in NaCl 3% Solution Copyright © 2011 SciRes. MSA 1264 Figure 4. Nyquist plots of copper-nickel 70 - 30 in NaCl 3% with and without various concentration of inhibitor. Figure 5 shows the impedance diagrams obtained in corrosive solution at the corrosion potential after the Cu 30 Ni electrode was exposed to the solution for different immersion times. It maintains the same shape after 30 min of immersion time. As can be seen in this figure, the impedance diagrams in the Nyquist plot become larger with time. It can be seen that Rt and Cd increased with time. The increase of Rt may be explained by the formation of copper chloride which protects the copper against the corrosion. With accumulation of corrosion products, the surface roughness increased leading to a higher Cd value. Figure 6 shows the impedance diagrams obtained in corrosive solution at 60 ppm of MATA at the corrosion potential after the Monel electrode was exposed to the solution for different immersion times. It maintains the same shape after 30 min of immersion time. As can be seen in this figure, the impedance diagrams in the Ny- quist plot become larger with time. The increase of the polarisation resistance with the immersion period is often reported for the inhibiting action of heterocyclic on cop- per corrosion. At 30 min of immersion time, we have a loop in high frequencies (HF) and dispersion in low fre- quencies range. If we allot the HF loop to the charge transfer resistance Rt, then associated resistance is about 42 kOhm·cm2. The capacity is of the order 14.8 F·cm2. The effect of increasing immersion time on impedance spectra is characterised by the increasing size of the two capacitive loops observed, reaching a maximum in 20 h. The percentage inhibition efficiency (E%) is calcu- lated from the charge transfer resistance values using the following equation : % ctct inh ct RR ER100 (6) where Rct(inh) and Rct are the charge transfer resistance values with and without inhibitor respectively. The im- pedance parameters derived from these investigations are given in Tables 3 and 4. It is found that Rct values in- creased in the presence of inhibitor and with immersion time, whereas Cdl values found to be decreased. The de- crease in Cdl values was due to the adsorption of MATA on the metal surface. The inhibition efficiency reached 88.8 and 96.3% at 40 and 60 ppm respectively after 30 min of immersion, and it was not affected by the immer- sion time (95.2% after 20 h in the presence of inhibitor at 60 ppm). 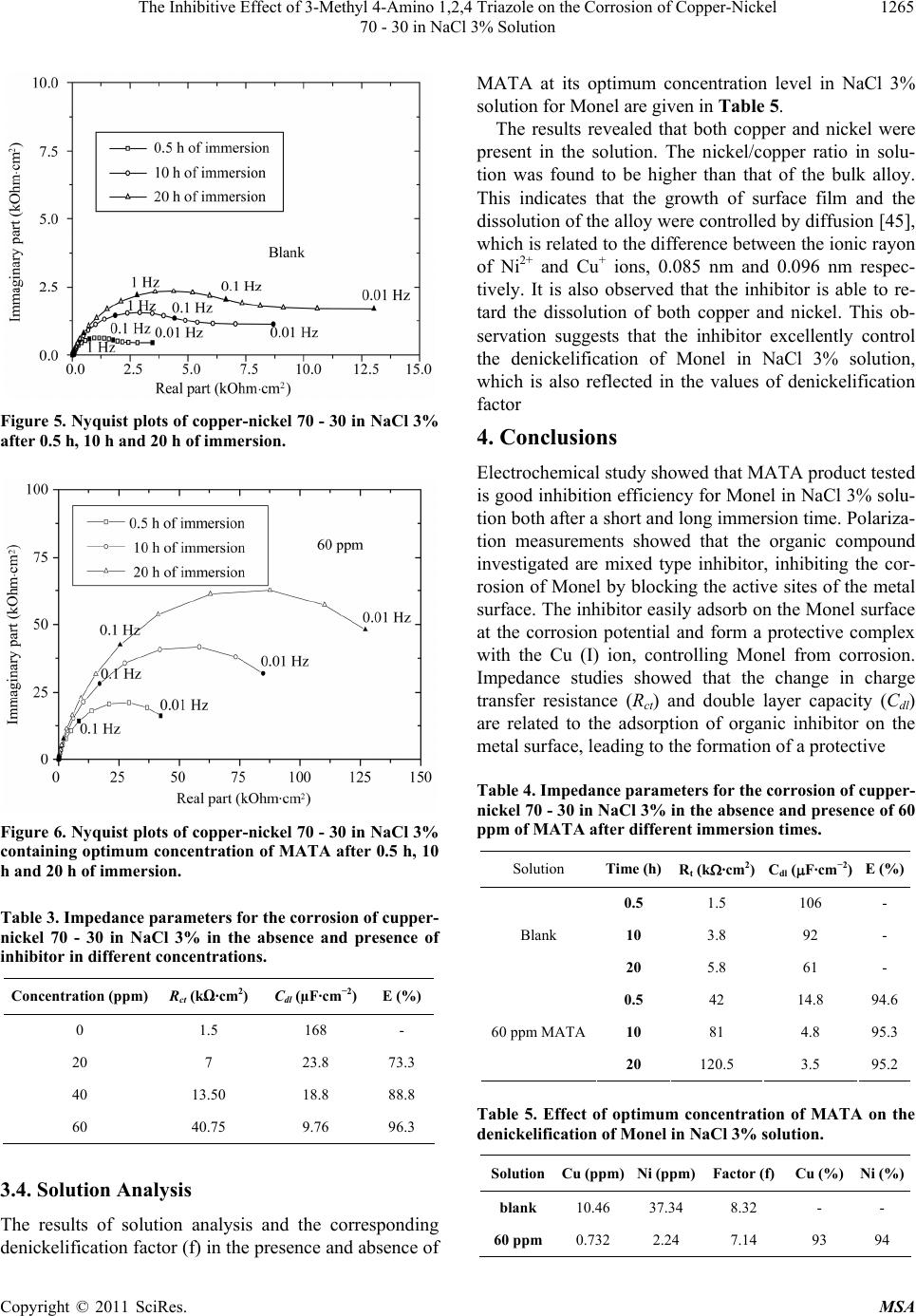 The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 1265 70 - 30 in NaCl 3% Solution Figure 5. Nyquist plots of copper-nickel 70 - 30 in NaCl 3% after 0.5 h, 10 h and 20 h of immersion. Figure 6. Nyquist plots of copper-nickel 70 - 30 in NaCl 3% containing optimum concentration of MATA after 0.5 h, 10 h and 20 h of immersion. Table 3. Impedance parameters for the corrosion of cupper- nickel 70 - 30 in NaCl 3% in the absence and presence of inhibitor in different concentrations. Concentration (ppm) Rct (k·cm2) Cdl (µF·cm2) E (%) 0 1.5 168 - 20 7 23.8 73.3 40 13.50 18.8 88.8 60 40.75 9.76 96.3 3.4. Solution Analysis The results of solution analysis and the corresponding denickelification factor (f) in the presence and absence of MATA at its optimum concentration level in NaCl 3% solution for Monel are given in Table 5. The results revealed that both copper and nickel were present in the solution. The nickel/copper ratio in solu- tion was found to be higher than that of the bulk alloy. This indicates that the growth of surface film and the dissolution of the alloy were controlled by diffusion [45], which is related to the difference between the ionic rayon of Ni2+ and Cu+ ions, 0.085 nm and 0.096 nm respec- tively. It is also observed that the inhibitor is able to re- tard the dissolution of both copper and nickel. This ob- servation suggests that the inhibitor excellently control the denickelification of Monel in NaCl 3% solution, which is also reflected in the values of denickelification factor 4. Conclusions Electrochemical study showed that MATA product tested is good inhibition efficiency for Monel in NaCl 3% solu- tion both after a short and long immersion time. Polariza- tion measurements showed that the organic compound investigated are mixed type inhibitor, inhibiting the cor- rosion of Monel by blocking the active sites of the metal surface. The inhibitor easily adsorb on the Monel surface at the corrosion potential and form a protective complex with the Cu (I) ion, controlling Monel from corrosion. Impedance studies showed that the change in charge transfer resistance (Rct) and double layer capacity (Cdl) are related to the adsorption of organic inhibitor on the metal surface, leading to the formation of a protective Table 4. Impedance parameters for the corrosion of cupper- nickel 70 - 30 in NaCl 3% in the absence and presence of 60 ppm of MATA after different immersion times. Solution Time (h)Rt (k·cm2) Cdl (F·cm2)E (%) 0.5 1.5 106 - 10 3.8 92 - Blank 20 5.8 61 - 0.5 42 14.8 94.6 10 81 4.8 95.360 ppm MATA 20 120.5 3.5 95.2 Table 5. Effect of optimum concentration of MATA on the denickelification of Monel in NaCl 3% solution. SolutionCu (ppm)Ni (ppm) Factor (f) Cu (%)Ni (%) blank 10.46 37.34 8.32 - - 60 ppm0.732 2.24 7.14 93 94 Copyright © 2011 SciRes. MSA  The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 1266 70 - 30 in NaCl 3% Solution film, which grows with increasing exposure time. Solu- tion analysis revealed that the MATA excellently control the denickelification of monel in NaCl 3% solution. REFERENCES [1] E. Stupnisek-Lisac, A. Loncarich Bozich and I. Cafuk, “Low-Toxicity Copper Corrosion Inhibitors,” Corrosion, Vol. 54, No. 9, 1998, Document ID 98090713. [2] R. Gasparac, C. R. Martin and E. Stupnisek-Lisac, “In Situ Studies of Imidazole and Its Derivatives as Copper Corrosion Inhibitors. I. Activation Energies and Thermo- dynamics of Adsorption,” Journal of the Electrochemical Society, Vol. 147, No. 2, 2000, pp. 551-548. doi:10.1149/1.1393230 [3] A. Zaki, “Electrochemical Behaviour of Copper-Silver Alloys in Sodium Carbonate Aqueous Solution,” British Corrosion Journal, Vol. 36, No. 1, 2001, pp. 59-64. doi:10.1179/000705901101501505 [4] R. F. North and M. J. pryar, “The Influence of Corrosion Product on the Corrosion Rate of Cu-Ni Alloys,” Journal of Corrosion Science, Vol. 10, 1970, pp. 297-311. [5] M. A. Elmorsi, M. Y. El Sheikh, A. M. Bastweesy and M. M. Ghoneim, “Effect of Hydroxyl Compounds on Corro- sion Behaviour of Cu and Cu-Zn Alloys in 3% NaCl So- lutions,” Bulletin of Electrochemistry, Vol. 7, 1991, p. 158. [6] M. M. Osman, “Corrosion Inhibitor of Aluminum.-Brass in 3.5% NaCl Solution and Sea Water,” Journal of Mate- rials Chemistry and Physics, Vol. 71, 2001, p. 12. [7] M. A. Qureishi, I. H. Farooqi and P. A. Saini, “Inhibition of Dezincification of 70-30 Brass by Aminoalkyl Mer- captotriazoles,” British Corrosion Journal, Vol. 35, No. 1, 2000, pp. 78-80. [8] H. C. Shih and R. J. Tzou, “Effect of Benzotriazole of the Stress Corrosion Cracking and the Electrochemical Po- larization of 70/30 Brass,” Journal of Electrochemical Society, Vol. 138, No. 4, 1991, pp. 958-961. doi:10.1149/1.2085753 [9] G. Quartarone, G. Moretti and T. Bellami, “Using Indole to Inhibit Copper Corrosion in Aerated 0.5M Sulfuric Acid,” Corrosion, Vol. 54, No. 8, 1998, Document ID 98080606. [10] D. D. Macdonald, B. C. Syrett and S. S. Wing, “The Corrosion of Cu-Ni Alloys 706 and 715 Flowing Sea Water,” Corrosion, Vol. 35, No. 8, 1979, pp. 367-378. [11] D. D. Macdonald, B. C. Syrett and S. S. Wing, “Annual Report of Naval Research,” ONR Contrat, No. 00014-77- 0046, NR 36-116, 1979. [12] B. C. Syrett and D. D. Macdonald, “The Validity of Elec- trochemical Methods for Measuring Corrosion Rates of Copper-Nickel Alloys in Sea Water,” Corrosion, Vol. 35, No. 11, 1979, pp. 505-509. [13] B. Trachli, , “Etude sur la Corrosion du Cuivre en Milieu NaCl 0.5 M et sa Protection par des Inhibiteurs Organiques et des Films Polyméres Obtenus par Électro- polymerisation,” Cotutelle Thesis, P&M Curie University– Kénitra University, Paris–Kénitra, 2001. [14] R. Francis, “Effect of Pollutants on Corrosion of Copper Alloys in Sea Water,” British Corrosion Journal, Vol. 20, No. 4, 1985, pp. 167-173. [15] D. A. Jones, “Principles and Prevention of Corrosion,” Maxwell Macmillan Pub., New York, 1992. [16] M. A. Elmorsi and A. M. Hassanein, “Corrosion Inhibi- tion of Copper by Heterocyclic Compounds,” Journal of Corrosion Science, Vol. 41, 1999, pp. 2337-2352. [17] F. Ammeloot, C. Fiaud and E. M. M. Sutter, “Characteri- zation of the Oxide Layers on a Cu-13Sn Alloy in NaCl Aqueous Solution without and with 0.1 M Benzotria- zole,” Journal of Electrochemica Acta, Vol. 44, No. 15, 1999, pp. 2549-2558. [18] Y. I. Kusnetzov, “Organic Inhibitors of Corrosion of Metals,” Plenum Press, New York, 1996. [19] N. K. Patel, J. Franco and I. S. Patel, “Corrosion of 63/37 Brass in Citric Acid Solution and Its Inhibition by Az- ole-Type Compounds,” Journal of India Chemical Soci- ety, Vol. 54, 1997, p. 815. [20] C. W. Yan, H. C. Lin and C. N. Cao, “Investigation of Inhibition of 2-Mercaptobenzotriazole for Copper Corro- sion,” Journal of Electrochemica Acta, Vol. 45, No. 17, 2000, pp. 2815-2821. [21] D. Zhang, L. Gao and G. Zhou, “Inhibition of Copper Corrosion by Bis-(-Benzotriazolmethylene)-(2,5 Thiadi- azoly)-Disulfide in Chloride Media,” Journal of Applied Surface Science, Vol. 225, 2004, pp. 287-293. [22] G. Xue, G. Ding, P. Lu and J. Dong, “SERS, XPS, and Electrochemical Studies of the Chemisorption of Ben- zotriazole on a Freshly Etched Surface and an Oxidized Surface of Copper,” Journal of Physical and Chemistry, Vol. 95, 1991, pp. 7380-7384. [23] N. Bellakhal and M. Dechraoui, “Study of Benzotriazole Efficiency as Corrosion Inhibitor of Copper in Humid Air Plasma,” Journal of Materials Chemistry and Physics, Vol. 85, No. 2-3, 2004, pp. 366-369. [24] R. Yuda, H. Nishihara and K. Aramaki, “A SERS Study on Inhibition Mechanisms of Benzotriazole and Its Deri- vates for Copper Corrosion in Sulfate Solutions,” Journal of Corrosion Science, Vol. 28, No. 1, 1998, pp. 87-96. [25] J. Bukowska and A. Kudelski, “The Use of Surface En- hanced Raman Scatting (SERS) to Probe the Interaction of Imidazole with the Silver Electrode Surface,” Journal of Electroanalytical Chemistry and Interfacial Chemistry, Vol. 309, No. 1-2, 1991, pp. 251-261. [26] D. Tromans and J. C. Silva, “The Anodic Behaviour of Copper in Chloride/Tolyltriazole and Chloride Benzotri- azole Solution,” Journal of Corrosion, Vol. 53, 1997, pp. 16-25. [27] S.K Bag, S. B. Shakraborty, A. Roy and S. R. Chaudhury, “2-Aminobenzimidazole as Corrosion Inhibitor for 70-30 Brass in Ammonia,” British Corrosion Journal, Vol. 31, No. 3, 1996, pp. 207-212. Copyright © 2011 SciRes. MSA  The Inhibitive Effect of 3-Methyl 4-Amino 1,2,4 Triazole on the Corrosion of Copper-Nickel 70 - 30 in NaCl 3% Solution Copyright © 2011 SciRes. MSA 1267 [28] J. Shukla and K. S. Pitre, “Electrochemical Behavior of Brass in Acid Solutions and the Inhibitive Effect of Imi- dazole,” Corrosion Reviews, Vol. 20, No. 3, 2002, pp. 217-229. [29] R. Walker, “Aqueous Corrosion of Tin-Bronze and Inhi- bition by Benzotriazole,” Corrosion, Vol. 56, No. 12, 2000, pp. 1211-1219. [30] A. M. Fenelon and C. B. Brezlin, “An Electrochemical Study of the Formation of Benzotriazole Surface Films on Copper, Zinc and Copper-Zinc Alloy,” Journal of Applied Electrochemistry, Vol. 31, No. 5, 2001, pp. 509-516. doi:10.1023/A:1017503031045 [31] K. Aramaki, T. Kiuchi, T. Simuyoshi and H. Nishihara, “Surface Enhanced RAMAN Scattering and Impedance Studies on the Inhibition of Copper Corrosion in Sulfate Solution by 5-Substituted Benzotriazoles,” Journal of Corrosion Science, Vol. 32, No. 5-6, 1991, pp. 593-607. [32] R. Subramanian and V. Lakshiminarayanan, “Effect of Adsorption of Some Azoles on Copper Passivation in Alkaline Medium,” Journal of Corrosion Science, Vol. 44, No. 3, 2002, pp. 535-554. [33] B. Trachli, M. Keddam, H. Takenouti and A. Srhiri, “Protective Effect of Electropolymerized 3-Amino-1,2,4- Triazole towards Corrosion of Copper in 0.5 M NaCl So- lution,” Journal of Corrosion Science, Vol. 44, No. 5, 2002, pp. 997-1008. [34] A. Nagiub and F. Mansfeld, “Evaluation of Corrosion Inhibition of Brass in Chloride Media Using EIS and ENA,” Journal of Corrosion Science, Vol. 43, No. 11, 2001, pp. 2147-2171. [35] W. Qafsaoui, C. Blanc, N. Pebere, H. Takenouti, A. Srhiri and G. Mankowski, “Quantitative Characterization of Protective Films Grown on Copper in the Presence of Different Triazole Derivative Inhibitors,” Journal of Electrochemica Acta, Vol. 47, No. 27, 2002, pp. 4339-4345. [36] F. M. El-Kharafi and B. G. Ateya, “Effect of Sulfides on the Electrochemical Impedance of Copper in Benzotria- zole-Inhibited Media,” Journal of the Electroc Society, Vol. 149, No. 6,hemica 2002, pp. B206-B210. l doi:10.1149/1.1470656 [37] L. Tommesani, G. Brunoro, A. Fringnani, C. Monticelli and M. Dal Colle, “On the Protective Action of 1, 2, 3 Benzotriazole Derivative Films against Copper Corro- sion,” Journal of Corrosion Science, Vol. 39, No. 7, 1997, Society, Perkin itish Corrosion ol. 113, No. 7, 1966, pp. 1221-1237. [38] A. R. katritzky, S. Rachwal and B. Rachwal, “The Chem- istry of Benzotriazole. Part 3. The Aminoalkylation of Benzotriazole,” Journal of the Chemical Transactions, Vol. 1, 1987, pp. 799-804. [39] M. G. Fontana, “Corrosion Engineering,” 3rd Edition, McGraw-Hill Book Company, New York, 1987. [40] G. Petrova, E. Sokolova, S. Raicheva and P. Ivanov, “In- hibition of Copper Corrosion in a Simulated Cooling Water by Pyrophthalone Compounds,” Br Journal, Vol. 31, No. 1, 1996, pp. 55-60. [41] N. Hackemen, E. S. Snavelly and J. S. Payne, “Effects of Anions on Corrosion Inhibition by Organic Compound,” Journal of Electrochemical Society, V pp. 677-681. doi:10.1149/1.2424089 [42] R. G. Lakshmi, “Electrochemical and Wear Behaviour of Surface Modified Orthopaedic Titanium Alloys,” Ph.D. sion Journal, Vol. 35, Thesis, Anna University, India, 2003. [43] B. Ramesh Babou and R. Holz, “Corrosion and Hydrogen Permeation Inhibition for Mild Steel in HCl by Isomers of Organic Compounds,” British Corro No. 3, 2000, pp. 204-209. doi:10.1179/000705900101501254 [44] K. Es-Salah, F. Said, M. Benmessoud, N. Hajjaji, M. Aouial, H. Takenouti and A. Srhiri, “Corrosion of Cop- per-Nickel (Cu-30Ni) Alloy in 3% NaCl Solution, in Presence of Ammoniac to pH 9, 25: The Inhibition Effect of the Bitriazole (BITA),” Physical Chemical News, Vol. o. 1, 1977, pp. 1-18. doi:10.1016/0376-4583(77)90019-X 23, 2005, pp. 108-118. [45] W. J. Van Oojj, “Surface Composition, Oxidation and Sulfidation of Cold-Worked Brass and Brass-Coated Steel Wire as Studied by X-Ray Photoelectron Spectros- copy I. Surface Composition of Commercial Cold- Worked Brass,” Surface Technology, Vol. 6, N
|