 Materials Sciences and Applicatio ns, 2011, 2, 1243-1255 doi:10.4236/msa.2011.29168 Published Online September 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1243 The Role of Magnesium in Superalloys—A Review Kumkum Banerjee Research and Development Division, Tata Steel Limited, Jamshedpur, India. Email: kumkum.banerjee@tatasteel.com Received February 17th, 2011; revised May 20th, 2011; accepted June 17th, 2011. ABSTRACT The role of magnesium (Mg) in improving the high temperature mechanical properties of the superalloys, like creep, fatigue, tensile ductility, impact toughness etc. have been vividly studied by several authors. On the other hand, very few authors have contradicted the view of any beneficial effect of Mg on the mechanical properties. This review presents a summary of the open literature related to the effect of Mg on the microstructu re and mechanical properties of su peral- loys and from whi ch f urt her metallurgi cal re search on the unexamined topics are prop o sed. Keywords: Superalloy, Magnesium 1. Introduction Over more than three decades several studies have been made on the influence of micro alloying of wrought and cast superalloys with magnesium. It was well recognized that a small addition of minor elements such as B, Mg, Ca and rare earths etc. could improve the stress rupture and creep properties significantly [1]. The results with Mg addition indicate that an optimum addition of mag- nesium causes enhancement in high temperature me- chanical properties. e.g. creep life, creep rupture duc- tile= ity, high temperature tensile ductility, cyclic stress rupture properties creep-fatigue interaction, crack propa- gation and hot workability [2-4]. Mg has been shown to improve the creep properties and particularly the high temperature ductility of the wrought alloys due to re- finement of the grain boundary carbides and equilibrium segregation [5]. Magnesium addition can also prolong secondary creep stage and develop tertiary creep stage and simultaneously alloys possess longer stress rupture life than the alloys without Mg [6]. The detrimental ef- fect of sulfur has been found to be reduced by the addi- tion of Mg. The results on cast IN-718 superalloy showed that a small amounts of Mg improved impact toughness and decreased Nb segregation by decreasing secondary arm spacing, which resulted in less and smaller interdendritic Laves and MC eutectics [5]. Moreover, magnesium also decreased the quantity of eutectic by segregating to the phase boundaries and thus refining the eutectic. Small amounts of Mg produced a more spherical as well as more dispersive MC phase. In contrast to this, detrimental effects of Mg on creep life and ductility has been reported in some studies [7,8]. In the attempt to achieve favourable properties many invest- tigations have been made to assess the optimum Mg concentration in the alloys and its relationship to micro- structure and properties [1,9-16]. However, common conclusions have not yet been achieved on this problem. This might be due to the different procedures used to determine the Mg percent and lack of comparability of the influence of magnesium in small-scale laboratory test and industrial ingot. Thus, the unique effect of Mg has been established for many superalloys and the beneficial influence of Mg has been attributed to the refinement of carbides, -phase and Laves phase on grain boundary and reducing the detri- mental effect of sulfur. In this paper, the existing findings in this area are critically reviewed and the unexamined areas are pointed out for carrying out further research in this direction. 2. Influence of Mg on Mechanical Properties and Weldability of Superalloys 2.1. Mechanical Properties The high temperature symmetrical low cycle fatigue (LCF) (R = –1) and unsymmetrical LCF (R = 0.42) were studied by Xie et al. [15] on IN- 718 iron and nickel-base superalloys and it was reported that the micro alloying of Mg (30-50 ppm) showed beneficial effect on unsymmet- rical LCF, where crack growth rate decreased by a factor of 3 - 7. However, symmetrical tension-compression stress 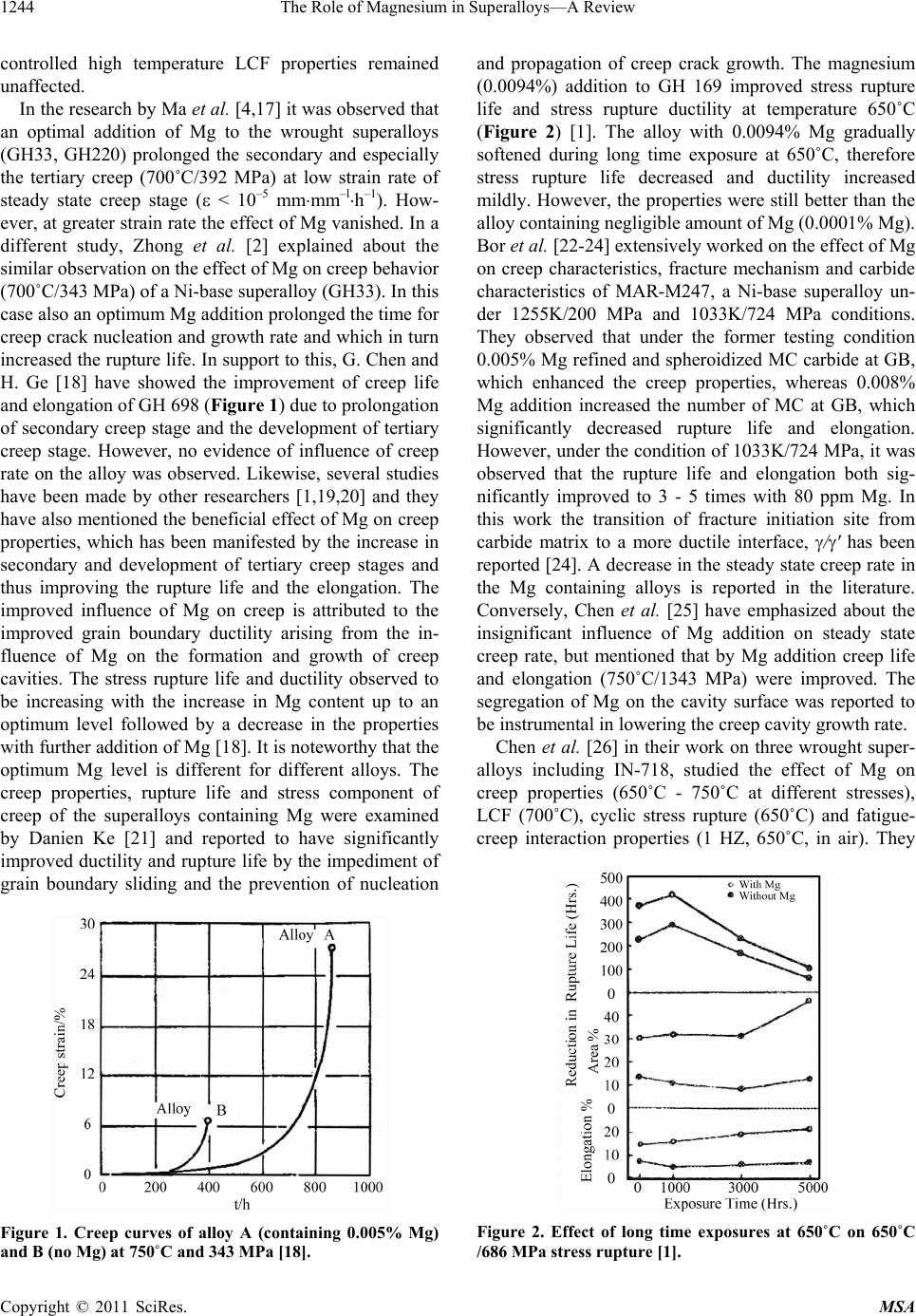 The Role of Magnesium in Superalloys—A Review 1244 controlled high temperature LCF properties remained unaffected. In the research by Ma et al. [4,17] it was observed that an optimal addition of Mg to the wrought superalloys (GH33, GH220) prolonged the secondary and especially the tertiary creep (700˚C/392 MPa) at low strain rate of steady state creep stage ( < 10–5 mmmm–lh–1). How- ever, at greater strain rate the effect of Mg vanished. In a different study, Zhong et al. [2] explained about the similar observation on the effect of Mg on creep behavior (700˚C/343 MPa) of a Ni-base superalloy (GH33). In this case also an optimum Mg addition prolonged the time for creep crack nucleation and growth rate and which in turn increased the rupture life. In support to this, G. Chen and H. Ge [18] have showed the improvement of creep life and elongation of GH 698 (Figure 1) due to prolongation of secondary creep stage and the development of tertiary creep stage. However, no evidence of influence of creep rate on the alloy was observed. Likewise, several studies have been made by other researchers [1,19,20] and they have also mentioned the beneficial effect of Mg on creep properties, which has been manifested by the increase in secondary and development of tertiary creep stages and thus improving the rupture life and the elongation. The improved influence of Mg on creep is attributed to the improved grain boundary ductility arising from the in- fluence of Mg on the formation and growth of creep cavities. The stress rupture life and ductility observed to be increasing with the increase in Mg content up to an optimum level followed by a decrease in the properties with further addition of Mg [18]. It is noteworthy that the optimum Mg level is different for different alloys. The creep properties, rupture life and stress component of creep of the superalloys containing Mg were examined by Danien Ke [21] and reported to have significantly improved ductility and rupture life by the impediment of grain boundary sliding and the prevention of nucleation Figure 1. Creep curves of alloy A (containing 0.005% Mg) and B (no Mg) at 750˚C and 343 MPa [18]. and propagation of creep crack growth. The magnesium (0.0094%) addition to GH 169 improved stress rupture life and stress rupture ductility at temperature 650˚C (Figure 2) [1]. The alloy with 0.0094% Mg gradually softened during long time exposure at 650˚C, therefore stress rupture life decreased and ductility increased mildly. However, the properties were still better than the alloy containing negligible amount of Mg (0.0001% Mg). Bor et al. [22-24] extensively worked on the effect of Mg on creep characteristics, fracture mechanism and carbide characteristics of MAR-M247, a Ni-base superalloy un- der 1255K/200 MPa and 1033K/724 MPa conditions. They observed that under the former testing condition 0.005% Mg refined and spheroidized MC carbide at GB, which enhanced the creep properties, whereas 0.008% Mg addition increased the number of MC at GB, which significantly decreased rupture life and elongation. However, under the condition of 1033K/724 MPa, it was observed that the rupture life and elongation both sig- nificantly improved to 3 - 5 times with 80 ppm Mg. In this work the transition of fracture initiation site from carbide matrix to a more ductile interface, /' has been reported [24]. A decrease in the steady state creep rate in the Mg containing alloys is reported in the literature. Conversely, Chen et al. [25] have emphasized about the insignificant influence of Mg addition on steady state creep rate, but mentioned that by Mg addition creep life and elongation (750˚C/1343 MPa) were improved. The segregation of Mg on the cavity surface was reported to be instrumental in lowering the creep cavity growth rate. Chen et al. [26] in their work on three wrought super- alloys including IN-718, studied the effect of Mg on creep properties (650˚C - 750˚C at different stresses), LCF (700˚C), cyclic stress rupture (650˚C) and fatigue- creep interaction properties (1 HZ, 650˚C, in air). They Figure 2. Effect of long time exposures at 650˚C on 650˚C /686 MPa stress rupture [1]. Copyright © 2011 SciRes. MSA 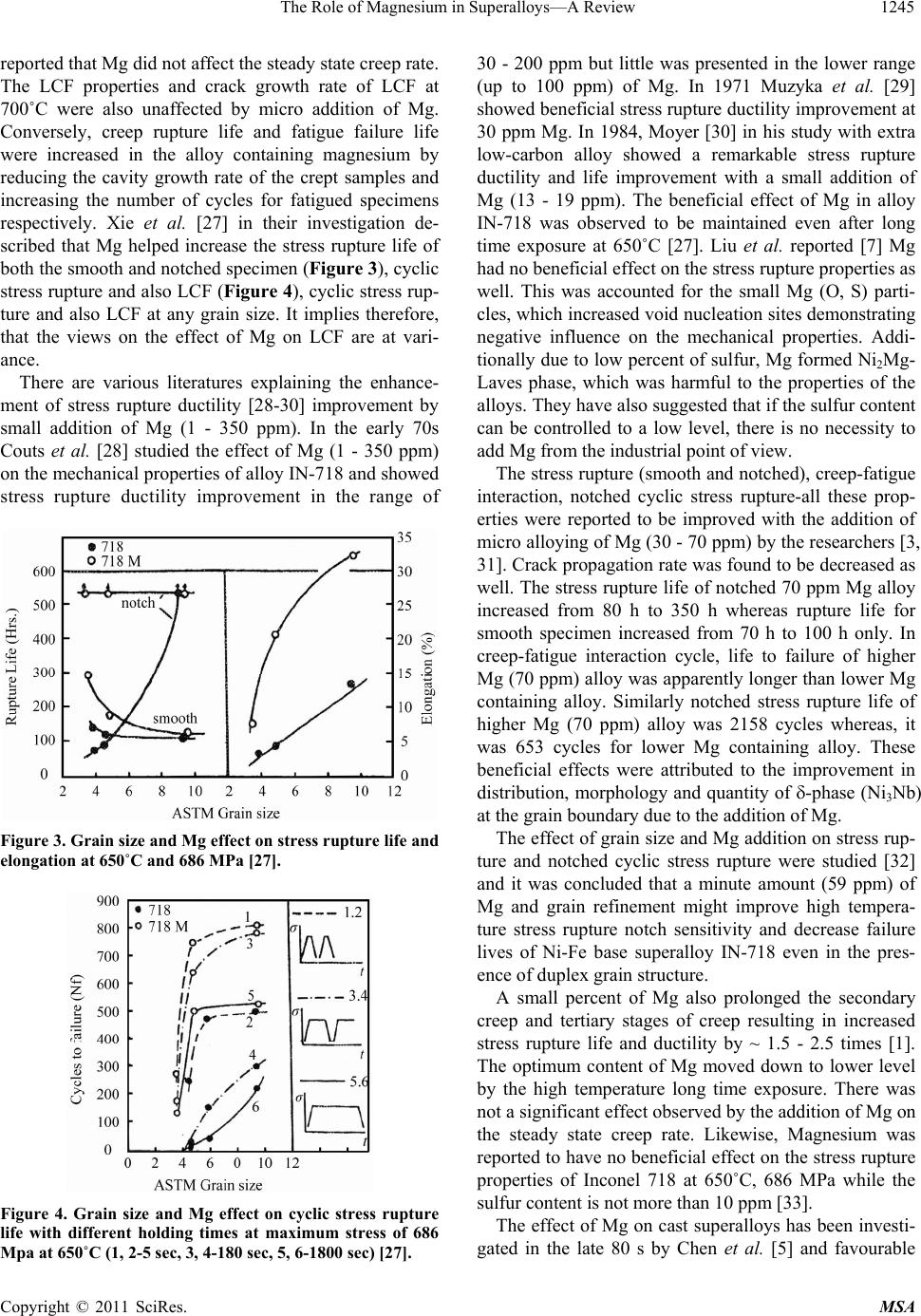 The Role of Magnesium in Superalloys—A Review 1245 reported that Mg did not affect the steady state creep rate. The LCF properties and crack growth rate of LCF at 700˚C were also unaffected by micro addition of Mg. Conversely, creep rupture life and fatigue failure life were increased in the alloy containing magnesium by reducing the cavity growth rate of the crept samples and increasing the number of cycles for fatigued specimens respectively. Xie et al. [27] in their investigation de- scribed that Mg helped increase the stress rupture life of both the smooth and notched specimen (Figure 3), cyclic stress rupture and also LCF (Figure 4), cyclic stress rup- ture and also LCF at any grain size. It implies therefore, that the views on the effect of Mg on LCF are at vari- ance. There are various literatures explaining the enhance- ment of stress rupture ductility [28-30] improvement by small addition of Mg (1 - 350 ppm). In the early 70s Couts et al. [28] studied the effect of Mg (1 - 350 ppm) on the mechanical properties of alloy IN-718 and showed stress rupture ductility improvement in the range of Figure 3. Grain size and Mg effect on stress rupture life and elongation at 650˚C and 686 MPa [27]. Figure 4. Grain size and Mg effect on cyclic stress rupture life with different holding times at maximum stress of 686 Mpa at 650˚C (1, 2-5 sec, 3, 4-180 sec, 5, 6-1800 sec) [27]. 30 - 200 ppm but little was presented in the lower range (up to 100 ppm) of Mg. In 1971 Muzyka et al. [29] showed beneficial stress rupture ductility improvement at 30 ppm Mg. In 1984, Moyer [30] in his study with extra low-carbon alloy showed a remarkable stress rupture ductility and life improvement with a small addition of Mg (13 - 19 ppm). The beneficial effect of Mg in alloy IN-718 was observed to be maintained even after long time exposure at 650˚C [27]. Liu et al. reported [7] Mg had no beneficial effect on the stress rupture properties as well. This was accounted for the small Mg (O, S) parti- cles, which increased void nucleation sites demonstrating negative influence on the mechanical properties. Addi- tionally due to low percent of sulfur, Mg formed Ni2Mg- Laves phase, which was harmful to the properties of the alloys. They have also suggested that if the sulfur content can be controlled to a low level, there is no necessity to add Mg from the industrial point of view. The stress rupture (smooth and notched), creep-fatigue interaction, notched cyclic stress rupture-all these prop- erties were reported to be improved with the addition of micro alloying of Mg (30 - 70 ppm) by the researchers [3, 31]. Crack propagation rate was found to be decreased as well. The stress rupture life of notched 70 ppm Mg alloy increased from 80 h to 350 h whereas rupture life for smooth specimen increased from 70 h to 100 h only. In creep-fatigue interaction cycle, life to failure of higher Mg (70 ppm) alloy was apparently longer than lower Mg containing alloy. Similarly notched stress rupture life of higher Mg (70 ppm) alloy was 2158 cycles whereas, it was 653 cycles for lower Mg containing alloy. These beneficial effects were attributed to the improvement in distribution, morphology and quantity of -phase (Ni3Nb) at the grain boundary due to the addition of Mg. The effect of grain size and Mg addition on stress rup- ture and notched cyclic stress rupture were studied [32] and it was concluded that a minute amount (59 ppm) of Mg and grain refinement might improve high tempera- ture stress rupture notch sensitivity and decrease failure lives of Ni-Fe base superalloy IN-718 even in the pres- ence of duplex grain structure. A small percent of Mg also prolonged the secondary creep and tertiary stages of creep resulting in increased stress rupture life and ductility by ~ 1.5 - 2.5 times [1]. The optimum content of Mg moved down to lower level by the high temperature long time exposure. There was not a significant effect observed by the addition of Mg on the steady state creep rate. Likewise, Magnesium was reported to have no beneficial effect on the stress rupture properties of Inconel 718 at 650˚C, 686 MPa while the sulfur content is not more than 10 ppm [33]. The effect of Mg on cast superalloys has been investi- gated in the late 80 s by Chen et al. [5] and favourable Copyright © 2011 SciRes. MSA 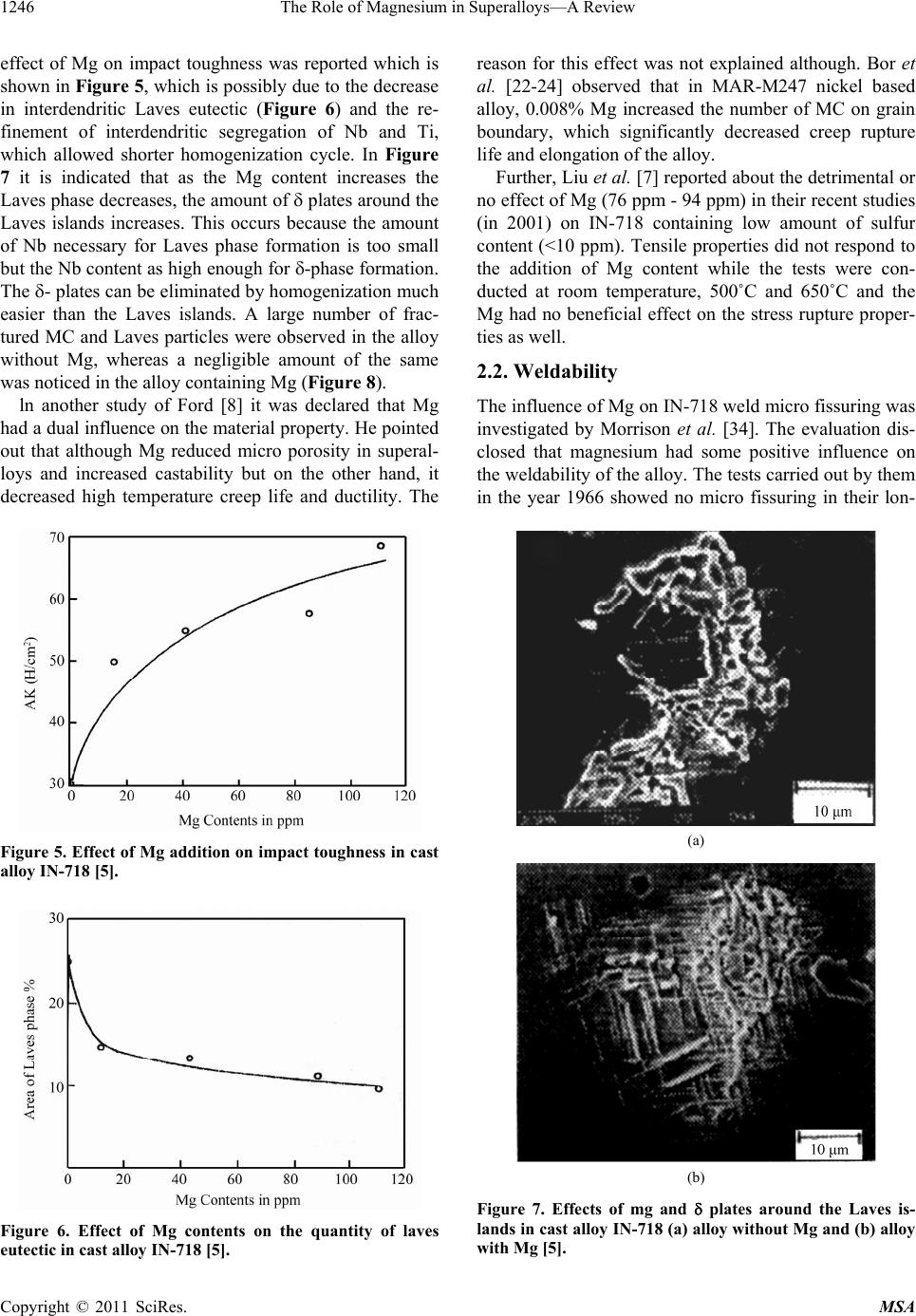 The Role of Magnesium in Superalloys—A Review Copyright © 2011 SciRes. MSA 1246 reason for this effect was not explained although. Bor et al. [22-24] observed that in MAR-M247 nickel based alloy, 0.008% Mg increased the number of MC on grain boundary, which significantly decreased creep rupture life and elongation of the alloy. effect of Mg on impact toughness was reported which is shown in Figure 5, which is possibly due to the decrease in interdendritic Laves eutectic (Figure 6) and the re- finement of interdendritic segregation of Nb and Ti, which allowed shorter homogenization cycle. In Figure 7 it is indicated that as the Mg content increases the Laves phase decreases, the amount of plates around the Laves islands increases. This occurs because the amount of Nb necessary for Laves phase formation is too small but the Nb content as high enough for -phase formation. The - plates can be eliminated by homogenization much easier than the Laves islands. A large number of frac- tured MC and Laves particles were observed in the alloy without Mg, whereas a negligible amount of the same was noticed in the alloy containing Mg (Figure 8). Further, Liu et al. [7] reported about the detrimental or no effect of Mg (76 ppm - 94 ppm) in their recent studies (in 2001) on IN-718 containing low amount of sulfur content (<10 ppm). Tensile properties did not respond to the addition of Mg content while the tests were con- ducted at room temperature, 500˚C and 650˚C and the Mg had no beneficial effect on the stress rupture proper- ties as well. 2.2. Weldability ln another study of Ford [8] it was declared that Mg had a dual influence on the material property. He pointed out that although Mg reduced micro porosity in superal- loys and increased castability but on the other hand, it decreased high temperature creep life and ductility. The The influence of Mg on IN-718 weld micro fissuring was investigated by Morrison et al. [34]. The evaluation dis- closed that magnesium had some positive influence on the weldability of the alloy. The tests carried out by them in the year 1966 showed no micro fissuring in their lon- (a) Figure 5. Effect of Mg addition on impact toughness in cast alloy IN-718 [5]. (b) Figure 7. Effects of mg and plates around the Laves is- lands in cast alloy IN-718 (a) alloy without Mg and (b) alloy with Mg [5]. Figure 6. Effect of Mg contents on the quantity of laves eutectic in cast alloy IN-718 [5].  The Role of Magnesium in Superalloys-A Review 1247 (a) (b) Figure 8. Micro cracks in impact samples (cast IN-718) (a) alloy without Mg and (b) alloy with Mg [5]. gitudinal and transverse sections of the alloy by the addi- tion of Mg. The tests carried out in 1967 on the prewelded specimens (1150 ˚C/1 hr./AC), welded by TIG showed no cracking with 37 ppm of Mg. However, when the tests were conducted at the same preweld heat treated condition with 52 ppm Mg, micro fissuring was observed. Whereas the preweld temperature of 1060˚C was com- patible with both the Mg contents. Their result indicated that around 20 ppm of Mg weldability improvelnent started and at around 30 ppm Mg and a plateau value was observed. Liu et al. [7] reported about the detrimental or no ef- fect of Mg (76 ppm-94 ppm) in their recent studies (in 2001) on IN-718 containing low amount of sulfur content (<10 ppm). Tensile properties did not respond to the ad- dition of Mg content while the tests were conducted at room temperature, 500˚C and 650˚C. High temperature tensile ductility of wrought heat resistant alloy EP 199, showed a consistency in ductility in the work of Topilin and Tsvetkeva [35]. A fairly high strength and ductility characteristics at 20˚C and 900˚C were obtained with Mg concentration of 150 - 290 ppm. They declared that there was a critical amount of Mg (0.005%) below which the ductility of the alloy decreased sharply. An improved high temperature tensile ductility in a Mg containing Ni-base alloy was also reported by Xu et al. [31], but a little effect was realized on tensile strength. The tensile properties at high temperature of 650˚C were reported to be improved with 30-70 ppm alloying of Mg [3,31]. A significant increase in reduction in area (45%) and elon- gation (27%) was reported. Further, the beneficial effect of higher yield and ultimate tensile strength observed at ambient temperature, due to a small amount of Mg addi- tion, was observed to be disappeared at higher tempera- ture [1]. Further, Xie et al. reported that Magnesium had almost no influence on tensile strength and ductility in Inconel 718 alloys with low content of sulfur at room temperature, 500˚C and 650˚C [33]. 2.3. Hot Ductility Behavior of Superalloys Liu and his group [36] performed a series of hot tensile tests to study the effect of S and Mg on hot ductility of IN-690 alloy and reported about the beneficial effect of an appropriate addition of Mg. It was observed that with the increase in S content (0 - 80 ppm) there was sharp decrease in ductility at high temperatures 900˚C - 1150˚C in the absence of Mg. However, a very low level of sul- fur content (<10 ppm) showed excellent ductility in the temperature range 900˚C - 1200˚C. But when the sulfur content exceeded 20 ppm, the value of reduction in area was less than 50% at 900˚C - 1000˚C, which corre- sponded to poor hot ductility. It was indicated that sulfur had less influence on ductility at relatively higher tem- peratures (>1050˚C), which was accounted for softening of the alloy matrix and the possibility of increase in sul- fur solubility at higher temperature. When sulfur was about 40 ppm, the ductility was reported to be better with 160 ppm of Mg. But at the same sulfur level higher Mg content had detrimental effect on the ductility. In the late 70’s Yamaguchi et al. [37] studied the effect of minor elements (S, Ca, Mg, Y and Zr) on hot workability of solid solution strengthened Ni-base superalloys. The hot workability of Ni-base superalloys is mainly controlled by dS = %S – 0.8 X %Ca – 0.3 X Mg % – 0.5 X% Y – 0.1 X %Zr. The dS corresponds to the residual amount of sulfur that is not fixed by strong sulfide forming ele- ments-Ca, Mg, Y and Zr. An excellent hot ductility can be achieved with 0.003 > dS > 0.004. The ductility de- creased gradually with dS < 0.004 and became extremely poor when dS > 0.003. Both dS > 0 and dS < 0 lowered hot ductility. Hence in order to achieve superior ductility dS should be nearly zero. In order to strengthen the grain boundaries and change the type of fracture from intergranular to transgranular Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review 1248 with a corresponding increase in ductility alloying boron, zirconia, magnesium and hafnium is recommended [38, 39] Boron atoms, which are not chemically bonded, to- gether with the atoms of zircon, hafnium and magnesium segregate to grain boundaries and contribute significantly to improving the high temperature properties of superal- loys at elevated temperatures [40]. From the aforementioned reported results it is implied that a small amount of Mg can significantly improve high temperature mechanical properties of some Ni and Fe-base superalloys. However, to maintain the optimum Mg concentration in various alloys, different techniques, e.g., VIM, VAR, ESR, VADER (Vacuum Arc Double Electrode Remelting) are being used and have been dis- cussed in the literature [41-43]. Yeniscavich and Fox [44] served a detrimental effect of Mg and Si on zero ductility while working on Hastelloy X. 3. Influence of Mg on Microstructure and Mechanisms Involved Magnesium has a great influence on the size, morphol- ogy, distribution and quantity of carbide and phases at grain boundaries. The studies indicated that Mg is a sur- face-active element and segregation of Mg takes place at the grain boundaries and the interphases like /carbide and / [45,46]. The segregation of Mg at the interfaces and interphases in turn accounted for the surface-active characteristic of Mg and hence its high propensity for segregation to interfaces and interphases [22]. Mg formed an enriched layer of Mg surrounding the carbide and might influence the transport of carbon and carbide forming elements. This resulted in an isotropic growth of carbides during solidification and eventually led to the refinement and spheroidization of MC carbides both at the grain boundary and within the grain interior [4, 22-24]. The spheroidization resulted from Mg addition improves the stress distribution state at grain boundary thus decreases the possibility of wedge crack at the grain boundary [17]. Hence the coalescence of grain boundary micro voids generated by carbides became the prominent mechanism of creep crack initiation and the crack initiation and propagation in the tertiary stages of creep were retarded and thus the rupture life was subsequently prolonged. The spheroidization of grain boundary carbides resulting from the micro alloying of Mg resisted the propagation of cracks and the carbides at the tip of the crack may become the nucleating centre for further growth of cracks. However, the stress concentration around the spher- oidized carbides is smaller than that of the plate-like car- bides and hence the possibility of crack nucleation is decreased lowering the crack growth rate. An optimal Mg addition inhibits the precipitation of coarse MC car- bide and causes the formation of a number of discrete M23C6 carbides at grain boundary. The morphology and distribution of carbides at grain boundaries are the major factors in determining the creep behaviour. These struc- ture changes decreased stress concentration at the inter- faces/grain boundaries during creep and hence prolong secondary and tertiary creep stages and simultaneously increases ductility at fracture. Generally a large amount of fine and discrete and less number of MC on grain boundary is beneficial for grain boundary strengthening and for inhibiting grain boundary migration. The discrete GB carbides are generally considered beneficial since they inhibit GB sliding and retard the onset of creep cavitation and rupture. Typically over addition of mag- nesium resulted in an exceptionally high amount of MC carbide at the grain boundary resulting in a sharp de- crease in M23C6 carbides. It has been reported that coarse GB MC carbide could weaken the boundaries and enhance intergranular cracking [47,48]. TEM results showed [6] that Mg addition to alloy GH36 could change grain boun- dary carbide distribution from continuous plate form to globular shapes and the M23C6 could be distributed in a non-continuous chain form (Figure 9(a), (b)). Fracto- graphic analysis showed that the intergranular cracks changed from wedge type to cavity type to cavity type (Figures 9(c), (d),) in a stress rupture test conducted at 650˚C, which implied Mg retarded intergranular crack propagation. SEM observation showed ductile fracture characteristics in modified GH 36 alloy in comparison with unmodified GH 36 (Figures 9(e), (f)). All these indicate that Mg addition strengthens the grain bounda- ries mainly due to the retardation in intergranular void initiation and the decrease in creep crack growth rate. Another mechanism of Mg micro addition in superal- loy causing the ductility enhancement, has been put for- ward by many researchers [1-2,6,49-51], is to purify the grain boundary through binding the detrimental elements like S, P, O etc. However, if the amount of the detrimen- tal species is far below 5 ppm, then this mechanism might not be applicable. The energy concept is a well-accepted mechanism by many researchers [2,42,50-52]. According to this mecha- nism, Mg segregates to the grain boundary and carbide/ matrix interfaces leading to the lowering of interfacial energy and consequently increasing the cohesion energy/ rupture energy between the carbide and the matrix or the boundary and the matrix [53,54]. Under this circum- stances crack path changes from the carbide/matrix in- terface to interface, a region having superior ductil- ity [55]. The crack initiation and propagation are even- tually retarded and rupture life and elongation are im- proved. The beneficial influence of Mg in changing the GB Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys-A Review 1249 (a) (b) (c) (d) (e) (f) Figure 9. Effect of magnesium on grain boundary carbide morphology (a, b), mode of grain boundary cracks (c, d) and char- acter of intergranular fracture (e, f) of GH 36 [6]. -Ni3Nb cellular precipitates is also reported in the lit- erature [1,4]. The continuous cellular precipitate/plate like precipitates of -Ni3Nb transforms to small amount of phases or discrete globular shapes in the presence of Mg hence retards intergranular crack growth, which si- multaneously increases stress rupture ductility and pro- longs failure life. The amount of -Ni3Nb depends on grain size, amount of Mg and heat treatment. However, the addition of Mg does not reported to have any signifi- cant influence on main strengthening phases and [1, 27]. Micro mechanical phase analysis results showed that the amount of strengthening phases of and phase is not affected by Mg addition or grain size in alloy IN-718 and 718M as shown in Figure 10 [27]. Mg-free and Mg-containing 718 M, both contained approximately 14% + independent of grain size. However, -Ni3Nb precipitation at grain boundaries increased with grain refinement and increase in amount of Mg. It has also been suggested that Mg with large atomic radius in the grain boundary areas might decrease the vacancy density and the diffusion coefficient of vacan- cies. The initiation and propagation rate of creep voids Copyright © 2011 SciRes. MSA 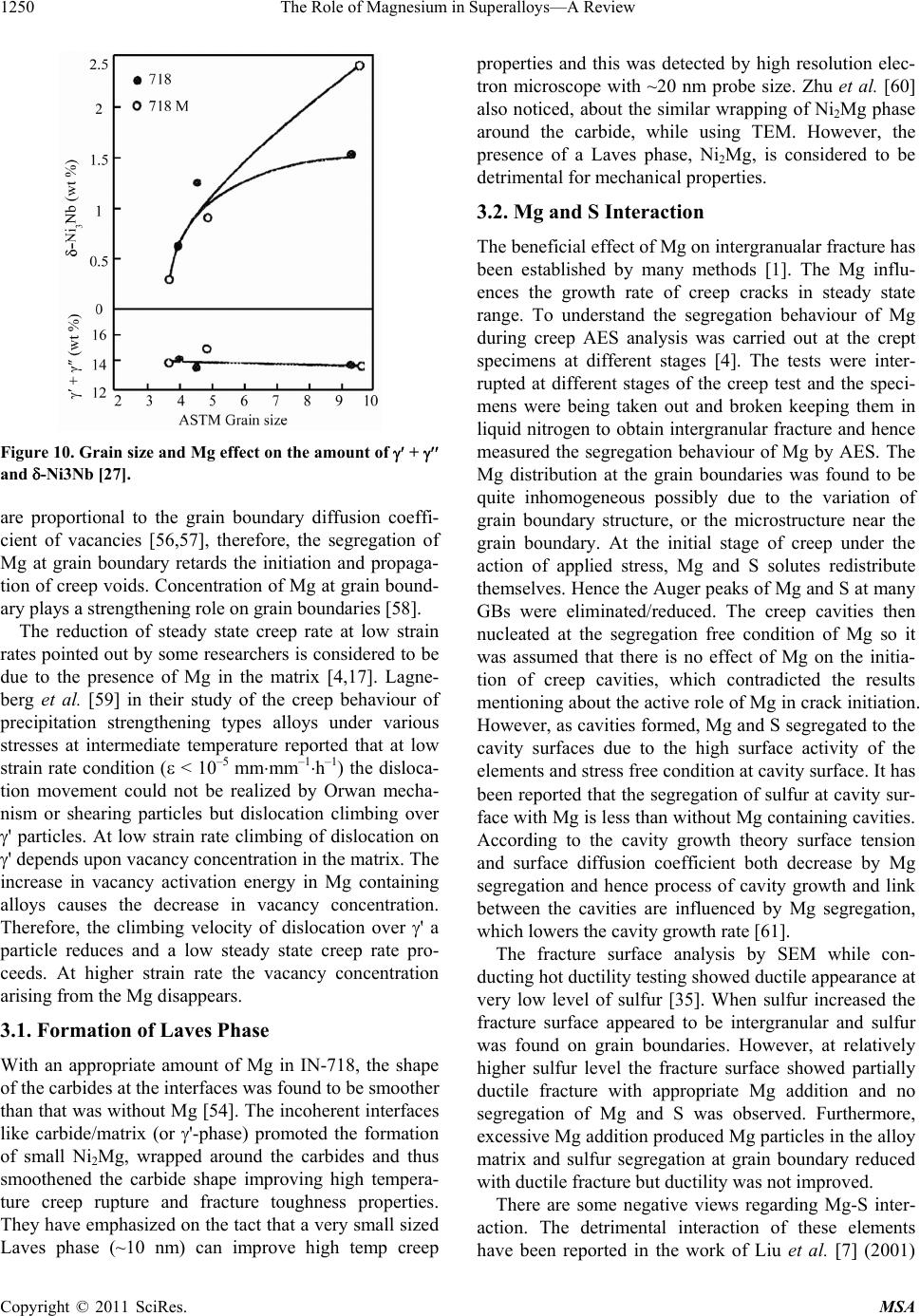 The Role of Magnesium in Superalloys—A Review 1250 Figure 10. Grain size and Mg effect on the amount of + and -Ni3Nb [27]. are proportional to the grain boundary diffusion coeffi- cient of vacancies [56,57], therefore, the segregation of Mg at grain boundary retards the initiation and propaga- tion of creep voids. Concentration of Mg at grain bound- ary plays a strengthening role on grain boundaries [58]. The reduction of steady state creep rate at low strain rates pointed out by some researchers is considered to be due to the presence of Mg in the matrix [4,17]. Lagne- berg et al. [59] in their study of the creep behaviour of precipitation strengthening types alloys under various stresses at intermediate temperature reported that at low strain rate condition ( < 10–5 mmmm–1h–1) the disloca- tion movement could not be realized by Orwan mecha- nism or shearing particles but dislocation climbing over ' particles. At low strain rate climbing of dislocation on ' depends upon vacancy concentration in the matrix. The increase in vacancy activation energy in Mg containing alloys causes the decrease in vacancy concentration. Therefore, the climbing velocity of dislocation over ' a particle reduces and a low steady state creep rate pro- ceeds. At higher strain rate the vacancy concentration arising from the Mg disappears. 3.1. Formation of Laves Phase With an appropriate amount of Mg in IN-718, the shape of the carbides at the interfaces was found to be smoother than that was without Mg [54]. The incoherent interfaces like carbide/matrix (or '-phase) promoted the formation of small Ni2Mg, wrapped around the carbides and thus smoothened the carbide shape improving high tempera- ture creep rupture and fracture toughness properties. They have emphasized on the tact that a very small sized Laves phase (~10 nm) can improve high temp creep properties and this was detected by high resolution elec- tron microscope with ~20 nm probe size. Zhu et al. [60] also noticed, about the similar wrapping of Ni2Mg phase around the carbide, while using TEM. However, the presence of a Laves phase, Ni2Mg, is considered to be detrimental for mechanical properties. 3.2. Mg and S Interaction The beneficial effect of Mg on intergranualar fracture has been established by many methods [1]. The Mg influ- ences the growth rate of creep cracks in steady state range. To understand the segregation behaviour of Mg during creep AES analysis was carried out at the crept specimens at different stages [4]. The tests were inter- rupted at different stages of the creep test and the speci- mens were being taken out and broken keeping them in liquid nitrogen to obtain intergranular fracture and hence measured the segregation behaviour of Mg by AES. The Mg distribution at the grain boundaries was found to be quite inhomogeneous possibly due to the variation of grain boundary structure, or the microstructure near the grain boundary. At the initial stage of creep under the action of applied stress, Mg and S solutes redistribute themselves. Hence the Auger peaks of Mg and S at many GBs were eliminated/reduced. The creep cavities then nucleated at the segregation free condition of Mg so it was assumed that there is no effect of Mg on the initia- tion of creep cavities, which contradicted the results mentioning about the active role of Mg in crack initiation. However, as cavities formed, Mg and S segregated to the cavity surfaces due to the high surface activity of the elements and stress free condition at cavity surface. It has been reported that the segregation of sulfur at cavity sur- face with Mg is less than without Mg containing cavities. According to the cavity growth theory surface tension and surface diffusion coefficient both decrease by Mg segregation and hence process of cavity growth and link between the cavities are influenced by Mg segregation, which lowers the cavity growth rate [61]. The fracture surface analysis by SEM while con- ducting hot ductility testing showed ductile appearance at very low level of sulfur [35]. When sulfur increased the fracture surface appeared to be intergranular and sulfur was found on grain boundaries. However, at relatively higher sulfur level the fracture surface showed partially ductile fracture with appropriate Mg addition and no segregation of Mg and S was observed. Furthermore, excessive Mg addition produced Mg particles in the alloy matrix and sulfur segregation at grain boundary reduced with ductile fracture but ductility was not improved. There are some negative views regarding Mg-S inter- action. The detrimental interaction of these elements have been reported in the work of Liu et al. [7] (2001) Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review 1251 containing low sulfur content. The fractographic and micrographic analysis by SEM reveal that Mg had little effect on the microstructure of IN-718, i.e. grain size, -phase etc. The Auger research revealed no segregation of Mg at GB. The phase diagram of Ni-Mg shows no solution of Mg in Ni-matrix and Mg tends to segregate at the GB of the matrix. However, it is a matter of con- troversy since IN-718 is a multicomponent system and there is complex interaction among the alloying ele- ments. The sites at grain boundaries, which can absorb solute atoms, are limited. Hence the diffusion velocity of Mg is lower than that of the other elements and there is no room for Mg segregation. In this case, Mg appears either as MgO, MgS or Mg (O, S). However, Mg (O, S) is supposed to increase void nucleation sites, which is detrimental for, mechanical properties. This is the reason put forward by the authors [7] for the opposite effects on mechanical properties of high and low sulfur levels. Moreover, if the sulfur is very low, Mg forms Ni2Mg type of Laves phase. Magnesium had no effect on stress rupture properties of Inconel 718 at 650˚C, 686 MPa while the S ≤ 10 ppm [33]. Liu et al. [7] also reported about the detrimental or no effect of Mg (76 ppm - 94 ppm) in their recent studies on IN-718 containing S < 10 ppm). 3.3. Magnesium Segregation The segregation behaviour of Mg to phase interfaces has been established by many using electron micro- probe technique, AES and EDS analysis on the TEM thin film and the results showed Mg segregation at MC/, '/ [62,63]. The beneficial effects of Mg addi- tion on grain boundaries are related to the grain bound- ary segregation behaviour and its influence on the grain boundary properties. Due to the segregation of Mg to the grain boundary, the following changes in the grains may occur [18]: 1) Grain boundary cohesive bond is intensified in terms of the increase and more homogeneous distribu- tion in electron density due to Mg segregation. 2) Grain boundary dislocation mobility decreases due to Mg atom segregation to dislocation cores and which may influence the creep rate if the grain boundary creep predominates over the creep in grains. 3) The vacancy formation energy is increased due to Mg segregation, which results in the decrease in grain boundary vacancy concentration and grain boundary diffusion coefficient. 4) The morphology of grain boundary precipitates may be changed due to the decrease in grain boundary energy, and thus the uniform granular grain boundary precipitates decreased the mobility of grain boundary migration, which results in the retardation of creep void initiation and growth. By EPM analysis, Mg concentration at the phase in- terface, interior of MC and the matrix of a Ni base su- peralloy has been measured –2.55 × 10–2, 0.437 × 10–2 and 0.956 × 10–2 % respectively. This indicates that Mg is enriched at the interface of the phases and not the in- terior of it. TEM analysis of ' interface, interior of ' and in the matrix were respectively 0.555, 0.252 and 0.256. Again the segregation of Mg at the interface is pronounced. Furthermore, AES results of phase interface also showed Mg segregation at the interface. The Mg segregation thickness at MC carbide interface is thicker than ' phase interface. This is because the lattice mis- match between the carbide and matrix is larger. The structure of MC carbide interface is incoherent and has a large deformed area. It creates a favourable thermody- namic condition for the Mg atom to segregate to MC phase interface. If Mg atom enters MC phase it increases Gibbs free energy, as a result it segregates at the inter- faces instead of the interior of the carbide. On the other hand, ' phase interface is semi-coherent and the struc- ture of the interface consists of dislocation arrays and impurities and the degree of distortion is also less-hence the segregation of Mg is easier in this case. Basically, the driving force for grain boundary segre- gation of an element is the elastic distortion energy, which is proportional to the square of the mismatch be- tween the atomic radius and the unoccupied hole radius. Hence, Mg should have a strong tendency to segregate to the GB due to its larger atomic radius compared with Nb and Mo. In addition, from the segregation theory, the solubility of an element in a matrix is an indication of the ability of an element to segregate, i.e., lower the solubility the stronger the tendency to segregate. Since the solubility of Mg is much less than Nb, Mo and W, Mg segregates to the grain boundary. As the mismatch of' Mg and Nb both are positive, a repulsive interaction between Mg and Nb segregation can be expected. Mg causes an additional lattice distortion and this distortion assumed to have promoted “B” and “C” segregation at GB. It is therefore, assumed that Mg segregation causes Nb segregation to decrease and the segregation of ‘B’ and “C” to increase. Additional potential energy gener- ated from the lattice distortion caused by Mg atoms may accelerate the segregation of' “B” at the GB [64]. Hence Mg helps eliminate the thick lamellae [65] or brittle strips of NbC and -Ni3Nb along the GB [52,65]. An example of globular type Ni3Nb along grain boundary of Mg (0.0094) containing GH 169 alloy is presented as opposed to cellular precipitates at grain boundary of alloy with negligible amount of Mg (0.0001) (Figure 11) [1]. Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review 1252 (a) (b) Figure 11. The influence of Mg on grain boundary -Ni3Nb precipitation in GH 169 (a) without Mg (0.0001) (b) with Mg (0.0094) [1]. 3.4. Mg in Carbides and Matrix The literatures [17,52,66] indicate that Mg not only seg- regates along GB but also into the carbides of GB and ' phase either along grain boundary or in the grains. The Mg dissolved in the ' phase and carbides lead to the composition change, i.e., increases the contents of W, Mo and Ti in the phases and therefore changes their lat- tice constants and increases the elastic energy, which is proportional to the increase in M23C6 thickness by caus- ing a large distortion due to the large atomic radius of Mg. Thus as the thickness of M23C6 reaches a critical value, the coherent interface becomes semi or incoherent, resulting in decrease in long range stress field and a de- crease in total energy [66]. It is noteworthy that a pre- cipitate phase always tends to minimize its surface en- ergy. Hence the M23C6 with incoherent interface gradu- ally becomes granular-which is beneficial for mechanical properties. However, over addition of Mg caused M23C6 lamellae to precipitate due to the increase of supersatura- tion of carbon at the GB [1]. 3.5. Behaviour of Mg in Cast Superalloys Mg improves the solidification structure of cast superal- loys [5]. It segregates to phase boundaries and refines the interdendritic MC carbides and ' eutectic-decreasing quantity of ' eutectic. The addition of Mg influences the grain size and decrease the secondary arm spacing. As a result, the interdendritic precipitates like Laves phase and MC eutectic are reduced. A decrease in Nb segregation was also reported by using electron microprobe analysis and this was considered good since MC eutectic and Laves phase were the results of Nb segregation. The morphology of MC also changed to spheroidal due to the addition of Mg. Hence an optimum amount of Mg de- creases Nb segregation and decreases initial cast segre- gation. which shortens homogenization cycle? However, the amount of -Ni3Nb plate around laves island in- creased. This occurs because the Nb available for Laves phase formation is too low but the Nb content for plate formation is high. Although both the phases are generally considered to be detrimental, however, the elimination of -Ni3Nb by homogenization is much easier than the elimination of laves islands. 4. Level of Mg in Superalloys The content of Mg from 30 - 70 ppm showed beneficial effect in improving plasticity, high temperature tensile ductility, stress rupture life, notched cyclic stress ru~1ture life and creep fatigue interaction ability [3]. The effect of Mg from 1 - 350 ppm on mechanical properties was studied and 30 - 200 ppm was reported to have beneficial effect on stress rupture ductility improvement —no data was presented in the lower range (up to 100 ppm) [28]. An enhanced stress rupture ductility effect was reported by addition of 30 ppm Mg [29]. A remark- able stress rupture ductility and life improvement was noticed with a small addition of Mg (13 - 19 ppm) [30]. The mechanical properties like tensile and stress rupture ductilities, smooth and notch stress-rupture lives, fatigue- creep interaction properties were reported to have in- creased by 59 ppm addition of Mg (S-40 ppm) [27]. Stress rupture and creep properties improve by the addi- tion of 94 ppm Mg in a vacuum melted IN-718 (S-50 ppm) [1]. The effect of Mg on cast alloys was reported to be beneficial in the optimum range of 40 - 80 ppm Mg addition [5]. By the addition of 20 ppm Mg impact Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review 1253 toughness was reported to have improved and influenced favourably the distribution of interdendritic Laves and MC particles. The materials contained Mg in the range of 0 - 110 ppm. In a study with 50 ppm Mg level it was concluded that Mg had no effect on LCF but creep prop- erties enhanced (S-40 ppm) [26]. High temperature creep properties were increased by the addition of 59 ppm Mg [32]. An improved influence of Mg on stress rupture, high tem- perature tensile ductility, fatigue creep interac- tion properties were reported with 59 ppm Mg [5]. No effect or detrimental effect of Mg has been re- ported in some literature [7]. The Mg did not show any influence on tensile strengths and ductilities and stress rupture life reported to have decreased when sulfur is less than 10 ppm. Mg added was 0, 76 and 94 ppm (S-10 ppm). 5. Thrust Areas for Further Work From the literature review it is obvious that the studies have been made on the high temperature tensile, creep, fatigue and impact toughness properties of Mg contain- ing superalloys, including IN-718 alloy in question. However, the studies on weldability of the material are scarce if non-existent. In late 1960s the weldability measurement by conducting gleeble hot ductility test was performed by Morrison et al. [34], and the beneficial role of Mg on weldability was reported. However, there was no mention about the production route of the material. Since there is propensity for the material to pick up Mg during melting practice—from the furnace lining, cruci- ble or the slag-the amount of optimum Mg reported by their study, which was considered to have beneficial in- fluence on the weldability of the Mg containing IN-718 was debatable. Moreover, Laves phase formation present in the dendritic structure poses difficulty during welding of IN-718 and as it has been observed in the literature that an optimum amount of Mg can modify the Laves phase and improve mechanical properties it is again worthwhile to study the role of Mg in influencing the weldability of IN-718. Furthermore, Mg being a sur- face-active element has the tendency to segregate at the interfaces and grain boundaries and hence it might have great impact in affecting intergranular liquation cracking in HAZ region of the weldments. In addition it is impera- tive to study the interactive effect of Mg with the detri- mental elements like S, P, and especially B in assessing the weldability behaviour of superalloys produced by using the vacuum technological routes (VIM, VAR and VADER). 6. Conclusions 1) Microalloying of Mg shows beneficial effect of de- creased low cycle fatigue crack growth rate by a factor of 3-7. 2) Mg prolongs secondary and tertiary creep at low strain rate ( < 10–5 mmmm–1s–1) of steady state and also prolongs rupture creep strength and elongation. 3) Mg refines and spheroidizes MC carbide at GB, thus improving the stress distribution state at GB, which in turn has decreases the possibility of wedge crack at the GB. This enhances the creep properties. 4) Beneficial and detrimental effects of Mg addition on rupture life and elongation, depend upon the operating stress and temperature. 5) Mg can improve tensile strength and ductility at high as well as low temperature. 6) Mg has been found to improve impact toughness, which is possibly due to the decrease in interdendritic Laves eutectic and the refinement of interdendritic seg- regation of Nb and Ti, which allowed shorter homogeni- sation cycle. 7) The effect of Mg on hot ductility behaviour is re- ported to be controversial. 8) Ni2Mg (Laves phase) wrapping around MC has been found to smoothen the carbide shape, thus improv- ing high temperature creep and fracture properties. 9) Mg scavenges sulfur giving ductile fracture with no magnesium and sulfur segregation on GB. 10) Mg helps eliminate thick lamellae or brittle strips of NbC and -Ni3Nb along the GB. 11) Segregation of Mg has been shown to occur into M23C6 and apart from along grain boundary and other surfaces. Thus the Mg containing M23C6 phase with in- coherent interface becomes granular, which is beneficial for mechanical properties. 12) In cast superalloys the segregation of Mg to phase boundaries improves the solidification structure and thus refining MC and eutectic. Mg also decreases secondary arm spacing and thus reducing interdendritic precipitates like MC and Laves eutectic. 13) The study of the effect of Mg in influencing HAZ liquation cracking and weldability of superalloys has been proposed. REFERENCES [1] G. Chen, D. Wang and Z. Xu, “Proceedings Superalloys 1984,” In: M. Gell, C. S. Kortovich, R. H. Bricknell, W. B. Kent and J. F. Radavich, Eds., The Metallurgical Soci- ety, AIME, Warrendale, PA 15086, 1984, pp. 611-620. [2] Z. Zhong, P. Ma, J. Zhuang, Y. Yuan and N. Jim, Pro- ceedings Superalloy 1992, TMS, Warrendale, PA, 1992, pp. 629-635. [3] J. Dong, W. Xie, Z. Xu, X. Xie and S. Zhang, Acta Metallurgica Sinica, Vol, 6. No. 6, 1993, pp. 405-409. [4] P. Ma, Y. Yuan and Z. Zhong, Acta Metallurgica Sinica,Vol. 3, No. 3, 1990, pp. 208-212. Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review 1254 [5] G. Chen, Q. Zhu, D. Wang, X. Xi and J. F. Radavich, Proceedings Superalloy 718, E. A. Loria, Ed., TMS, Warrendale, PA, 1989, pp. 545-551. [6] X . Xie, J. Liang and H. Jiang, Elsevier Applied Science, 1987, pp. 719-723. [7] X. Liu, J. Dong, X. Xie and K. Chang, “The Appearance of Magnesium and Its Effect on the Mechanical Proper- ties of Inconel 718 with Low Sulfur Content,” Materials Science and Engineering A, Vol. 303, 2001, pp. 262-266. doi:10.1016/S0921-5093(00)01789-5 [8] D. A. Ford, Metals Technology, Vol. 11, No. 10, 1984, pp. 438-445. [9] Z. M. Kalinina, et al., Production of Electric Steel’ (Pro- izvodstvo Clektrostali), 1972, pp. 94-100. [10] Z. M. Kalinina, et al., Production of Electric Steel' (Pro- izvodstvo Clektrostali), 1972, pp. 89-94. [11] Z. M. Kalinina, et al., “Effect of Microalloying with Magnesium on Structure and Properties of Nickel Base Alloys,” Proceedings Production of Steels and Alloys in Vacuum, Electroslag and Electron Beam Furnaces (in Russian), Part 2, Chermetinformatsiya, 1971, pp. 30-32. [12] O. V. Rutes, G. S. Chernyak and S. B. Maslenkov, “Ef- fect of Mg, Ca and Ba on structure and properties of heat-resistant alloys hardened with Ni3Al,” Special Steels and Alloys (in Russian), Metallurgiya, No. 2, 1973, pp. 41-47. [13] G. S. Chyernyak, O. V. Rutes and S. B. Maslenkov, “Ef- fect of Magnesium and Calcium on Structure and Proper- ties of Heat-Resistant Alloys,” Special Steels and alloys (in Russian), Metallurgiya, No. 1, 1972, pp. 89-97. [14] Z. M. Kalinina, et al., Metall (Metalli) SSSR, No. 4, 1973, pp. 193-196. [15] V. V. Topilin and V. K. Versina, Quality Steels and Al- loys, Moscow, 1976, pp. 108-113. [16] V. V. Topilin and V. K. Versina, Metalloved. Term Obrab Met., Vol. 11, 1977, pp. 10-13. [17] P. Ma, Y. Yuan and Z. Zhong, “Creep Behavior of Mag- nesium Microalloyed Wrought Superalloys,” Proceedings Superalloys 1988, The Metallurgical Soci ety/A IME, 1988, pp. 625-633. [18] G. Chen and H. Ge, Journal of University of Science & Technology, Beijing, Vol. 2, No. 2, 1995, pp. 84-91. [19] Y. Li, C. Sun, J. Liu and G. Chen, High Temperature Technology, Vol. 5, No. 4, November 1997, pp. 201-204. [20] Guo E. and F. Xu, Acta Metallurgica Sinica, October 1988, pp. 957-962. [21] D. Ke, Acta Metallurgica Sinica, Vol. 19, No. 5, October 1983, pp. a377-a384. [22] H. Y. Bor, C. Y. Ma and C. G. Chao, “The Influence of Mg on Creep Properties and Fracture Behaviors of Mar-M247 Superalloy under 1255 K/200 MPa”, Metal- lurgical and Materials Transactions A, Vol. 31A, May 2000, pp. 1365-1373. [23] H. Y. Bor, C. G. Chao and C. Y. Ma, “The Influence of Magnesium on Carbide Characteristics and Creep Be- havior of the Mar-M247 Superalloy,” Scripta Materialia, Vol. 38, No. 2, 1998, pp. 329-335. [24] H. Y. Bor, C. G. Chao and C. Y. Ma, “The Effects of Mg Micro Addition on the Mechanical Behavior and Fracture Mechanism of MAR-M247 Superalloy at Elevated Tem- peratures,” Metallurgical and Materials Transactions A, Vol. 30A, No. 3, 1999, pp.55l-561. [25] G. L. Chen, T. H. Zhang and W. Y. Yang, High Tem- perature Technology, Vol. 6, No. 3, 1988, pp. 149-152. [26] G. Chen, X. Xie, Z. Xu, J. Zhang, Chinese Journal of Metal Science and Technology, Vol. 7, No. 6, 1991, pp. 435-441. [27] X. Xie, Z. Xu, B. Qu, G. Chen and J. F. Radavich, Super- alloys 1988, S. Reichman, D. N. Duhl, G. Maurer, S. An- tolovich and C. Lund, Eds., AIME, Warrendale, PA, ,The Metallurgical Society/AIME, 420 C'wealth Dr., Warren- dale, PA, 1988, pp. 635-642. [28] W. H. Couts Jr., et al, “Effect of Mg as an Alloying Ele- ment in IN-718,” Report AEML-TR-7s-76, 1971. [29] D. R. Muzyka and C. R. Whitney, “Process for Making Ni-Base Precipitation Hardenable Alloys,” U.S. Patent 3,575,734, 20 April 1971. [30] J. M. Moyer, “Extra Low Carbon Alloy 718,” Proceed- ings Superalloys 1984, The metallurgical Society/AIME, 420 C'wealth Dr. Warrendale, PA 15086, pp. 223-254. [31] Z. Xu, X. Xie, B. Qu, G. Chen and J. F. Radavich, Sci- ence and Technology Beijing, Vol. 2, No. 6, 1989, pp. 560-567. [32] Z. Xu, B. Xu, X. Xie, S. Zhou and X. F. Cheng, Journal of Beijing University of Iron and Steel Technology, Vol. 9, No. 4, 1987, pp. 38-44. [33] X. S. Xie, J. X. Dong and M. C. Zhang, “Research and Development of Inconel 718 Type Superalloy,” Materials Science Forum, Vol. 539-543, March 2007, pp. 262-269. [34] T. J. Morrison, et al, Proceedings Symposium of Welding Research Council, Department of Mechanical Engineer- ing, University of Manitoba, Winnipeg, Canada R3T 2N2, 1969, pp. 47-67. [35] V. V. Topilin and Tsvetkova, Metalloved. Ternl. Obrab. Met., Vol. 2, February 1980, pp. 62-64. [36] K. Liu, et al., Proceedings 7th Symposium on Environ- mental Degradation, Breckenridge, Co, USA, 1995, pp. 509-517. [37] S. Yamaguchi, H. Kobayashi, T. Matsumiya and S. Ha- yami, Metals Technology, May 1979, pp. 170-175. [38] E. O. Ezugwu. J. Bonney and Y. Yamane, “An Overview of the Machinability of Aeroengine Alloys,” Journal of Materials Processing Technology, Vol. 134, 2003, pp. 233-253. doi:10.1016/S0924-0136(02)01042-7 [39] T. Baumgartner, K. Bothe, S. Hurta, W. M. Laanemae and V. Gerold, “Thermomechanical Fatigue of Nimonic 80A,” Advanced Materials and Processes—Proceedings of the First European Conference. EUROMAT ’89. Vol. 1, Aachen, FRG, 22-24 November 1989. pp. 535-540. [40] R. Sunulahpasic and M. Oruc, Metallurgica, Vol. 50, No. Copyright © 2011 SciRes. MSA  The Role of Magnesium in Superalloys—A Review Copyright © 2011 SciRes. MSA 1255 3, 2011, pp. 155-158. [41] J. Fu, H. Wang and E. P. Chen, Proceedings 7th ICVM, Tokyo, Japan, 1982, p.1226. [42] J. Fu and L. Gao, J. Vacuum Science and Technology, Vol. 5A, No. 4, Part 4, 1987, p. 2687. [43] J. Fu and L. Song, Proceedings 10th International Con- ference on Vacuum, Metallurgical Industry Press-Peoples Republic of China, Beijing, China, 1991, pp. 160-168. [44] W. Yeniscavich and C. W. Fox, Proceedings Symposium of Welding Research Council, Department of Mechanical Engineering, University of Manitoba, Winnipeg, Canada R3T 2N2, 1969, pp. 24-35. [45] F. Wang, Metal Physics, Mechanical Industry Press, Bei- jing, 1981, p. 47. [46] D. Ke and Z. Zhong, Acta Metallurgica Sinica, 1983, p. A377. [47] P. S. Kotval, J. D. Venables and R. W. Calder, Metallur- gical Transactions A, Vol. 3, 1972, pp. 453-458. [48] G. R. Leverant and M. Gell, Transactions TMS-AIME, Vol. 245, 1969, pp. 1167-1173. [49] G. S. Chyernyak, A. V. Smirnova and S. B. Maslenkov, Metall.(Metalli.) SSSR, No. 1, 1973, pp. 98-104. [50] D. Ke, Masters Thesis, CISRI, Beijing, China, 1981. [51] V. V. Topilin, Steel USSR, 1978, pp. 643-646. [52] D. Ke and Z. Zhong, Acta Metallurgica Sinica, Vol. 19, 1983, p. A377. [53] P. Ma J. Zhuang, J. Yang and L. Gao, Acta Metallurgica Sinica, Vol. 23, 1987, p. A195. [54] D. Ke and Z. Zhong, CISRI Technical Bulletin, Vol. 73, 1983, p. 3. [55] J. Zhu, Z. Y. Cheng and H. Q. Ye, “The Distribution and Morphology of Trace Mg at a Grain Boundary in a Ni-Base Superalloy,” Scripta Metallurgica, Vol. 23, 1989, pp. 1537-1542. doi:10.1016/0036-9748(89)90124-5 [56] K. L. Gasko, G. M. Janowski and B. J. Pletka, “The In- fluence of γ-γ′ Eutectic on the Mechanical Properties of Conventionally Cast MAR-M247,” Materials Science and Engineering, Vol. 104A, October 1988, pp. 1-8. [57] A. S. Argon, I-W. Chen and C.-W. Lan, Proceedings Creep-Fatigue Environmental Interaction, AIME, New York, 1980, pp. 46. [58] R. J. Raj, Engineering Materials Technology, Vol. 98, 1976, p. 132. doi:10.1115/1.3443355 [59] R. Lagneberg and B. Bergman, “The Stress/Creep Rate Behaviour of Precipitation-Hardened Alloys,” Metals Science, Vol. 10, No. 1, 1976, pp. 20-28. doi:10.1179/030634576790431462 [60] J. Zhu, P. Ma, S. Zhang and L. Gao, Proceedings XIth International Conference on Electron Microscopy, Kyoto, Japan, 1986, pp. 851-852. [61] T-J. Chuang and J. R. Rice, “The Shape of Intergranular Creep Cracks Growing by Surface Diffusion,” Acta Met- allurgica, Vol. 21, 1973, pp. 1625-1628. . doi:10.1016/0001-6160(73)90105-3 [62] Q. Zhu, D. Wang, H. Ge and G. Chen, Acta Metallurgica Sinica, June 1989, pp. A185-A189. [63] Q. Zhu, D. Wang, H. Ge and G. Chen, Acta Metallurgica Sinica, Vol. 2A, No. 6, 1989, pp. 408-411. [64] V. V. Topilin, Stahl, Vol. 11, 1978, p. 1074. [65] Y. Li, B. Xu, High Temperature Technology, Vol. 6, No. 4, November 1988, pp. 203-209. [66] R. Ming, “Physical Basis of Crystal Growth”, Shanghai Science & Technology Press, Shanghai, 1982, p. 364.
|